Quartz Sand Filter Media with Special Wettability for Continuous and Efficient Oil/Water Separation and Dye Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Filter Media
2.3. Preparation of Quartz Sand Filter Media with Special Wettability
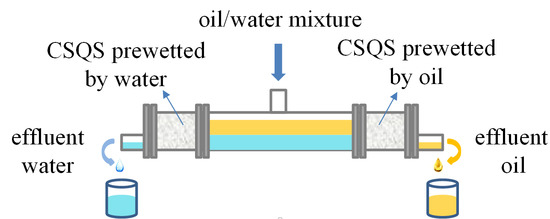
2.4. Oil/Water Separation Device and Experimental Method
2.5. Dye Adsorption Experiment
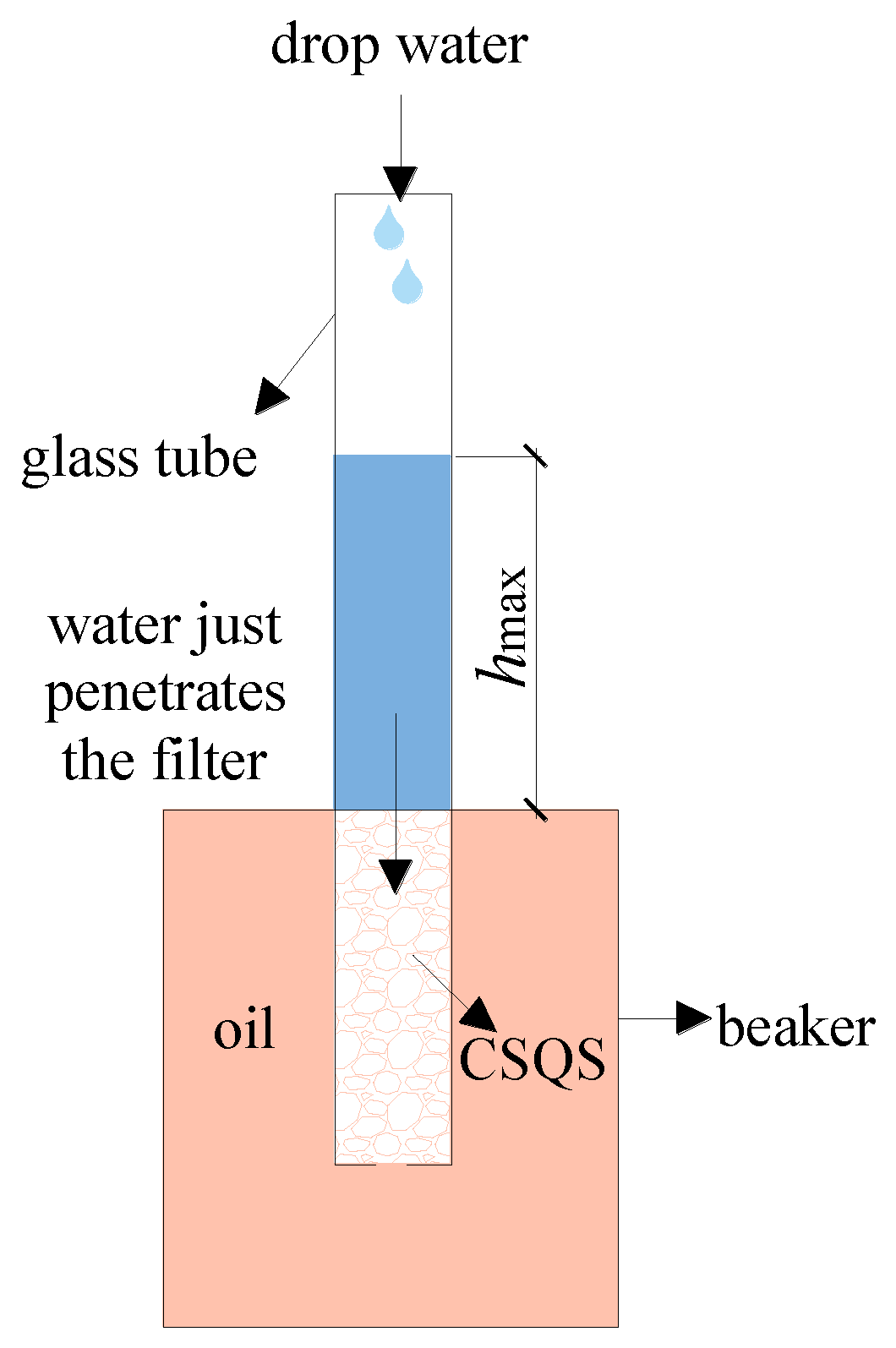
2.6. Study on Wetting Stability
3. Result and Discussion
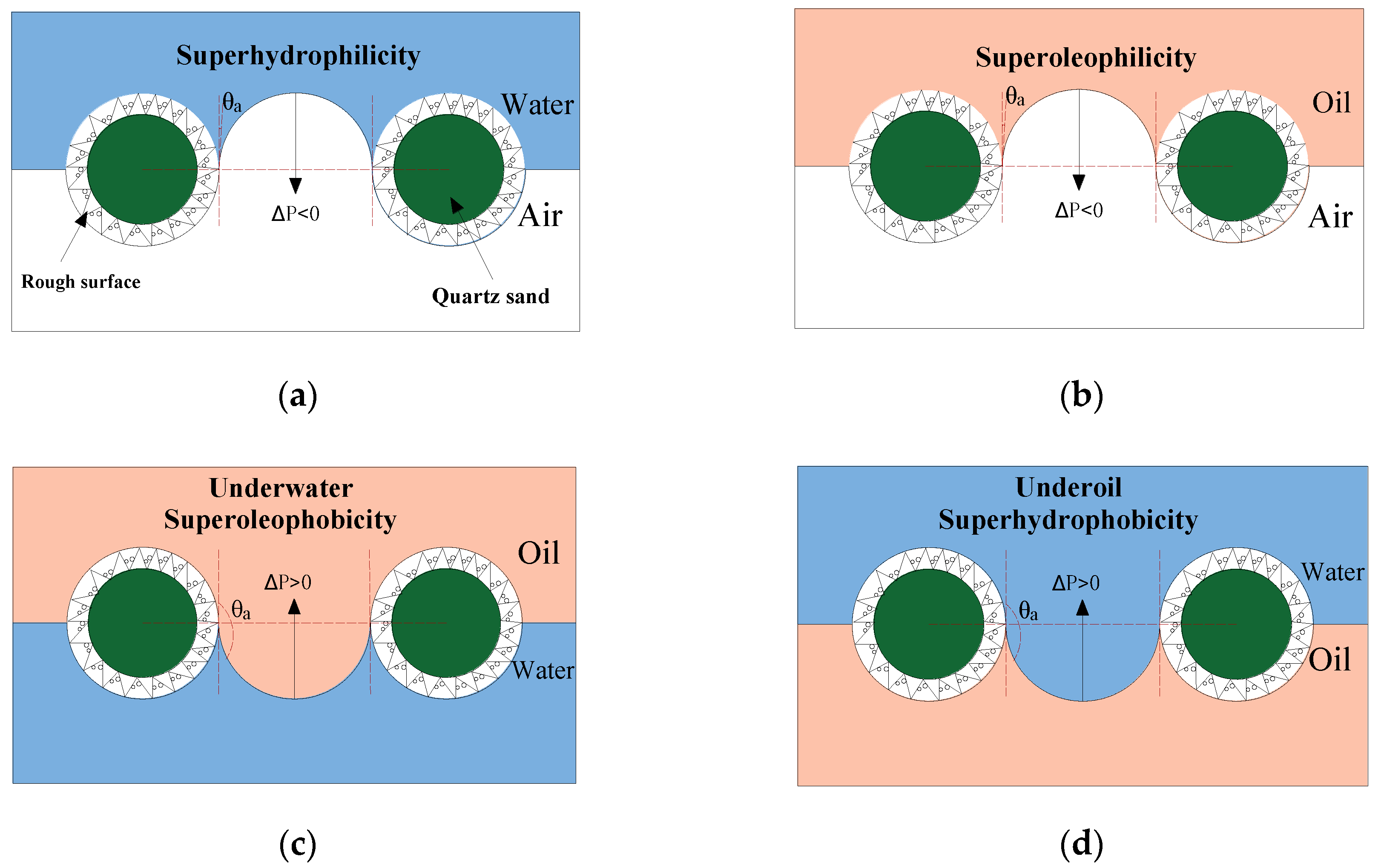
3.1. Modified Mechanism
3.2. Surface Wettability
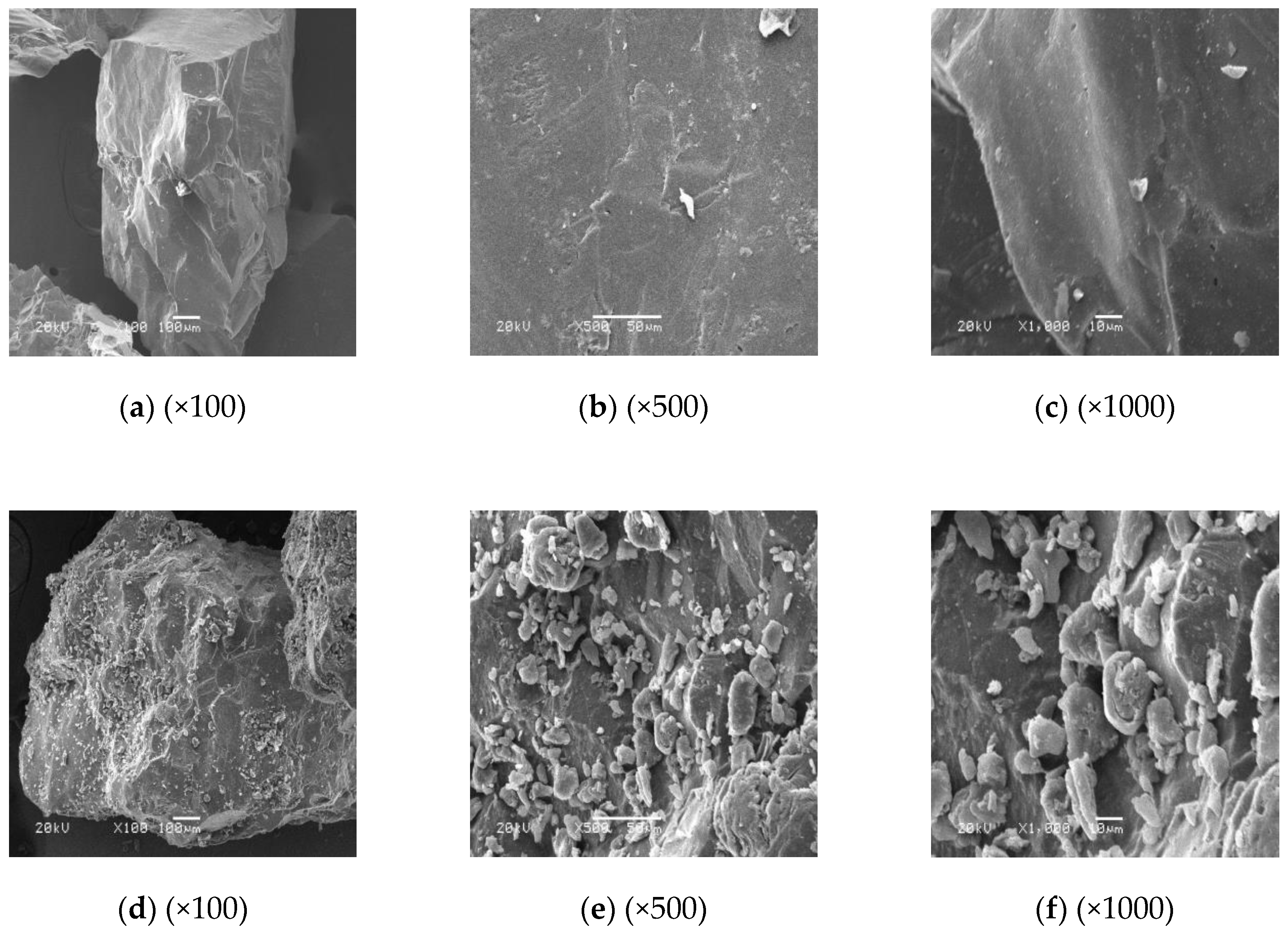
3.3. SEM Analysis
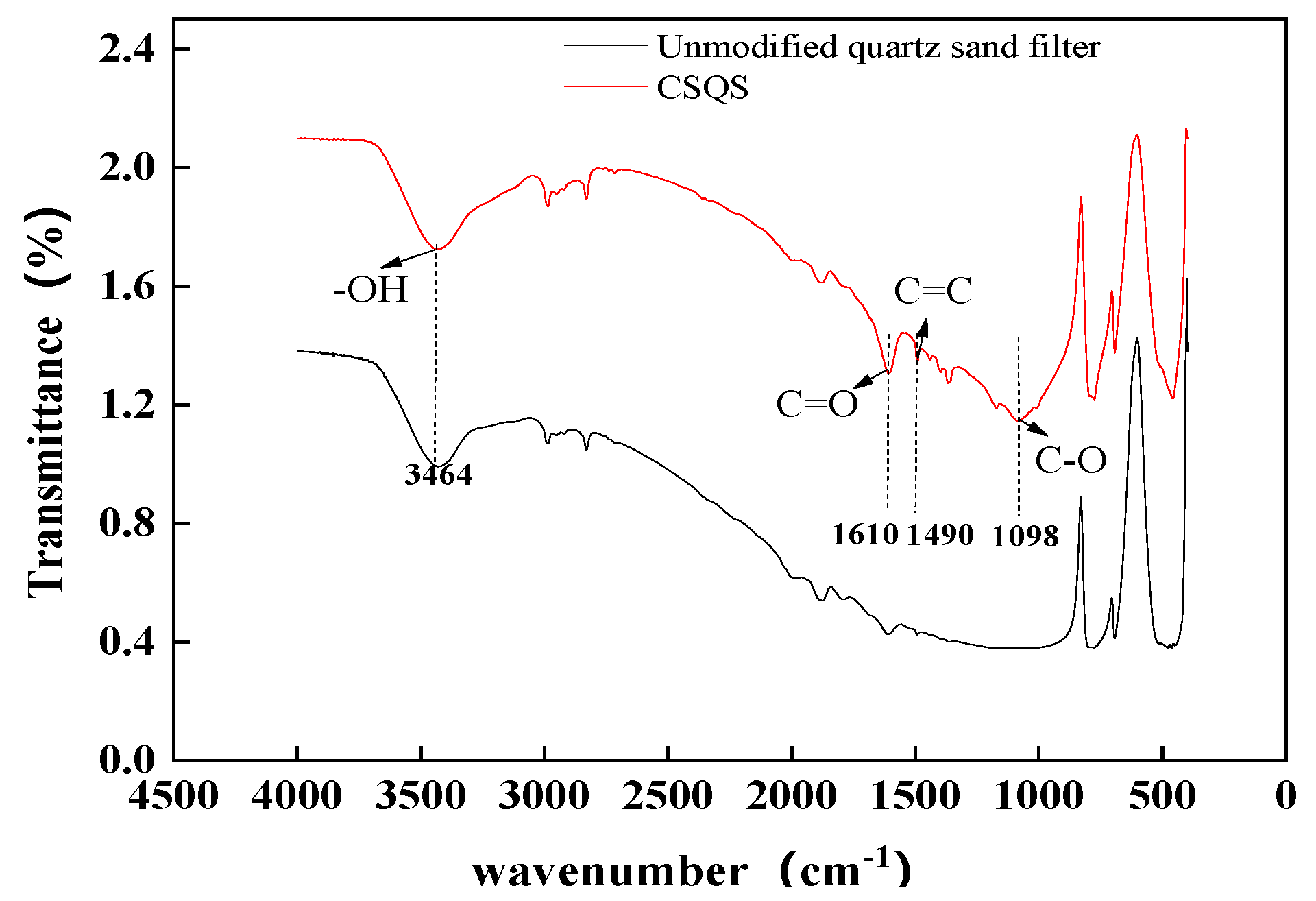
3.4. FTIR Analysis
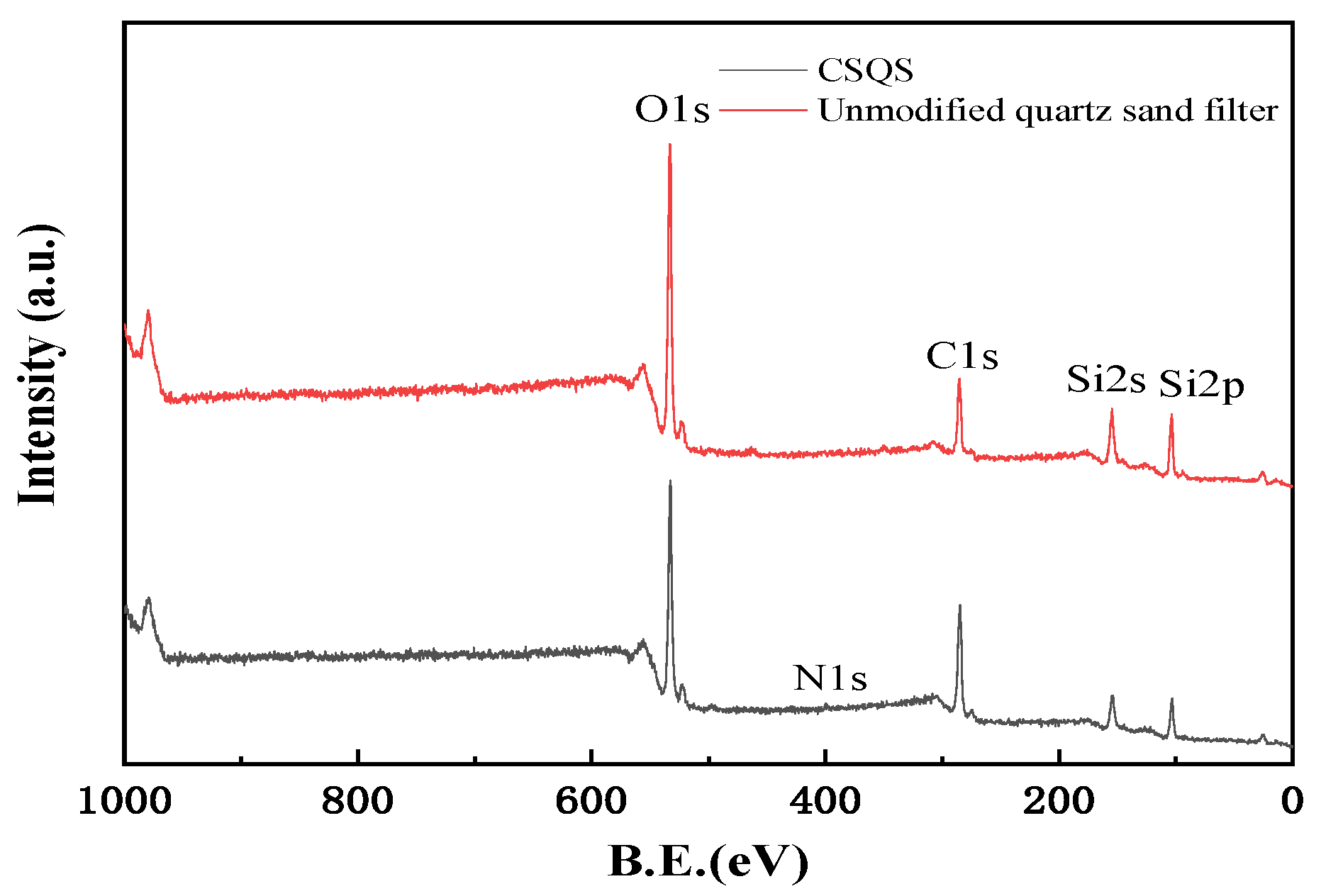
3.5. XPS Analysis
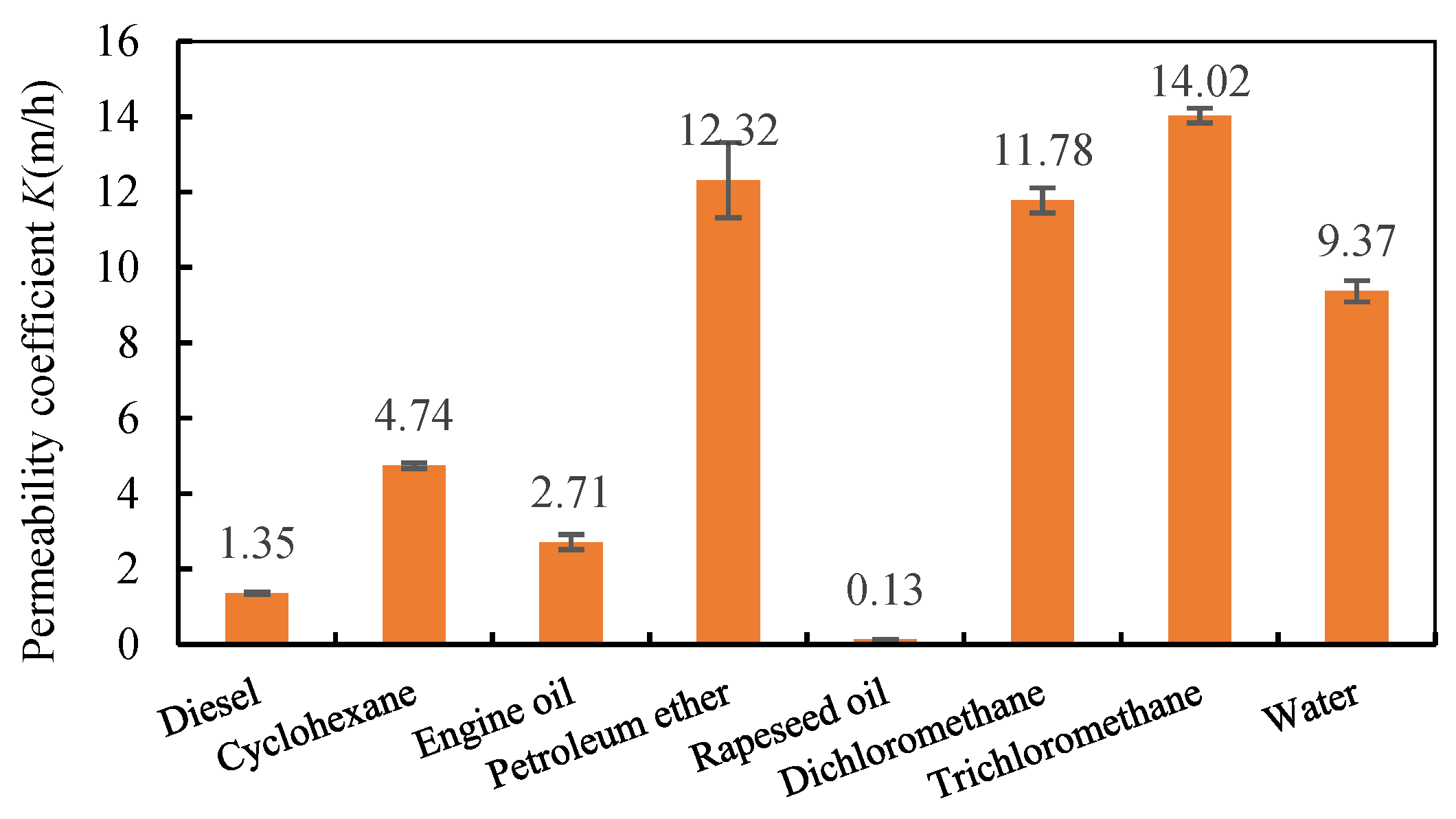
3.6. Oil/Water Separation Performance
3.7. Adsorbed Dyes
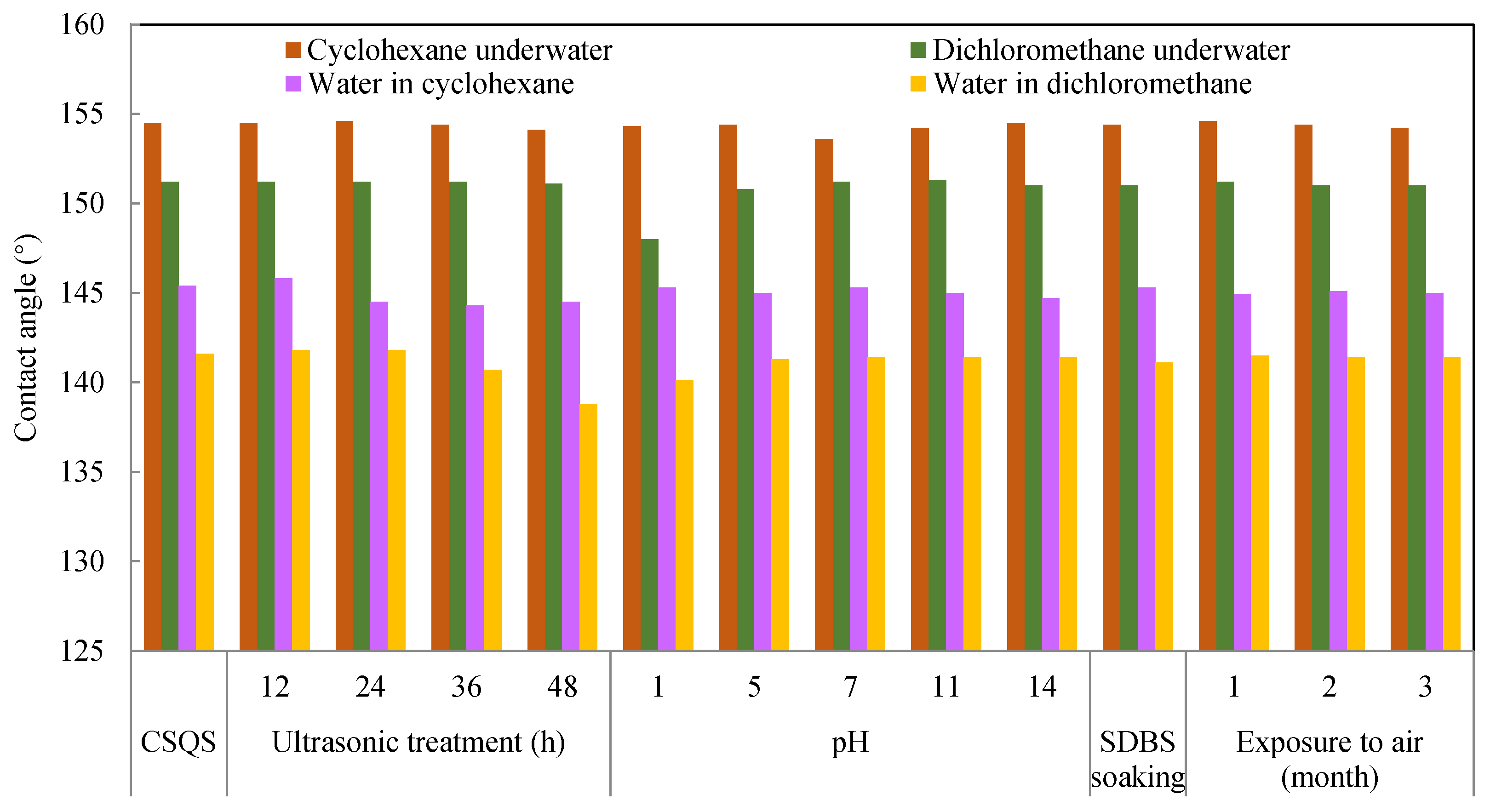
3.8. Stability Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem. Eng. J. 2019, 358, 1539–1551. [Google Scholar] [CrossRef]
- Rosell-Mele, A.; Moraleda-Cibrian, N.; Cartro-Sabate, M.; Colomer-Ventura, F.; Mayor, P.; Orta-Martinez, M. Oil pollution in soils and sediments from the Northern Peruvian Amazon. Sci. Total Environ. 2018, 610, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Raymond, T.; King, C.K.; Raymond, B.; Stark, J.S.; Snape, I. Oil Pollution in Antarctica. In Oil Spill Science and Technology, 2nd ed.; Elsevier: Cambridge, MA, USA, 2017; pp. 759–803. [Google Scholar]
- Alshavef, M.S.; Javed, A. Assessment of Relative Tectonics Activity Zones in Masila Oil Field, Yemen. JGSA 2020, 4, 16. [Google Scholar]
- Abed, R.M.M.; Al-Kindi, S. Effect of disturbance by oil pollution on the diversity and activity of bacterial communities in biological soil crusts from the Sultanate of Oman. Appl. Soil Ecol. 2017, 110, 88–96. [Google Scholar] [CrossRef]
- Fox, C.H.; O’Hara, P.D.; Bertazzon, S.; Morgan, K.; Underwood, F.E.; Paquet, P.C. A preliminary spatial assessment of risk: Marine birds and chronic oil pollution on Canada’s Pacific coast. Sci. Total Environ. 2016, 573, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Chequer, F.M.; Lizier, T.M.; de Felicio, R.; Zanoni, M.V.; Debonsi, H.M.; Lopes, N.P.; de Oliveira, D.P. The azo dye Disperse Red 13 and its oxidation and reduction products showed mutagenic potential. Toxicol. Vitr. 2015, 29, 1906–1915. [Google Scholar] [CrossRef]
- Bruschweiler, B.J.; Merlot, C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharm. 2017, 88, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Shamaei, L.; Khorshidi, B.; Perdicakis, B.; Sadrzadeh, M. Treatment of oil sands produced water using combined electrocoagulation and chemical coagulation techniques. Sci. Total Environ. 2018, 645, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, M.G.H.; Manilal, A.M.; Soloman, P.A. Control of Electrocoagulation batch reactor for oil removal from automobile garage wastewater. Procedia Technol. 2016, 24, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Li, R.; Xu, R.; Si, D.; Shang, Y.; Ye, H.; Zhang, Y.; Ye, H.; Xin, Q. Antifouling slippery liquid-infused membrane for separation of water-in-oil emulsions. J. Membr. Sci. 2020, 611, 118289. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z. Novel and cutting-edge applications for a solvent-responsive superoleophobic–superhydrophilic surface: Water-infused omniphobic surface and separating organic liquid mixtures. Chem. Eng. J. 2020, 381, 122629. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Xie, A.; Dai, X.; Yan, Y.; Dai, J. Facile surface coating of metal-tannin complex onto PVDF membrane with underwater Superoleophobicity for oil-water emulsion separation. Surf. Coat. Technol. 2020, 389, 125630. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N.; et al. Preparation of magnetic, superhydrophobic/superoleophilic polyurethane sponge: Separation of oil/water mixture and demulsification. Chem. Eng. J. 2020, 384. [Google Scholar] [CrossRef]
- Zou, L.; Phule, A.D.; Sun, Y.; Zhu, T.Y.; Wen, S.; Zhang, Z.X. Superhydrophobic and superoleophilic polyethylene aerogel coated natural rubber latex foam for oil-water separation application. Polym. Test. 2020, 85, 123339. [Google Scholar] [CrossRef]
- Nine, M.J.; Kabiri, S.; Sumona, A.K.; Tung, T.T.; Moussa, M.M.; Losic, D. Superhydrophobic/superoleophilic natural fibres for continuous oil-water separation and interfacial dye-adsorption. Sep. Purif. Technol. 2020, 233, 116062. [Google Scholar] [CrossRef]
- Priyanka, M.; Saravanakumar, M.P. Ultrahigh adsorption capacity of starch derived zinc based carbon foam for adsorption of toxic dyes and its preliminary investigation on oil-water separation. J. Clean. Prod. 2018, 197, 511–524. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Wang, L.; Li, H.; Xiong, W. Design and preparation of superhydrophobic NiS nanorods on Ni mesh for oil-water separation. Sep. Purif. Technol. 2020, 234, 116126. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and fabrication of superwetting fiber-based membranes for oil/water separation applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, C.; Dai, M.; Lin, D.; Ali, I.; Alhewairini, S.S.; Zheng, X.; Chen, G.; Li, J.; Naz, I. Recent development of super-wettable materials and their applications in oil-water separation. J. Clean. Prod. 2020, 266, 121624. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H.; Sun, Y.; Zhang, L.; Xu, L.; Hao, L.; Yang, H. Covalent layer-by-layer grafting (LBLG) functionalized superhydrophobic stainless steel mesh for oil/water separation. Appl. Surf. Sci. 2017, 406, 150–160. [Google Scholar] [CrossRef]
- Cheng, Y.; Barras, A.; Lu, S.; Xu, W.; Szunerits, S.; Boukherroub, R. Fabrication of superhydrophobic/superoleophilic functionalized reduced graphene oxide/polydopamine/PFDT membrane for efficient oil/water separation. Sep. Purif. Technol. 2020, 236, 116240. [Google Scholar] [CrossRef]
- Tang, W.; Sun, D.; Liu, S.; Li, B.; Sun, W.; Fu, J.; Li, B.; Hu, D.; Yu, J. One step electrochemical fabricating of the biomimetic graphene skins with superhydrophobicity and superoleophilicity for highly efficient oil-water separation. Sep. Purif. Technol. 2020, 236, 116293. [Google Scholar] [CrossRef]
- Miao, W.; Jiao, D.; Wang, C.; Han, S.; Shen, Q.; Wang, J.; Han, X.; Hou, T.; Liu, J.; Zhang, Y. Ethanol-induced one-step fabrication of superhydrophobic-superoleophilic poly(vinylidene fluoride) membrane for efficient oil/water emulsions separation. J. Water Process Eng. 2020, 34, 101121. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Z.; Qi, H. Superhydrophobic-superoleophilic SiC membranes with micro-nano hierarchical structures for high-efficient water-in-oil emulsion separation. J. Membr. Sci. 2020, 601, 117842. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Yunos, M.Z.; Ismail, A.F.; Salleh, W.N.W. Facile fabrication of superhydrophobic and superoleophilic green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash for oil/water separation. Arab. J. Chem. 2020, 13, 3558–3570. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Xie, H.; Huang, H.-X.; Turng, L.-S. Magnetically driven superhydrophobic silica sponge decorated with hierarchical cobalt nanoparticles for selective oil absorption and oil/water separation. Chem. Eng. J. 2018, 337, 541–551. [Google Scholar] [CrossRef]
- Huang, A.; Kan, C.-C.; Lo, S.-C.; Chen, L.-H.; Su, D.-Y.; Soesanto, J.F.; Hsu, C.-C.; Tsai, F.-Y.; Tung, K.-L. Nanoarchitectured design of porous ZnO@copper membranes enabled by atomic-layer-deposition for oil/water separation. J. Membr. Sci. 2019, 582, 120–131. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Chen, M.; Xu, H.; Luo, W.; Zhang, F. Robust functionalization of underwater superoleophobic PVDF-HFP tubular nanofiber membranes and applications for continuous dye degradation and oil/water separation. J. Membr. Sci. 2020, 596, 117583. [Google Scholar] [CrossRef]
- Li, X.; Shan, H.; Zhang, W.; Li, B. 3D printed robust superhydrophilic and underwater superoleophobic composite membrane for high efficient oil/water separation. Sep. Purif. Technol. 2020, 237, 116324. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Phase inversion/sintering-induced porous ceramic microsheet membranes for high-quality separation of oily wastewater. J. Membr. Sci. 2020, 595, 117477. [Google Scholar] [CrossRef]
- Chen, C.; Wang, B.; Liu, H.; Chen, T.; Zhang, H.; Qiao, J. Synthesis of 3D dahlia-like Co3O4 and its application in superhydrophobic and oil-water separation. Appl. Surf. Sci. 2019, 471, 289–299. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Xu, C.; Tian, H.; Wu, Y.; Zha, F. Continuous, high-flux and efficient oil/water separation assisted by an integrated system with opposite wettability. Appl. Surf. Sci. 2018, 433, 374–380. [Google Scholar] [CrossRef]
- Xie, A.; Cui, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Capillarity-driven both light and heavy oil/water separation via combined system of opposite superwetting meshes. Sep. Purif. Technol. 2019, 215, 1–9. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Zhu, M.; Ouyang, T.; Avtar, R.; Dzarfan Othman, M.H.; Mohammad, B.T.; Albadarin, A.B. Removal of acetaminophen from synthetic wastewater in a fixed-bed column adsorption using low-cost coconut shell waste pretreated with NaOH, HNO3, ozone, and/or chitosan. J. Environ. Manag. 2018, 226, 365–376. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Wu, Z.; Zhu, S.; Gao, Y.; Shi, C. The effect of surface modification on chemical and crystalline structure of the cellulose III nanocrystals. Carbohydr. Polym. 2020, 235, 115962. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef]








| Characteristic | Density (g/mL) | Viscosity (mPa·s) |
|---|---|---|
| Water | 1 | 1 × 10−9 |
| Diesel oil | 0.81–0.855 | 2.87 |
| Cyclohexane | 0.779 | 1 |
| Engine oil | 0.88~0.89 | 105.4 |
| Petroleum ether | 0.64–0.66 | 0.3 |
| Rapeseed oil | 0.923 | 12.5–12.9 |
| Dichloromethane | 1.325 | 0.429 |
| Trichloromethane | 1.484 | 0.563 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, B.; Luo, X.; Song, X.; Guo, H.; Dai, L.; Zhang, H.; Wang, G. Quartz Sand Filter Media with Special Wettability for Continuous and Efficient Oil/Water Separation and Dye Adsorption. Processes 2020, 8, 1083. https://doi.org/10.3390/pr8091083
Wei B, Luo X, Song X, Guo H, Dai L, Zhang H, Wang G. Quartz Sand Filter Media with Special Wettability for Continuous and Efficient Oil/Water Separation and Dye Adsorption. Processes. 2020; 8(9):1083. https://doi.org/10.3390/pr8091083
Chicago/Turabian StyleWei, Bigui, Xuying Luo, Xiaosan Song, Hanyue Guo, Liang Dai, Hongwei Zhang, and Gang Wang. 2020. "Quartz Sand Filter Media with Special Wettability for Continuous and Efficient Oil/Water Separation and Dye Adsorption" Processes 8, no. 9: 1083. https://doi.org/10.3390/pr8091083