Gas Turbine Intercoolers: Introducing Nanofluids—A Mini-Review
Abstract
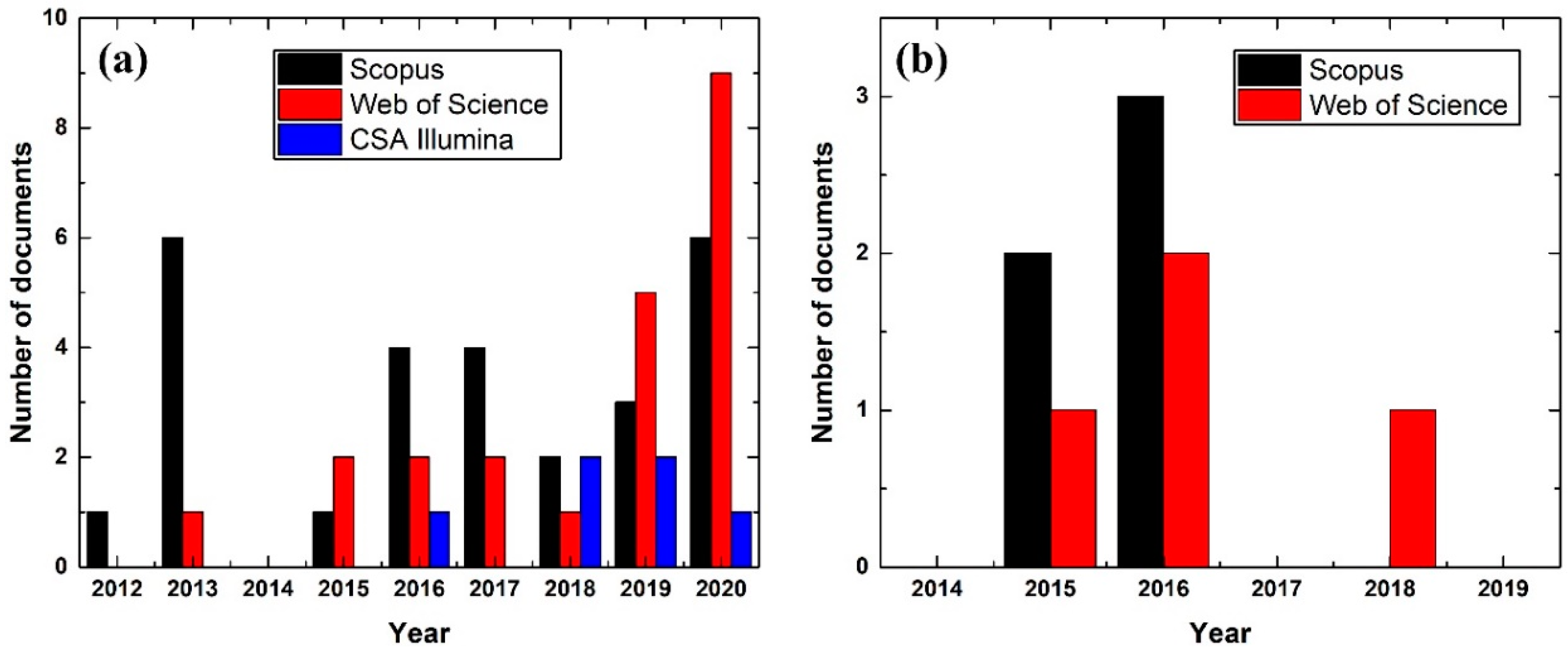
:1. Introduction
- The gas turbine industry has not yet opened up to this sort of advanced working fluids;
- The researchers working on the nanofluid field may have preferred to use the term HE to refer to intercoolers, as there are 1313 documents (1996–2020) on ‘Heat exchanger nanofluid’ in the Scopus database;
- The term ‘Nanofluid’ is often replaced by other terms, such ‘Suspension’, ‘Dispersion’, and ‘Mixture’; and/or
- Other publications do exist but are not covered by the explored search engines.
2. Gas Turbines Intercooled System and Their Designs
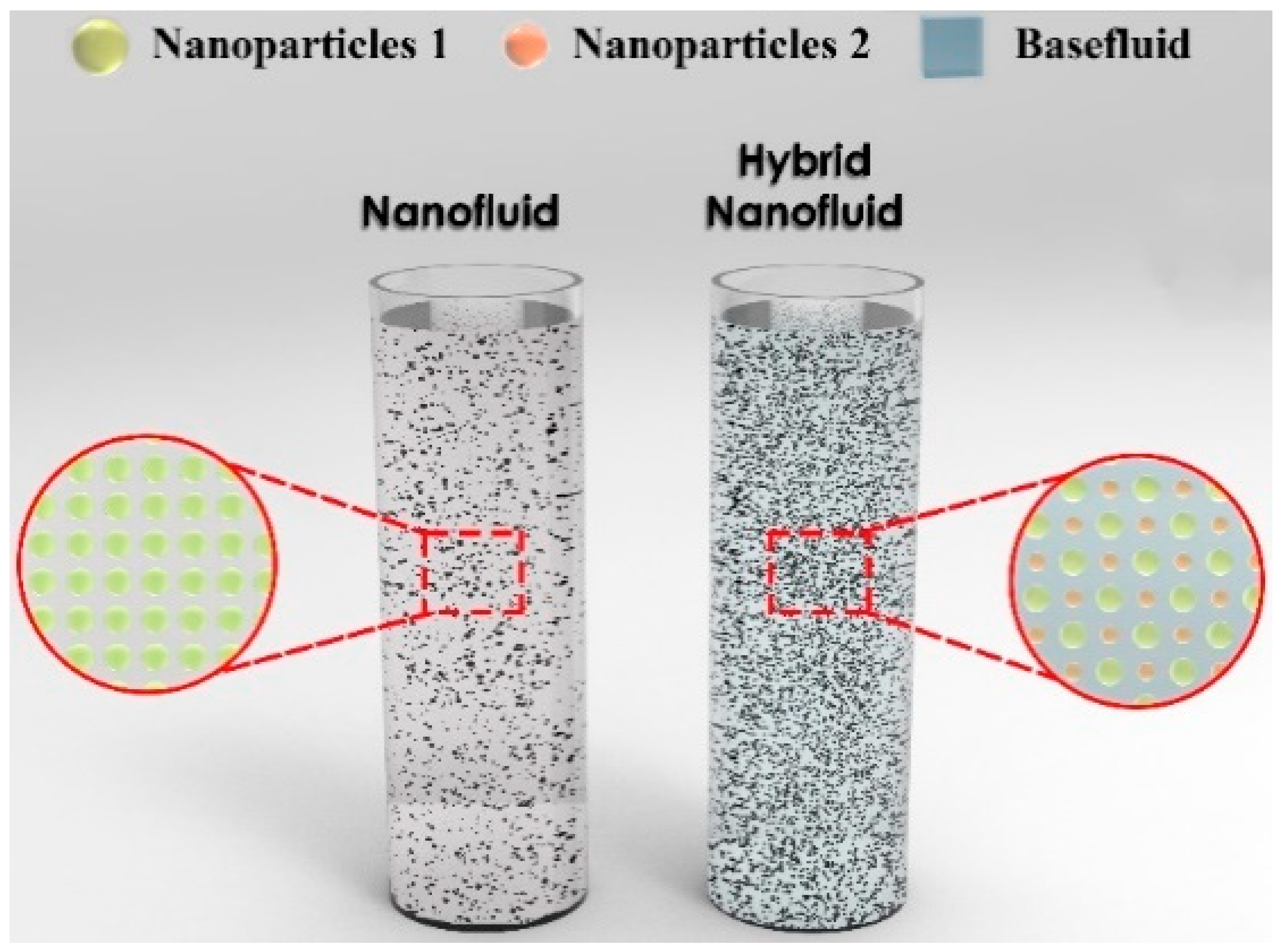
3. Nanofluids Types and Fabrication Processes
4. Dispersion Stability and Thermophysical Properties
5. Utilization of Nanofluids in Intercoolers
6. Discussion and Future Directions
- A feasibility study on utilizing different types of nanofluids needs to be conducted. It should consider the net cost of the starting materials (e.g., NPs and basefluids) against the gained performance enhancement of the system. At this point, there does not exist any such study on intercooler HEs [85].
- One of the most important aspects that is associated with the use of nanofluids is their environmental impact. This includes the different stages that the nanofluids undergo, such as NPs synthesis, nanofluids production, suspension transfer and employment in the system, and disposal. Currently, such studies are very limited, and at the same time, not available for intercoolers [86].
- Further investigation in terms of both experimental and theoretical approach should be conducted on the effect of employing nanofluids in gas turbine intercooler systems. This is because the current literature is not adequate for introducing such heat transfer fluids for the industry.
- In terms of dispersion stability, the chemical routes used for improving the stability of nanofluids, such as surfactants and NPs functionalization, would also cause their effective thermal conductivity to reduce [48,87]. The level of degradation in such thermal property is crucial when it comes to their thermal transport capability, and hence more investigations are needed in this area.
- Generally, in any application of elevated temperature, the working fluid tends to form fouling layers on the attached surfaces. Such layer formation is more rapidly seen when using nanofluids except for the thin film in such case would mostly contain deposited NPs that were initially hosted by the working fluid [2]. Researchers [88,89,90] have reported that the wettability of the surface changes with the type of film formed, layer thickness, and temperature of the attached fluid. Understanding how nanofluids fouling affect the wettability of different surfaces is important as it is directly linked to the pumping power required by the system, and hence the overall efficiency of the system.
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| CNT | Carbon nanotube |
| EG | Ethylene glycol |
| Temperature fitting parameter | |
| HE | Heat exchanger |
| Thermal conductivity (W/m.K) | |
| Length of nanoplatelet (nm) | |
| ND | Nanodiamond |
| NP | Nanoparticle |
| P&FHE | Plate-and-frame heat exchanger |
| PFHE | Plate-fin heat exchanger |
| PHE | Plate heat exchanger |
| Radius (nm) | |
| Impact of interfacial resistance | |
| Re | Reynolds number |
| SANSS | Submerged arc nanoparticles synthesis system |
| SS | Stainless steel |
| Mixture temperature (°C or K) | |
| Average flatness ratio | |
| Nanolayer Thickness (nm) | |
| VEROS | Vacuum evaporation onto a running oil substrate |
| vol. % | Scanning electron microscope |
| wt. % | Weight percentage |
| Greek letters | |
| Nanoplatelet thickness (nm) | |
| Dynamic viscosity (kg/m.s) | |
| Subscripts | |
| Basefluid | |
| Effective | |
| Nanoparticles | |
| Particle equivalent |
References
- Bhargava, R.K.; Bianchi, M.; Peretto, A.; Spina, P.R. A Feasibility Study of Existing Gas Turbines for Recuperated, Intercooled, and Reheat Cycle. J. Eng. Gas Turbines Power 2004, 126, 531–544. [Google Scholar] [CrossRef]
- Almurtaji, S.; Ali, N.; Teixeira, J.A.; Addali, A. On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers—A Review. Nanomaterials 2020, 10, 734. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Application of nanofluids in plate heat exchanger: A review. Energy Convers. Manag. 2015, 105, 1017–1036. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K. Alteration of Thermal Conductivity and Viscosity of Liquid by Dispersing Ultra-Fine Particles. Dispersion of Al2o3, Sio2 and Tio2 Ultra-Fine Particles. Netsu Bussei 1993, 7, 227–233. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995; PBD: Lemont, IL, USA, 1995. ANL/MSD/CP-84938. [Google Scholar]
- Norris, M.; Oppenheim, C. Comparing alternatives to the Web of Science for coverage of the social sciences’ literature. J. Inf. 2007, 1, 161–169. [Google Scholar] [CrossRef]
- Scopus-Database. Search Results for Documents Containing the Phrase ‘Gas Turbine Nanofluid’ from 2012 to 2020, in Elsevier. 2020. Available online: www.scopus.com (accessed on 1 September 2020).
- Scopus-Database. Search Results for Documents Containing the Phrase ‘Interrcooler Nanofluid’ from 2014 to 2019, in Elsevier. 2020. Available online: www.scopus.com (accessed on 1 September 2020).
- Web-of-Science-Database. Search Results for Documents Containing the Phrase ‘Gas Turbine Nanofluid’ from 2012 to 2020, in Clarivate Analytics. 2020. Available online: www.webofknowledge.com (accessed on 1 September 2020).
- CSA-Illumina-Database. Search Results for Documents Containing the Phrase ‘Gas Turbine Nanofluid’ from 2012 to 2020, in ProQuest. 2020. Available online: www.proquest.com (accessed on 1 September 2020).
- Web-of-Science-Database. Search Results for Documents Containing the Phrase ‘Interrcooler Nanofluid’ from 2014 to 2019, in Clarivate Analytics. 2020. Available online: www.webofknowledge.com (accessed on 1 September 2020).
- Alqallaf, J.; Ali, N.; Teixeira, J.A.; Addali, A. Solid Particle Erosion Behaviour and Protective Coatings for Gas Turbine Compressor Blades—A Review. Processes 2020, 8, 984. [Google Scholar] [CrossRef]
- Razak, A.M.Y. Gas turbine performance modelling, analysis and optimisation. In Modern Gas Turbine Systems; Jansohn, P., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 423–514. [Google Scholar]
- Alajmi, A.E.S.E.T.; Adam, N.M.; Hairuddin, A.A.; Abdullah, L.C. Fuel atomization in gas turbines: A review of novel technology. Int. J. Energy Res. 2019, 43, 3166–3181. [Google Scholar] [CrossRef]
- Zohuri, B.; McDaniel, P. Combined Cycle Driven Efficiency for Next Generation Nuclear Power Plants: An Innovative Design Approach; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Boggia, S.; Rüd, K. Intercooled Recuperated Gas Turbine Engine Concept. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005; p. 4192. [Google Scholar]
- Jeong, J.H.; Kim, L.S.; Lee, J.; Ha, M.; Kim, K.S.; Ahn, Y.C. Review of heat exchanger studies for high-efficiency gas turbines. In Turbo Expo: Power for Land, Sea, and Air; ASME: Montreal, QC, Canada, 2007; pp. 833–840. [Google Scholar]
- Schütz, U. New Mobile Gas Turbine with a Wide Range of Applications. Mech. Eng. 2018, 140, S54–S55. [Google Scholar] [CrossRef] [Green Version]
- Shepard, S.B.; Bowen, T.L.; Chiprich, J.M. Design and Development of the WR-21 Intercooled Recuperated (ICR) Marine Gas Turbine. J. Eng. Gas. Turbines Power 1995, 117, 557–562. [Google Scholar] [CrossRef]
- Jansohn, P. Modern Gas Turbine Systems: High Efficiency, Low Emission, Fuel Flexible Power Generation; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Pordanjani, A.H.; Aghakhani, S.; Wongwises, S.; Mahmoudi, B.; Mahian, O.; Wongwises, S. An updated review on application of nanofluids in heat exchangers for saving energy. Energy Convers. Manag. 2019, 198, 111886. [Google Scholar] [CrossRef]
- Mukherjee, R. Effectively design shell-and-tube heat exchangers. Chem. Eng. Prog. 1998, 94, 21–37. [Google Scholar]
- Wang, L.-S.; Pan, L. Optimum Peak Cycle Pressure for the Intercooled-Supercharged Gas Turbine Engine. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 1995; p. V002T003A003. [Google Scholar]
- Guo, J.; Cai, J.; Wang, H. Performance analysis of an irreversible regenerative intercooled Brayton cycle. Int. J. Exergy 2012, 11, 271. [Google Scholar] [CrossRef]
- Doori, W.H.A.R. Parametric Performance of Gas Turbine Power Plant with Effect Intercooler. Mod. Appl. Sci. 2011, 5, 173. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar] [CrossRef]
- Sekrani, G.; Poncet, S. Ethylene- and Propylene-Glycol Based Nanofluids: A Litterature Review on Their Thermophysical Properties and Thermal Performances. Appl. Sci. 2018, 8, 2311. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ji, W.; Mao, M.; Huang, J.-N. An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J. Clean. Prod. 2020, 257, 120408. [Google Scholar] [CrossRef]
- Saidina, D.S.; Abdullah, M.Z.; Hussin, M. Metal oxide nanofluids in electronic cooling: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 4381–4398. [Google Scholar] [CrossRef]
- Ali, H.M.; Babar, H.; Shah, T.R.; Sajid, M.U.; Qasim, M.A.; Javed, S. Preparation Techniques of TiO2 Nanofluids and Challenges: A Review. Appl. Sci. 2018, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Stability of Heat Transfer Nanofluids—A Review. ChemBioEng Rev. 2018, 5, 312–333. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Mamat, R.; Usri, N.; Najafi, G. Investigation of Al2O3 Nanofluid Viscosity for Different Water/EG Mixture Based. Energy Procedia 2015, 79, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Asadi, A.; Alarifi, I.M.; Foong, L.K. An experimental study on characterization, stability and dynamic viscosity of CuO-TiO2/water hybrid nanofluid. J. Mol. Liq. 2020, 307, 112987. [Google Scholar] [CrossRef]
- Aghahadi, M.H.; Niknejadi, M.; Toghraie, D. An experimental study on the rheological behavior of hybrid Tungsten oxide (WO3)-MWCNTs/engine oil Newtonian nanofluids. J. Mol. Struct. 2019, 1197, 497–507. [Google Scholar] [CrossRef]
- Kakavandi, A.; Akbari, M. Experimental investigation of thermal conductivity of nanofluids containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. Aluminium Nanofluids Stability: A Comparison between the Conventional Two-Step Fabrication Approach and the Controlled Sonication Bath Temperature Method. J. Nanomater. 2019, 2019, 3930572. [Google Scholar] [CrossRef] [Green Version]
- Halelfadl, S.; Maré, T.; Estellé, P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp. Therm. Fluid Sci. 2014, 53, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Pastoriza-Gallego, M.J.; Casanova, C.; Páramo, R.; Barbés, B.; Legido, J.L.; Piñeiro, M.M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Saini, A.; Sandhu, H.; Sharma, S.; Dasaroju, G. Nanofluids: A Review Preparation, Stability, Properties and Applications. Int. J. Eng. Res. Technol. IJERT 2016, 5, 11–16. [Google Scholar]
- Cabaleiro, D.; Graciafernandez, C.; Legido, J.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transf. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Sabiha, M.; Mostafizur, R.; Saidur, R.; Mekhilef, S. Experimental investigation on thermo physical properties of single walled carbon nanotube nanofluids. Int. J. Heat Mass Transf. 2016, 93, 862–871. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Conceptions for heat transfer correlation of nanofluids. Int. J. Heat Mass Transf. 2000, 43, 3701–3707. [Google Scholar] [CrossRef]
- Akilu, S.; Baheta, A.T.; Sharma, K.V.; Said, M.A. Experimental determination of nanofluid specific heat with SiO2 nanoparticles in different base fluids. AIP Conf. Proc. 2017, 1877, 090001. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Yu, H.; Hermann, S.; Schulz, S.E.; Gessner, T.; Dong, Z.; Li, W.J. Optimizing sonication parameters for dispersion of single-walled carbon nanotubes. Chem. Phys. 2012, 408, 11–16. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Zhang, S.; Han, X. Effect of different surface modified nanoparticles on viscosity of nanofluids. Adv. Mech. Eng. 2018, 10, 1687814018762011. [Google Scholar] [CrossRef]
- Naser, A.; Teixeira, J.A.; Addali, A. New pH Correlations for Stainless Steel 316L, Alumina, and Copper(I) Oxide Nanofluids Fabricated at Controlled Sonication Temperatures. J. Nano Res. 2019, 58, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Mashali, F.; Languri, E.M.; Davidson, J.; Kerns, D.; Johnson, W.; Nawaz, K.; Cunningham, G. Thermo-physical properties of diamond nanofluids: A review. Int. J. Heat Mass Transf. 2019, 129, 1123–1135. [Google Scholar] [CrossRef]
- Paul, G.; Chopkar, M.; Manna, I.; Das, P.K. Techniques for measuring the thermal conductivity of nanofluids: A review. Renew. Sustain. Energy Rev. 2010, 14, 1913–1924. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Tawfik, M.M. Experimental studies of nanofluid thermal conductivity enhancement and applications: A review. Renew. Sustain. Energy Rev. 2017, 75, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-J.; Li, M.-J.; He, Y.-L.; Tao, W.-Q. A novel semi-empirical model on predicting the thermal conductivity of diathermic oil-based nanofluid for solar thermal application. Int. J. Heat Mass Transf. 2019, 138, 1002–1013. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Sasmito, A.P.; Mujumdar, A.S. Measurement and modeling of thermal conductivity of graphene nanoplatelet water and ethylene glycol base nanofluids. Int. J. Heat Mass Transf. 2018, 123, 97–109. [Google Scholar] [CrossRef]
- Xue, Q. Model for thermal conductivity of carbon nanotube-based composites. Phys. B Condens. Matter 2005, 368, 302–307. [Google Scholar] [CrossRef]
- Doganay, S.; Alsangur, R.; Turgut, A. Effect of external magnetic field on thermal conductivity and viscosity of magnetic nanofluids: A review. Mater. Res. Express 2019, 6, 112003. [Google Scholar] [CrossRef]
- Yiamsawas, T.; Mahian, O.; Dalkilic, A.S.; Kaewnai, S.; Wongwises, S. Experimental studies on the viscosity of TiO2 and Al2O3 nanoparticles suspended in a mixture of ethylene glycol and water for high temperature applications. Appl. Energy 2013, 111, 40–45. [Google Scholar] [CrossRef]
- Walters, K.; Jones, W.M. Measurement of Viscosity. In Instrumentation Reference Book, 4th ed.; Boyes, W., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2010; Chapter 7; pp. 69–75. [Google Scholar]
- Żyła, G.; Cholewa, M. On unexpected behavior of viscosity of diethylene glycol-based MgAl2O4 nanofluids. RSC Adv. 2014, 4, 26057–26062. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Narahari, M.; Pendyala, R. Rheological characteristics of ultrastable diamond-thermal oil nanofluids. J. Mol. Liq. 2020, 309, 113098. [Google Scholar] [CrossRef]
- Akbari, M.; Afrand, M.; Arshi, A.; Karimipour, A. An experimental study on rheological behavior of ethylene glycol based nanofluid: Proposing a new correlation as a function of silica concentration and temperature. J. Mol. Liq. 2017, 233, 352–357. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Moravej, M.; Aberoumand, H.; Javaherdeh, K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl. Therm. Eng. 2016, 101, 362–372. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Wang, X.; Wang, X. Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys. Lett. A 2011, 375, 1323–1328. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass Transf. 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Rashidi, A.; Shariaty-Niassar, M. Convective heat transfer enhancement of graphene nanofluids in shell and tube heat exchanger. Exp. Therm. Fluid Sci. 2014, 53, 136–141. [Google Scholar] [CrossRef]
- Singh, D.; Timofeeva, E.V.; Yu, W.; Routbort, J.L.; France, D.M.; Smith, D.Y.; Lopez-Cepero, J.M. An investigation of silicon carbide-water nanofluid for heat transfer applications. J. Appl. Phys. 2009, 105, 064306. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.G.; Grulke, E.A.; Anderson, W.B.; Wu, G. Heat transfer properties of nanoparticle-in-fluid dispersions (nanofluids) in laminar flow. Int. J. Heat Mass Transf. 2005, 48, 1107–1116. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Timofeeva, E.V.; Singh, D.; Routbort, J.L. Comparative review of turbulent heat transfer of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 5380–5396. [Google Scholar] [CrossRef]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.J.; Townsend, J.; Christianson, R.J.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-W.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, E.; Singh, D.; Yu, W.; France, D. Engineered Nanofluids for Heat Transfer and Novel Applications. In Proceedings of the TechConnect 2013, NSTI Nanotechnology Conference and Expo 2013, Washington, DC, USA, 12–16 May 2013; pp. 404–406. [Google Scholar]
- Yu, W.; France, D.M.; Timofeeva, E.V.; Singh, D.J. Effective Thermal Conductivity Models for Carbon Nanotube-Based Nanofluids. J. Nanofluids 2013, 2, 69–73. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Yu, W.; France, D.M.; Singh, D.; Routbort, J.L. Base fluid and temperature effects on the heat transfer characteristics of SiC in ethylene glycol/H2O and H2O nanofluids. J. Appl. Phys. 2011, 109, 014914. [Google Scholar] [CrossRef] [Green Version]
- Timofeeva, E.; Singh, D.; Yu, W. Percolation theory at work—Boosting the heat transfer performance of graphitic nanofluids. In Proceedings of the TechConnect World 2014, NSTI Nanotechnology Conference and Expo 2014, NSTO-Nanotech 2014, Washington, DC, USA, 15–18 June 2014; pp. 420–423. [Google Scholar]
- Akhavan-Zanjani, H.; Saffar-Avval, M.; Mansourkiaei, M.; Ahadi, M.; Sharif, F. Turbulent Convective Heat Transfer and Pressure Drop of Graphene-Water Nanofluid Flowing Inside a Horizontal Circular Tube. J. Dispers. Sci. Technol. 2014, 35, 1230–1240. [Google Scholar] [CrossRef]
- Li, Z.; Asadi, S.; Karimipour, A.; Abdollahi, A.; Tlili, I. Experimental study of temperature and mass fraction effects on thermal conductivity and dynamic viscosity of SiO2-oleic acid/liquid paraffin nanofluid. Int. Commun. Heat Mass Transf. 2020, 110, 104436. [Google Scholar] [CrossRef]
- Zhao, N.; Wen, X.; Li, S. An Evaluation of the Application of Nanofluids in Intercooled Cycle Marine Gas Turbine Intercooler. In Proceedings of the ASME Turbo Expo, Montreal, QC, Canada, 15–19 June 2015. [Google Scholar]
- Zhao, N.; Wen, X.; Li, S. An Evaluation of the Application of Nanofluids in Intercooled Cycle Marine Gas Turbine Intercooler. J. Eng. Gas. Turbines Power 2016, 138, 012201. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Vafajoo, L.; Salman, B. Performance of CNT-water nanofluid as coolant fluid in shell and tube intercooler of a LPG absorber tower. Int. J. Heat Mass Transf. 2016, 102, 45–53. [Google Scholar] [CrossRef]
- Estellé, P. Comment on Performance of CNT-water nanofluid as coolant fluid in shell and tube intercooler of a LPG absorber tower. Int. J. Heat Mass Transf. 2016, 103, 1378–1379. [Google Scholar] [CrossRef] [Green Version]
- Chintala, V.; Vikesh, S.; Karn, A. Efficiency and effectiveness enhancement of an intercooler of two-stage air compressor by low-cost Al2O3/water nanofluids. Heat Transf. 2020, 49, 2577–2594. [Google Scholar] [CrossRef]
- Zhao, N.-B.; Wen, X.-Y.; Li, S.-Y. Dynamic Time-Delay Characteristics and Structural Optimization Design of Marine Gas Turbine Intercooler. Math. Probl. Eng. 2014, 2014, 701843. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.-Q.; Zhao, M.-Q.; Qian, W.; Wei, F. Carbon Nanotube Mass Production: Principles and Processes. ChemSusChem 2011, 4, 864–889. [Google Scholar] [CrossRef]
- Said, Z.; Arora, S.; Bellos, E. A review on performance and environmental effects of conventional and nanofluid-based thermal photovoltaics. Renew. Sustain. Energy Rev. 2018, 94, 302–316. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A.; Saeed, M.A.; Al-Zubi, F.; Sedaghat, A.; Bahzad, H. Deposition of Stainless Steel Thin Films: An Electron Beam Physical Vapour Deposition Approach. Materials 2019, 12, 571. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Teixeira, J.A.; Addali, A. Effect of Water Temperature, pH Value, and Film Thickness on the Wettability Behaviour of Copper Surfaces Coated with Copper Using EB-PVD Technique. J. Nano Res. 2019, 60, 124–141. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A.; Al-Zubi, F.; Shaban, E.; Behbehani, I. The effect of aluminium nanocoating and water pH value on the wettability behavior of an aluminium surface. Appl. Surf. Sci. 2018, 443, 24–30. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institusional affiliations. |




| Nanofluid Fabrication Methods | ||
|---|---|---|
| Production Route | Single-step | Two-step |
| Advantages |
|
|
| Disadvantages |
|
|
| Particles Dispersion |
|
|
| Property | Method | Device/Equation * | Source |
|---|---|---|---|
| Effective thermal conductivity | Experimental |
| [50,51,52,53] |
| Formula * | [54] | ||
| [55] | |||
| [56] | |||
| Effective viscosity | Experimental |
| [57,58,59,60] |
| Formula * | [61] | ||
| [62] | |||
| [63] |
| Researcher (/s) | NPs | Basefluid | Concentration | Additional Information | Finding |
|---|---|---|---|---|---|
| Yu et al. [64] | Graphene | EG | 2–5 vol. % | Two-step controlled temperature fabrication from 10 to 60 °C | The 5 vol. % suspension produced at 60 °C was 86% higher in thermal conductivity than pure EG at similar temperature. |
| Ghozatloo et al. [66] |
| Water | 0.01–0.05 wt. % |
|
|
| Zhang et al. [67] |
| Ionic liquid | – | Dispersion of particles was done through two stages. The first was through magnetic stirring (15 min), and the second by ultrasonication (1 h). |
|
| Timofeeva et al. [75] | Silicon carbide | EG–water | – |
|
|
| Timofeeva et al. [76] | f-graphite nanoplatelets | EG–water | Up to 5 wt. % |
| Increasing the NPs thickness and diameter have caused the effective thermal conductivity to improve up to 80% over the basefluid but had also raised the effective viscosity 100 times more. |
| Akhavan-Zanjani et al. [77] | Graphene | Water | 0.005–0.02 wt. % |
| 0.02 wt. % nanofluid showed an increase of 4.95% in viscosity compared to the basefluid. |
| Li et al. [78] | Silicon dioxide | Liquid paraffin–oleic acid | 0.005–5 wt. % |
| significant increases in the 5 wt. % nanofluids viscosity over the basefluid, precisely 331% (at 25 °C) and 495% (at 70 °C). |
| Scholar (/s) | NPs Type | Basefluid | NPs Concentration (vol. %) | Performance Enhancement | Additional Notes |
|---|---|---|---|---|---|
| Zhao et al. [79,80] |
| Water | 1–5 | Pumping power
| The study was performed theoretically on a marine gas turbine system that is integrated with an intercooler HE. |
| Masoud Hosseini et al. [81] |
| Water | 0.0055–0.278 | Heat transfer rate 10.3% | Theoretical modelling of an industrial LPG absorber tower utilizing CNTs nanofluids. |
| Estellé [82] |
| Water | 0.0055–0.278 | – | The author commented on the previous work of Masoud Hosseini et al. [81], where he pointed out that the effective viscosity equation used in the modeling process was inappropriate for the task. This is because the researchers used the formula for dispersed Al2O3 NPs instead of CNTs, which will only affect the results of nanofluids with ≥0.111 vol. % |
| Chintala et al. [83] |
| Water | 0.5–1 | Intercooler efficiency 36.1% at 4 bar compressor load | Experimental investigation on a double stage air compressor fitted with an intercooler HE. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsayegh, A.; Ali, N. Gas Turbine Intercoolers: Introducing Nanofluids—A Mini-Review. Processes 2020, 8, 1572. https://doi.org/10.3390/pr8121572
Alsayegh A, Ali N. Gas Turbine Intercoolers: Introducing Nanofluids—A Mini-Review. Processes. 2020; 8(12):1572. https://doi.org/10.3390/pr8121572
Chicago/Turabian StyleAlsayegh, Ali, and Naser Ali. 2020. "Gas Turbine Intercoolers: Introducing Nanofluids—A Mini-Review" Processes 8, no. 12: 1572. https://doi.org/10.3390/pr8121572






