Adsorption as a Process for Produced Water Treatment: A Review
Abstract
:1. Introduction
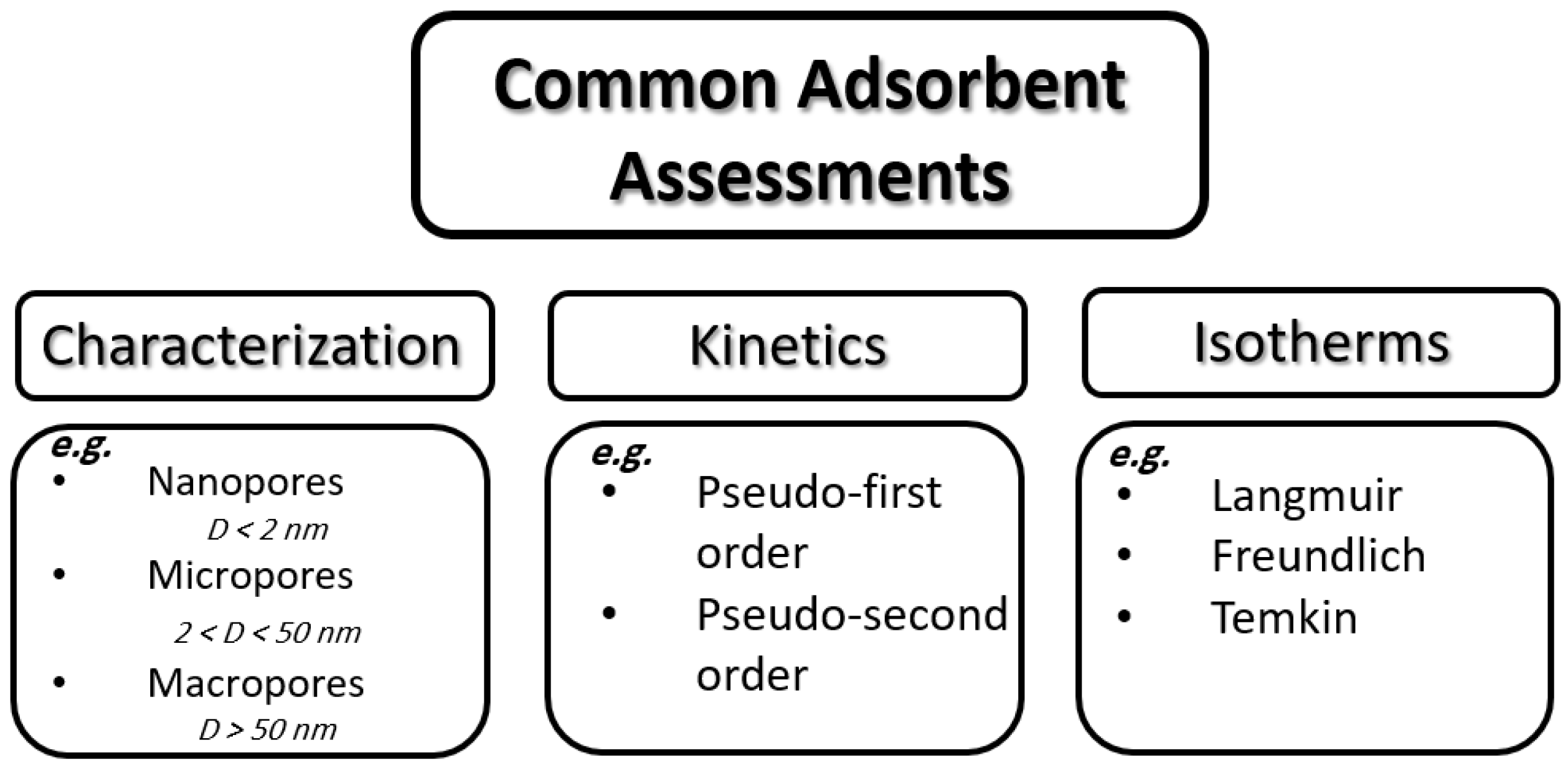
2. Adsorption
2.1. Characterization
2.2. Kinetics
2.3. Isotherms
2.3.1. Langmuir Isotherm
2.3.2. Freundlich Isotherm
2.3.3. Sips Isotherm
2.3.4. Dubinin–Radushkevich Isotherm
2.3.5. Temkin Isotherm
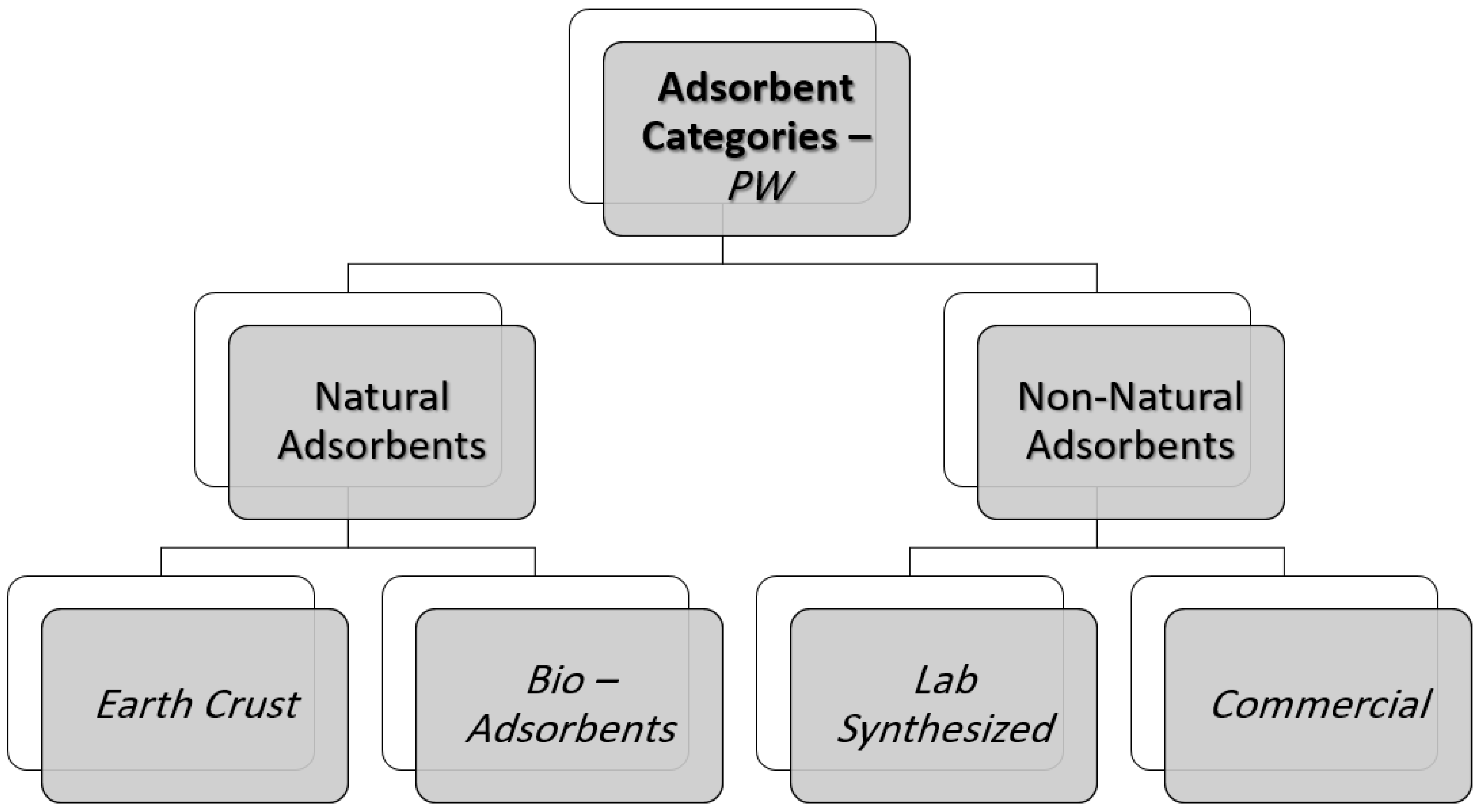
3. Produced Water Adsorbents
3.1. Natural Adsorbents
Earth Crust Adsorbents
3.2. Bio-Adsorbents
3.3. Non-Natural Adsorbents
3.3.1. Lab Synthesized Adsorbents
3.3.2. Commercial Adsorbents
4. Summary and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Veil, J.A. Produced Water Management Options and Technologies. In Produced Water: Environmental Risks and Advances in Mitigation Technologies; Springer: New York, NY, USA, 2011; pp. 537–571. [Google Scholar] [CrossRef]
- Hansen, B.R.; Davies, S.R. Review of potential technologies for the removal of dissolved components from produced water. Chem. Eng. Res. Des. 1994, 72, 176–188. [Google Scholar]
- Abdalla, M.; Nasser, M.; Kayvani Fard, A.; Qiblawey, H.; Benamor, A.; Judd, S. Impact of combined oil-in-water emulsions and particulate suspensions on ceramic membrane fouling and permeability recovery. Sep. Purif. Technol. 2019, 212, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Zsirai, T.; Qiblawey, H.; Buzatu, P.; Al-Marri, M.; Judd, S. Cleaning of ceramic membranes for produced water filtration. J. Pet. Sci. Eng. 2018, 166, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Guerra, K.; Dahm, K.; Dundorf, S. Oil and Gas Produced Water Management and Beneficial Use in the Western United States; Science and Technology Program Report; US Department of the Interior, Bureau of Reclamation Denver: Denver, CO, USA, 2011. [Google Scholar]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.; Purkait, M. Cross-flow ultrafiltration of stable oil-in-water emulsion using polysulfone membranes. Chem. Eng. J. 2010, 165, 447–456. [Google Scholar] [CrossRef]
- Zsirai, T.; Al-Jaml, A.K.; Qiblawey, H.; Al-Marri, M.; Ahmed, A.; Bach, S.; Watson, S.; Judd, S. Ceramic membrane filtration of produced water: Impact of membrane module. J. Sep. Purif. Technol. 2016, 165. [Google Scholar] [CrossRef] [Green Version]
- Weschenfelder, S.E.; Louvisse, A.M.; Borges, C.P.; Meabe, E.; Izquierdo, J.; Campos, J.C. Evaluation of ceramic membranes for oilfield produced water treatment aiming reinjection in offshore units. J. Pet. Sci. Eng. 2015, 131, 51–57. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Veil, J.; Puder, M.; Elcock, D.; Redweik, R.J., Jr. A White Paper Describing Produced Water from Production of Crude Oil, Natural Gas, and Coal Bed Methane; Prepared by Argonne National Laboratory; Department of Energy, National Energy Technology Laboratory: Lemont, IL, USA, 2004. [Google Scholar] [CrossRef] [Green Version]
- Judd, S.; Qiblawey, H.; Al-Marri, M.; Clarkin, C.; Watson, S.; Ahmed, A.; Bach, S. The size and performance of offshore produced water oil-removal technologies for reinjection. Sep. Purif. Technol. 2014, 134, 241–246. [Google Scholar] [CrossRef]
- Suez in the UK Zero Liquid Discharge. Available online: https://www.suez.co.uk/en-gb/our-offering/businesses/what-are-you-looking-for/water-management/equipment-and-systems/zero-liquid-discharge-and-thermal-products (accessed on 15 February 2020).
- Norwegian Petroleum Discharges to the Sea. Available online: https://www.norskpetroleum.no/en/environment-and-technology/discharges-to-the-sea/#:~:text=Norway%20established%20a%20zero%2Ddischarge,been%20achieved%20for%20chemical%20additives.&text=Chemicals%20that%20are%20not%20discharged,are%20treated%20as%20hazardous%20waste. (accessed on 15 February 2020).
- AlBatrni, H. Novel Adsorbents for the Removal of Oil from Produced Water. Ph.D. Thesis, Qatar University, Al-Dafna, Qatar, 2019. [Google Scholar]
- NPDES Compliance Inspection Manual; EPA: Washington, DC, USA, 2004.
- Mohr, K. An Overview of US and International Regulations Regarding Hydrocarbons in Water Effluents. Proc. Water Environ. Fed. 2000, 2000, 158–166. [Google Scholar] [CrossRef]
- Pedenaud, P. TOTAL Experience to Reduce Discharge of Hydrocarbons Through Produced Water. In Proceedings of the SPE International Health, Safety Environment Conference, Abu Dhabi, UAE, 2–4 April 2006. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook; IEA: Paris, France, 2019. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.D.; Langhus, B.G.; Patel, C. Technical Summary of Oil and Gas Produced Water Treatment Technologies; EPA: Washington, DC, USA, 2005.
- Lenntech. Chemical Properties of Barium. Available online: https://www.lenntech.com/periodic/elements/ba.htm (accessed on 15 July 2020).
- Hardi, M.; Siregar, Y.I.; Anita, S.; Ilza, M. Determination of heavy metals concentration in produced water of oil field exploration in siak regency. J. Phys. Conf. Ser. 2019, 1156, 012009. [Google Scholar] [CrossRef] [Green Version]
- Qaiser, M.S.H.; Ahmad, I.; Ahmad, S.R.; Afzal, M.; Qayyum, A. Assessing Heavy Metal Contamination in Oil and Gas Well Drilling Waste and Soil in Pakistan. Pol. J. Environ. Stud. 2019, 28, 785–793. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.A. Fundamentals of Momentum, Heat and Mass; Welty, J.R., Wicks, C.E., Wilson, R.E., Eds.; Wiley: Hoboken, NJ, USA, 1976; Volume 789, pp. 27–55, Fundamental Principles of Heat; Whetaker, S., Ed.; Cambridge University Press: Cambridge, UK, 1978; pp. 793–794. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics. Published by Imperial College Press and Distributed by World Scientific Publishing CO. 1998. Available online: http://xxx.lanl.gov/abs/https://www.worldscientific.com/ (accessed on 15 February 2020).
- Králik, M. Adsorption, chemisorption, and catalysis. Chem. Pap. 2014, 68. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B. An overview of dyes removal via activated carbon adsorption process. Desalin. Water Treat. 2011, 19, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Eren, Z.; Acar, F.N. Adsorption of Reactive Black 5 from an aqueous solution: Equilibrium and kinetic studies. Desalination 2006, 194, 1–10. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; McKay, G. Elovich equation and modified second-order equation for sorption of cadmium ions onto bone char. J. Chem. Technol. Biotechnol. 2000, 75, 963–970. [Google Scholar] [CrossRef]
- Allen, S.J.; Brown, P.A. Isotherm analyses for single component and multi-component metal sorption onto lignite. J. Chem. Technol. Biotechnol. 1995, 62, 17–24. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Wainipee, W.; Weiss, D.J.; Sephton, M.A.; Coles, B.J.; Unsworth, C.; Court, R. The effect of crude oil on arsenate adsorption on goethite. Water Res. 2010, 44, 5673–5683. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Cortés, F.B. Removal of oil from oil-in-saltwater emulsions by adsorption onto nano-alumina functionalized with petroleum vacuum residue. J. Colloid Interface Sci. 2014, 433, 58–67. [Google Scholar] [CrossRef]
- Fard, A.K.; Rhadfi, T.; Mckay, G.; Al-marri, M.; Abdala, A.; Hilal, N.; Hussien, M.A. Enhancing oil removal from water using ferric oxide nanoparticles doped carbon nanotubes adsorbents. Chem. Eng. J. 2016, 293, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.P.; Vidal, C.B.; Barros, A.L.; Costa, L.S.; Vasconcellos, L.C.; Dias, F.S.; Nascimento, R.F. Adsorption of BTX (benzene, toluene, o-xylene, and p-xylene) from aqueous solutions by modified periodic mesoporous organosilica. J. Colloid Interface Sci. 2011, 363, 626–634. [Google Scholar] [CrossRef] [Green Version]
- El-Naas, M.H.; Al-Zuhair, S.; Alhaija, M.A. Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 2010, 173, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Cortés, F. Water Remediation Based on Oil Adsorption Using Nanosilicates Functionalized with a Petroleum Vacuum Residue. Adsorpt. Sci. Technol. 2014, 32, 197–207. [Google Scholar]
- Kunjirama, M.; Saman, N.; Johari, K.; Song, S.T.; Kong, H.; Cheu, S.C.; Lye, J.; Mat, H. Adsorption affinity and selectivity of 3-ureidopropyltriethoxysilane grafted oil palm empty fruit bunches towards mercury ions. Environ. Sci. Pollut. Res. Int. 2017, 24. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe; Kungliga Svenska Vetenskapsakad; Handlingar Bihang: Stockholm, Sweden, 1898. [Google Scholar]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- El-Khaiary, M.I. Least-squares regression of adsorption equilibrium data: Comparing the options. J. Hazard. Mater. 2008, 158, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Yang, Y.; Hu, G.; Jamal, L.; S.M., J.A. Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution. Processes 2019, 7, 565. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.; Szeto, Y.; Cheung, W.; McKay, G. Adsorption of acid dyes on chitosan equilibrium isotherm analyses. Process. Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. II. liquids.1. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Potential Theory of Adsorption of Gases and Vapors for Adsorbents with Energetically Nonuniform Surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Tempkin, M.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physiochim. URSS 2020, 12, 217–222. [Google Scholar]
- Al.Haddabi, M.; Znad, H.; Ahmed, M. Removal of Dissolved Organic Carbon from Oily Produced Water by Adsorption onto Date Seeds: Equilibrium, Kinetic, and Thermodynamic Studies. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- De Caprariis, B.; Filippis, P.D.; Hernandez, A.D.; Petrucci, E.; Petrullo, A.; Scarsella, M.; Turchi, M. Pyrolysis wastewater treatment by adsorption on biochars produced by poplar biomass. J. Environ. Manag. 2017, 197, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kaveeshwar, A.R.; Ponnusamy, S.K.; Revellame, E.D.; Gang, D.D.; Zappi, M.E.; Subramaniam, R. Pecan shell based activated carbon for removal of iron(II) from fracking wastewater: Adsorption kinetics, isotherm and thermodynamic studies. Process. Saf. Environ. Prot. 2018, 114, 107–122. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; Almomani, F.; Adham, S.; Khraisheh, M. Polymeric adsorbents for oil removal from water. Chemosphere 2019, 233, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Adamson, A.; Gast, A. Physical Chemistry of Liquid Surfaces; Interscience Publishers: New York, NY, USA, 1970; pp. 44–116. [Google Scholar] [CrossRef]
- Haghseresht, F.; Lu, G.Q. Adsorption Characteristics of Phenolic Compounds onto Coal-Reject-Derived Adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium Isotherm Studies for the Sorption of Divalent Metal Ions onto Peat: Copper, Nickel and Lead Single Component Systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Chen, S.G.; Yang, R.T. Theoretical Basis for the Potential Theory Adsorption Isotherms. The Dubinin-Radushkevich and Dubinin-Astakhov Equations. Langmuir 1994, 10, 4244–4249. [Google Scholar] [CrossRef]
- Crisafully, R.; Milhome, M.A.L.; Cavalcante, R.M.; Silveira, E.R.; Keukeleire, D.D.; Nascimento, R.F. Removal of some polycyclic aromatic hydrocarbons from petrochemical wastewater using low-cost adsorbents of natural origin. Bioresour. Technol. 2008, 99, 4515–4519. [Google Scholar] [CrossRef]
- Bury, N.R.; Boyle, D.; Cooper, C.A. 4—Iron. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 31, pp. 201–251. [Google Scholar] [CrossRef]
- Pearson, G.; Boyd, F.; Haggerty, S.; Pasteris, J.; Field, S.; Nixon, P.; Pokhilenko, N. The characterisation and origin of graphite in cratonic lithospheric mantle: A petrological carbon isotope and Raman spectroscopic study. Contrib. Mineral. Petrol. 1994, 115, 449–466. [Google Scholar] [CrossRef]
- Takeuchi, K.; Fujishige, M.; Kitazawa, H.; Akuzawa, N.; Medina, J.O.; Morelos-Gomez, A.; Cruz-Silva, R.; Araki, T.; Hayashi, T.; Terrones, M.; et al. Oil sorption by exfoliated graphite from dilute oil–water emulsion for practical applications in produced water treatments. J. Water Process. Eng. 2015, 8, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.K.; Verma, A. Chapter 3.4—Electrocatalyst Materials for Oxygen Reduction Reaction in Microbial Fuel Cell. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 451–483. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Sparks, D.L. 2—Inorganic Soil Components. In Environmental Soil Chemistry, 2nd ed.; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 43–73. [Google Scholar] [CrossRef]
- Ross, C.S.; Shannon, E.V. The Minerals of Bentonite and Related Clays and Their Physical Properties. J. Am. Ceram. Soc. 1926, 9, 77–96. [Google Scholar] [CrossRef]
- He, H.; Zhu, J. Chapter 10—Analysis of Organoclays and Organic Adsorption by Clay Minerals. In Infrared and Raman Spectroscopies of Clay Minerals; Gates, W., Kloprogge, J., Madejová, J., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 310–342. [Google Scholar] [CrossRef]
- Wimpenny, J. Clay Minerals. In Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth; White, W.M., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–11. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Wang, Q.; Sun, Y.; Li, G.; Wu, T.; Li, Y. Study of STAB- and DDAB-modified sepiolite tructures and their adsorption performance for emulsified oil in produced water. Colloid Interface Sci. Commun. 2020, 34, 100231. [Google Scholar] [CrossRef]
- Sueyoshi, M.; Al-Maamari, R.S.; Jibril, B.; Tasaki, M.; Okamura, K.; Kuwagaki, H.; Yahiro, H.; Sagata, K.; Han, Y. Preparation and characterization of adsorbents for treatment of water associated with oil production. J. Anal. Appl. Pyrolysis 2012, 97, 80–87. [Google Scholar] [CrossRef]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil-water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Emam, E. Modified Activated Carbon and Bentonite Used to Adsorb Petroleum Hydrocarbons Emulsified in Aqueous Solution. Am. J. Environ. Prot. 2013, 2, 161. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Xu, Y.; Papineau, D.; Yu, N.; Fan, Q.; Qiu, X.; Wang, H. Experimental evidence for abiotic formation of low-temperature proto-dolomite facilitated by clay minerals. Geochim. Cosmochim. Acta 2019, 247, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, C.; Mckenzie, J.; Bernasconi, S.; Grujic, D.; Tien, A. Microbial Mediation as a Possible Mechanism for Natural Dolomite Formation at Low-Temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Ghaemi, A.; Torab-Mostaedi, M.; Ghannadi-Maragheh, M. Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J. Hazard. Mater. 2011, 190, 916–921. [Google Scholar] [CrossRef]
- Shahruddin, M.Z.; Othman, N.H.; Alias, N.H.; Ghani, S.N.A. Desalination of Produced Water Using Bentonite as Pre-Treatment and Membrane Separation as Main Treatment. Procedia Soc. Behav. Sci. 2015, 195, 2094–2100. [Google Scholar] [CrossRef] [Green Version]
- Younker, J.M.; Walsh, M.E. Bench-scale investigation of an integrated adsorption–coagulation–dissolved air flotation process for produced water treatment. J. Environ. Chem. Eng. 2014, 2, 692–697. [Google Scholar] [CrossRef]
- Younker, J.M.; Walsh, M.E. Impact of salinity and dispersed oil on adsorption of dissolved aromatic hydrocarbons by activated carbon and organoclay. J. Hazard. Mater. 2015, 299, 562–569. [Google Scholar] [CrossRef]
- Elsherif, K.; Alkherraz, A.; Ali, A. Removal of Pb(II), Zn(II), Cu(II) and Cd(II) from aqueous solutions by adsorption onto olive branches activated carbon: Equilibrium and thermodynamic studies. Chem. Int. 2019, 6, 11–20. [Google Scholar] [CrossRef]
- Araújo, C.S.; Almeida, I.L.; Rezende, H.C.; Marcionilio, S.M.; Léon, J.J.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Khan, S.; Ali, J. 2—Chemical analysis of air and water. In Bioassays; Häder, D.P., Erzinger, G.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 21–39. [Google Scholar] [CrossRef]
- El-Naas, M.; Sulaiman, A.Z.; Alhaija, M. Removal of Phenol from Petroleum Refinery Wastewater through Adsorption on Date-Pit Activated Carbon. Chem. Eng. J. 2010, 162, 997–1005. [Google Scholar] [CrossRef]
- El-Nafaty, U.; Misau, I.; Abdulsalam, S. Biosorption and Kinetic Studies on Oil Removal from Produced Water Using Banana Peel. Civ. Environ. Res. 2013, 3, 125–136. [Google Scholar]
- Ibrahim, T.; Gulistan, A.; Khamis, M.; Ahmed, H.; Aidan, A. Produced water treatment using naturally abundant pomegranate peel. Desalin. Water Treat. 2016, 57, 6693–6701. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Song, S.T.; Saman, N.; Johari, K.; Mat, H. Surface chemistry modifications of rice husk toward enhancement of Hg(II) adsorption from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 1747–1755. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; McMillan, O.; Jin, F.; Al-Tabbaa, A. Characteristics and mechanisms of nickel adsorption on biochars produced from wheat straw pellets and rice husk. Environ. Sci. Pollut. Res. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudaibergenov, K.; Ongarbayev, Y.; Mansurov, Z.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non-Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, H.; Ren, H. Dissolved organic matter (DOM) removal from biotreated coking wastewater by chitosan-modified biochar: Adsorption fractions and mechanisms. Bioresour. Technol. 2020, 297, 122281. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Zuo, J.E.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Iranmanesh, S.; Harding, T.; Abedi, J.; Seyedeyn-Azad, F.; Layzell, D. Adsorption of naphthenic acids on high surface area activated carbons. J. Environ. Sci. Health 2014, 49, 913–922. [Google Scholar] [CrossRef]
- Behnood, R.; Anvaripour, B.; Fard, N.; Farasati, M. Petroleum hydrocarbons adsorption from aqueous solution by raw sugarcane bagasse. Int. J. Emerg. Sci. Eng. 2013, 1, 96–99. [Google Scholar]
- Venkatesan, J.; Kim, S. 10—Chitosan for bone repair and regeneration. In Bone Substitute Biomaterials; Mallick, K., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2014; pp. 244–260. [Google Scholar] [CrossRef]
- Grem, I.; Lima, B.; Carneiro, W.; Queiros, Y.; Mansur, C. Chitosan Microspheres Applied for Removal of Oil from Produced Water in the Oil Industry. Polímeros Ciência Tecnol. 2013, 23, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Hosny, R.; Fathy, M.; Ramzi, M.; Moghny, T.A.; Desouky, S.; Shama, S. Treatment of the oily produced water (OPW) using coagulant mixtures. Egypt. J. Pet. 2016, 25, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Misau, I.; El-Nafaty, U.; Abdulsalam, S.; Isa, Y. Removal of Oil from Oil Produced Water Using Eggshell. Civ. Environ. Res. 2012, 2, 52–63. [Google Scholar]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean. Prod. 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Yuan, M.; Tong, S.; Zhao, S.; Jia, C.Q. Adsorption of polycyclic aromatic hydrocarbons from water using petroleum coke-derived porous carbon. J. Hazard. Mater. 2010, 181, 1115–1120. [Google Scholar] [CrossRef]
- Asenjo, N.G.; Álvarez, P.; Granda, M.; Blanco, C.; Santamaría, R.; Menéndez, R. High performance activated carbon for benzene/toluene adsorption from industrial wastewater. J. Hazard. Mater. 2011, 192, 1525–1532. [Google Scholar] [CrossRef] [Green Version]
- Björklund, K.; Li, L.Y. Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge. J. Environ. Manag. 2017, 197, 490–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, C.; Yang, S.; Huang, G.; Zhao, S.; Zhang, P.; Yao, Y. Removal of sulfonated humic acid from aqueous phase by modified coal fly ash waste: Equilibrium and kinetic adsorption studies. Fuel 2016, 165, 264–271. [Google Scholar] [CrossRef]
- Jung, K.; Oh, S.; Bak, H.; Song, G.H.; Kim, H.T. Adsorption of Arsenic and Lead onto Stone Powder and Chitosan-Coated Stone Powder. Processes 2019, 7, 599. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M.S.; El-Toony, M.M. Integrated treatment for oil free petroleum produced water using novel resin composite followed by microfiltration. Sep. Purif. Technol. 2020, 234, 116058. [Google Scholar] [CrossRef]
- Konggidinata, M.I.; Chao, B.; Lian, Q.; Subramaniam, R.; Zappi, M.; Gang, D.D. Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto Ordered Mesoporous Carbon (OMC). J. Hazard. Mater. 2017, 336, 249–259. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, E. Adsorption of Lithium from Shale Gas Produced Water Using Titanium Based Adsorbent. Ind. Eng. Chem. Res. 2018, 57, 8381–8387. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Fard, A.K.; Mckay, G.; Manawi, Y.; Malaibari, Z.; Hussien, M.A. Outstanding adsorption performance of high aspect ratio and super-hydrophobic carbon nanotubes for oil removal. Chemosphere 2016, 164, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Masomeh, S.; Masooleh, S.; Bazgir, S.; Tamizifar, M. Adsorption of petroleum hydrocarbons on organoclay. J. Appl. Chem. Res. 2010, 4, 19–23. [Google Scholar]
- Fard, A.K.; Mckay, G.; Chamoun, R.; Rhadfi, T.; Preud’Homme, H.; Atieh, M.A. Barium removal from synthetic natural and produced water using MXene as two dimensional (2-D) nanosheet adsorbent. Chem. Eng. J. 2017, 317, 331–342. [Google Scholar] [CrossRef]
- Reddy, K.; McDonald, K.; King, H. A novel arsenic removal process for water using cupric oxide nanoparticles. J. Colloid Interface Sci. 2013, 397, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, C.H.; Lien, H.L.; Chung, J.S.; Yeh, H.D. Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J. Hazard. Mater. 2017, 322, 215–222. [Google Scholar] [CrossRef]
- Belbase, S.; Urynowicz, M.A.; Vance, G.F.; Dangi, M.B. Passive remediation of coalbed natural gas co-produced water using zeolite. J. Environ. Manag. 2013, 131, 318–324. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Stevens, G.W.; Mumford, K.A. Hydrocarbon adsorption performance and regeneration stability of diphenyldichlorosilane coated zeolite and its application in permeable reactive barriers: Column studies. Microporous Mesoporous Mater. 2020, 294, 109843. [Google Scholar] [CrossRef]
- Rosenblum, J.S.; Sitterley, K.A.; Thurman, E.M.; Ferrer, I.; Linden, K.G. Hydraulic fracturing wastewater treatment by coagulation-adsorption for removal of organic compounds and turbidity. J. Environ. Chem. Eng. 2016, 4, 1978–1984. [Google Scholar] [CrossRef] [Green Version]
- El-Naas, M.; Alhaija, M.; Sulaiman, A.Z. Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ. Sci. Pollut. Res. 2017, 24. [Google Scholar] [CrossRef] [PubMed]

| PW Source | Hydrocarbon Concentration (mg/L) | Reference |
|---|---|---|
| Yangtze Petrochemical Company (China) | 6000 | [4,6] |
| Oil India Limited (India) | 366 | [4,7] |
| Brazilian Oil Production (Brazil) | 250 | [8,9] |
| BP AG Refinery (Germany) | 200–1000 | [8,10] |
| Country | Hydrocarbon Discharge Regulatory Level (mg/L) | Reference |
|---|---|---|
| U.S.A | 42 | [15,16] |
| Australia | 30 | [15,17] |
| Colombia | 15 | |
| Argentina | 5 |
| Model Name | Equation | Mechanism |
|---|---|---|
| Pseudo—first order | [44] | Physical adsorption |
| Pseudo—second order | [45] | Chemisorption |
| Elovich | [34] | Chemisorption |
| Intraparticle diffusion | [36,46] | Intramolecular diffusion |
| Isotherm Name | Equation | Parameters |
|---|---|---|
| Langmuir | is the amount of solute adsorbed, is the maximum monolayer on the adsorbent, is the Langmuir isotherm constant and is the equilibrium concentration [50,59] | |
| Freundlich | is the Freundlich constant and the heterogeneity factor [51,60] | |
| Sips | is Sips isotherm constant, is Sips isotherm exponent and is Sips isotherm model constant [52,62] | |
| Dubinin–Radushkevick | is the Dubinin–Radushlkevich constant, R is the gas constant and T is the absolute temperature [53,63] | |
| Temkin | R is the universal gas constant, T is the absolute temperature, is the Temkin isotherm constant and is the Temkin isotherm equilibrium binding constant [54,59] |
| Material | Target | Adsorption Capacity | Reference |
|---|---|---|---|
| Attapulgite | oil | 155 mg-oil/g-adsorbent | [75] |
| Attapulgite with petroleum sludge | oil | 405 mg-oil/g-adsorbent | [75] |
| Date palm (700 C) | oil | 1330 mg-oil/g-adsorbent | [75] |
| Date palm (800 C) | oil | 1425 mg-oil/g-adsorbent | [75] |
| Organoclay | oil | 100 mg oil/L to 15 mg/L | [82] |
| Organoclay | naphthalene | 1 mg napthalene/L to 0.11 mg naphthalene/L | [82] |
| Organoclay | phenol | none | [82] |
| Organoclay | oil | 100 mg oil/L to 7 mg/L | [82] |
| Organoclay | naphthalene | 1 mg napthalene/L to 0.11 mg naphthalene/L | [82] |
| Sugarcane | oil | 6.6 g oil/g adsorbent | [99] |
| Chitosan Microspheres | oil | >90% removal | [101] |
| Chitosan | oil | 96.35% removal | [102] |
| Chitosan | oil | 99% removal | [102] |
| Chitosan | oil | 85% removal | [102] |
| Chitosan | Dissolved Organic Matter | 52% removal | [94] |
| Wheat Straw | Dissolved Organic Matter | 12% removal | [94] |
| zeolite | Toluene | 16.58 mg-toluene/g-adsorbent | [121] |
| Bentonite | oil | 96.5% removal | [76] |
| Bentonite | oil | 38.5 mg-hydrocarbon/g adsorbent | [77] |
| org-bentonite | oil | 48 mg-hydrocarbon/g adsorbent | [77] |
| Commercial Organoclay | Napthalene in freshwater | 13.76 L/g | [83] |
| Organoclay | Napthalene in freshwater | 16.42 L/g | [83] |
| Commercial Organoclay | Napthalene in saline water | 4.61 L/g | [83] |
| Organoclay | Napthalene in saline water | 14.17 L/g | [83] |
| Commercial Organoclay | Napthalene in oil water | 28 L/g | [83] |
| Organoclay | Napthalene in oil water | 29.39 L/g | [83] |
| Commercial Organoclay | Phenol in freshwater | 0.08 L/g | [83] |
| Organoclay | Phenol in freshwater | 1.01 L/g | [83] |
| Commercial Organoclay | Phenol in saline water | 0.09 L/g | [83] |
| Organoclay | Phenol in saline water | 2.25 L/g | [83] |
| Commercial Organoclay | Phenol in oil water | 0.1 L/g | [83] |
| Organoclay | Phenol in oil water | 1.74 L/g | [83] |
| Rice Husks | Nickel | 98% removal | [92] |
| Sepiolite | oil | 1013.5 mg-oil/g-adsorbent (>99% removal) | [74] |
| Sepiolite | oil | 958 mg-oil/g-adsorbent (>99% removal) | [74] |
| Wood Biochar | organic compounds (from bio process) | 141.2 mg-organic compounds/g-adsorbent | [56] |
| Wood Biochar | organic compounds (from bio process) | 175.4mg-organic compounds/g-adsorbent | [56] |
| Wood Biochar | organic compounds (from bio process) | 848.6 mg-organic compounds/g-adsorbent | [56] |
| Banana Peel | oil | 100% removal | [88] |
| Pomegranate Peel | oil | >92% removal | [89] |
| Date Seeds | Dissolved Organic Carbon | 82% removal | [55] |
| Eggshells | oil | 100% removal | [103] |
| Sulfur Functionalized Rice Husk | mercury | 89 mg- Hg(II)/g-adsorbent | [91] |
| OrganoSilane Functionalized Rice Husk | mercury | 118 mg-Hg(II)/g-adsorbent | [91] |
| Wheat Straw | Nickel | 98% removal | [92] |
| Sawdust | Copper | 27 L/g | [97] |
| Sawdust | Cadmium | 4.71 L/g | [97] |
| Olive Branches | Lead | 41.32 mg-lead/g-adsorbent | [84] |
| Olive Branches | Zinc | 34.97 mg-zinc/g-adsorbent | [84] |
| Olive Branches | Copper | 43.10 mg-copper/g-adsorbent | [84] |
| Olive Branches | Cadmium | 38.17 mg-cadmium/g-adsorbent | [84] |
| Pineapple Peel | chromium | 99.19% removal | [90] |
| Pineapple Peel | chromium | 82.63% removal | [90] |
| Pineapple Peel | chromium | 58.22%removal | [90] |
| Pineapple Peel | chromium | 40.78% removal | [90] |
| Corn Straw | Cadmium | 38.91 mg-cadmium/g-adsorbent (99.24% removal) | [96] |
| Corn Straw | Lead | 28.99 mg-cadmium/g-adsorbent (98.62% removal) | [96] |
| Loberia Fruit | Lead | 51.02 mg-lead/g-adsorbent | [85] |
| Oil Palm Branches | Methyl-mercury | 0.14 mmol-methylmercury/g-adsorbent | [43] |
| Oil Palm Branches | mercury | 0.773 mmol-mercury/g-adsorbent | [43] |
| Oil Palm Branches | Methyl-mercury | 0.09 mmol-methylmercury/g-adsorbent | [43] |
| Goethite | As | 97 umol/g | [37] |
| Camel Bones Nanocomposite | Lead | 344.8 mg-lead/g-adsobent | [104] |
| Camel Bones Nanocomposite | Cadmium | 322.6 mg-cadmium/g-adsorbent | [104] |
| Camel Bones Nanocomposite | Cobalt | 294.1 mg-cobalt/g-adsorbent | [104] |
| Rape Straw | Cadmium | 72.369 mg-Cadmium/g-adsorbent | [95] |
| Rape Straw | Cadmium | 81.1 mg-Cadmium/g-adsorbent | [95] |
| Rape Straw | Cadmium | 67.36 mg-Cadmium/g-adsorbent | [95] |
| Rape Straw | Cadmium | 32.74 mg-Cadmium/g-adsorbent | [95] |
| Graphene | Antimony | 158.2 mg-antimony/g-adsorbent | [69] |
| Oil Palm Branches | mercury | 0.226 mmol-mercury/g-adsorbent | [43] |
| Pecan Shell | Iron (II) | 41.66 mg-iron/g-adsorbent | [57] |
| Sawdust | Naphthenic Acid | 83% removal | [98] |
| Material | Target | Adsorption Capacity | Reference |
|---|---|---|---|
| Commcercial Activated Carbon | oil | 730 mg-oil/g-adsorbent | [75] |
| Exfoliated Graphite | oil | from 100 mg-oil/L to 0.1 mg-oil/L | [67] |
| Activated Carbon | Polyethylene glycols | 99.60% | [122] |
| Activated Carbon | total petroleum hydrocarbons | 92% removal | [122] |
| Activated Carbon | total petroleum hydrocarbons | 99% removal | [122] |
| phenyl epoxy/poly (vinyl pyrrolidone)/Fe3O4 | Oil | 99.9% removal | [110] |
| Functionalized Silica Nanoparticles (4% VR) | oil saltwater | 100% removal | [42] |
| Functionalized Silica Nanoparticles (2% VR) | oil saltwater | 100% removal | [42] |
| Silica Nanoparticles | oil saltwater | 93% removal | [42] |
| Functionalized Silica Nanoparticles (2% VR) | oil freshwater | 100% removal | [42] |
| Functionalized Silica Nanoparticles (4% VR) | oil freshwater | 100% removal | [42] |
| Alumina Nanoparticles | oil saltwater | 185.76 mg-oil/g adsorbent | [38] |
| Functionalized Alumina Nanoparticles (4% VR) | oil saltwater | 188.64 mg-oil/g-adsorbent | [38] |
| Functionalized Alumina Nanoparticles (2% VR) | oil saltwater | 193.77 mg-oil/g-adsorbent | [38] |
| Lewatit AF 5 | oil | >98% removal | [58] |
| Deposited Carbon | oil | 97.5% removal | [76] |
| Powder Activted Carbon | oil | 82.6% removal | [76] |
| Activated Carbon | oil | 30 mg-hydrocarbon/g adsorbent | [77] |
| Acidic Modified Activated Carbon | oil | 40 mg-hydrocarbon/g adsorbent | [77] |
| Carbon Nanotubes | oil | 87% removal | [39] |
| Carbon Nanotubes | oil | 98.52% removal | [39] |
| Carbon Nanotubes | oil | 87% removal | [115] |
| Produced-Carbon Nanotubes | oil | 97% removal | [115] |
| Amberlite XAD 7 | oil | >98% removal | [58] |
| optipore L493, | oil | >98% removal | [58] |
| Silica Nanoparticles | oil freshwater | 93% removal | [42] |
| Sewage Sludge | hydrophobic organic compounds (HOC) | 2800 mico gram-HOC/g-adsorbent | [107] |
| Ordered Mesoporous Carbon | Benzene | 5.1 mg-benzene/g-adsorbent | [111] |
| Ordered Mesoporous Carbon | Toluene | 18.2 mg-benzene/g-adsorbent | [111] |
| Ordered Mesoporous Carbon | Ethylbenzene | 31.7 mg-benzene/g-adsorbent | [111] |
| Ordered Mesoporous Carbon | xylene | 46 mg-benzene/g-adsorbent | [111] |
| Activated carbon | organic compounds (from bio process) | 318 mg-organic compounds/g-adsorbent | [56] |
| Organosilica | Benzene | 40% removal | [40] |
| Organosilica | o-xylene | >60% removal | [40] |
| Organosilica | p-xylene | >60% removal | [40] |
| Organosilica | Toluene | >60% removal | [40] |
| Amberlite IRA 958 | oil | <25% removal | [58] |
| Titanium-based Adsorbent | Lithium | 92.7% removal | [112] |
| CuO Nanoparticles | Arsenic | 99% removal | [118] |
| Dendrimer Magnetic Nanoparticles | Palladium | 3.6 mg-Pd(IV)/g-adsorbent | [119] |
| Dendrimer Magnetic Nanoparticles | Gold | 3.58 mg- gold/g-adsorbent | [119] |
| Dendrimer Magnetic Nanoparticles | Silver | 2.84 mg-silver/g-adsorbent | [119] |
| Coal Fly-Ash waste | Sulfonated Humic Acid | 92.83% removal | [108] |
| MXene Nanosheets | Barium | 100% removal | [117] |
| Zeolite | Na | 21 Na g/kg zeolite (bicarbonate) 18 Na g/kg zeolite (chloride) | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a Process for Produced Water Treatment: A Review. Processes 2020, 8, 1657. https://doi.org/10.3390/pr8121657
Yousef R, Qiblawey H, El-Naas MH. Adsorption as a Process for Produced Water Treatment: A Review. Processes. 2020; 8(12):1657. https://doi.org/10.3390/pr8121657
Chicago/Turabian StyleYousef, Roghayeh, Hazim Qiblawey, and Muftah H. El-Naas. 2020. "Adsorption as a Process for Produced Water Treatment: A Review" Processes 8, no. 12: 1657. https://doi.org/10.3390/pr8121657






