Melt Stable Functionalized Organosolv and Kraft Lignin Thermoplastic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Etherification Reaction
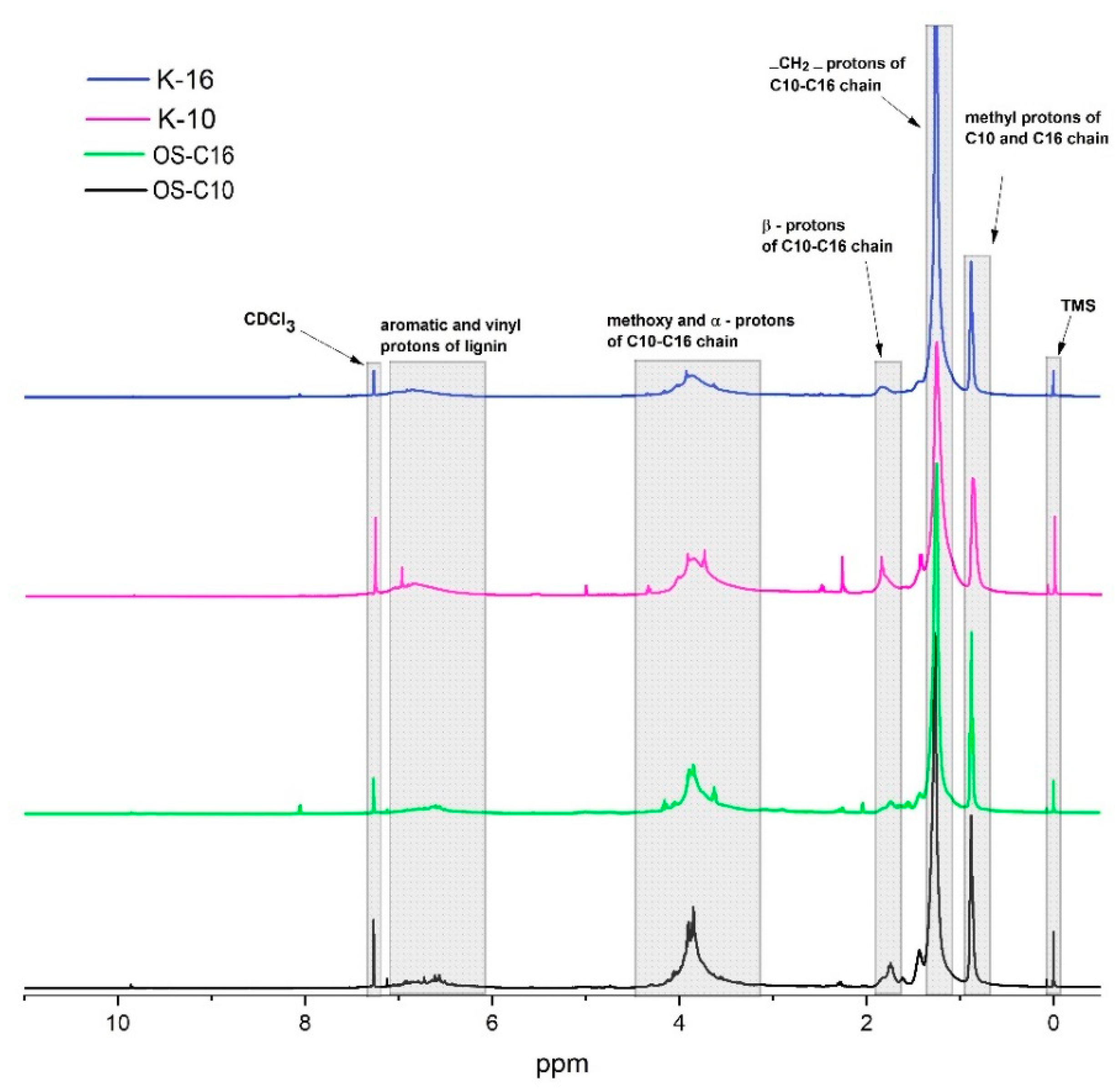
2.3. Nuclear Magnetic Resonance (NMR) Characterization
2.3.1. Quantitative 31P NMR
2.3.2. 1H and 13C NMR
2.4. Fourier Transform Infrared (FT-IR) Measurements
2.5. Thermogravimetric Analysis (TGA)
2.6. Differential Scanning Calorimetry (DSC) Analysis
2.7. Gel Permeation Chromatography (GPC)
3. Results and Discussion
3.1. Selection of Appropriate Functionalization Conditions
3.2. Characterization of Lignins
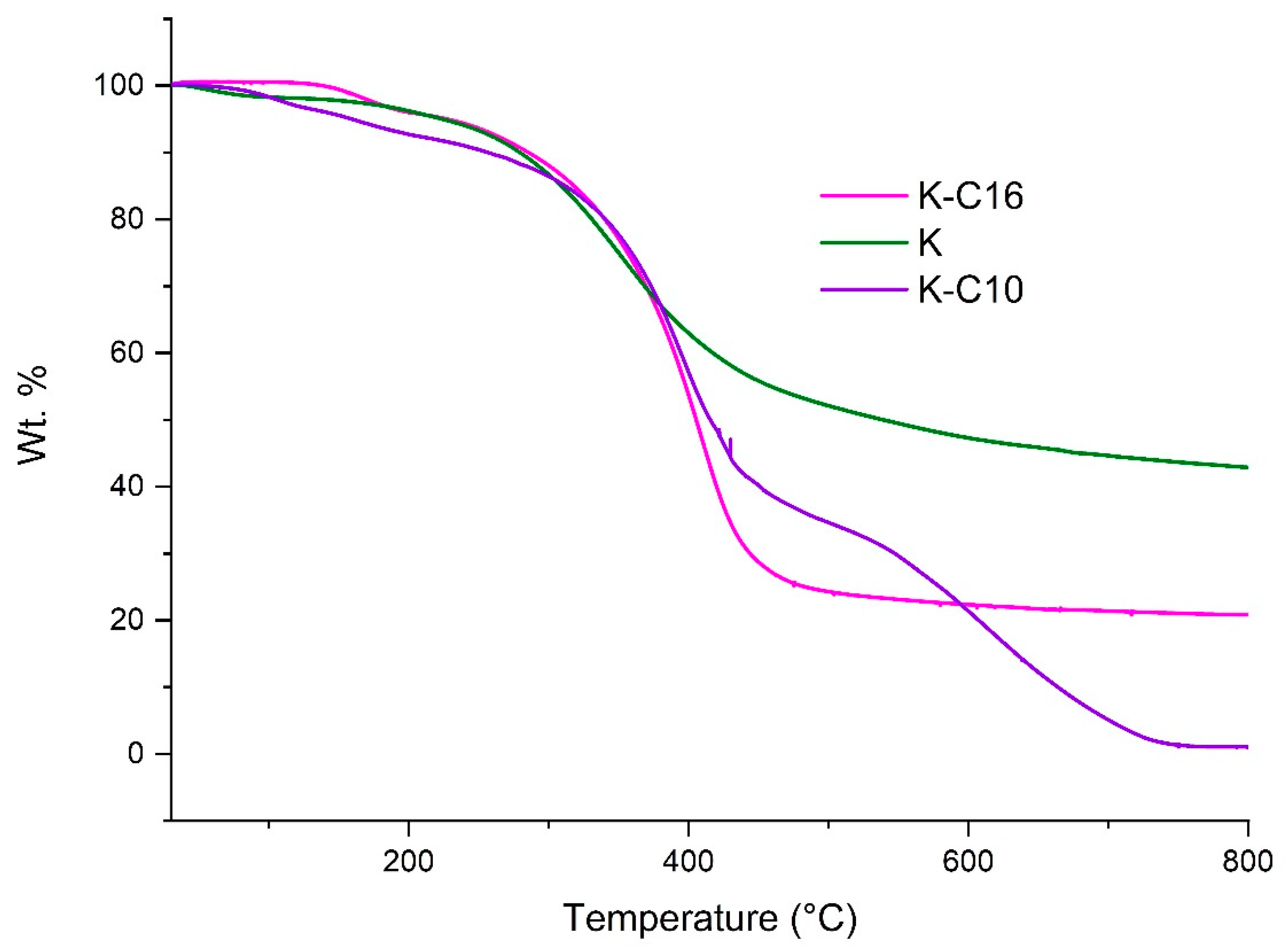
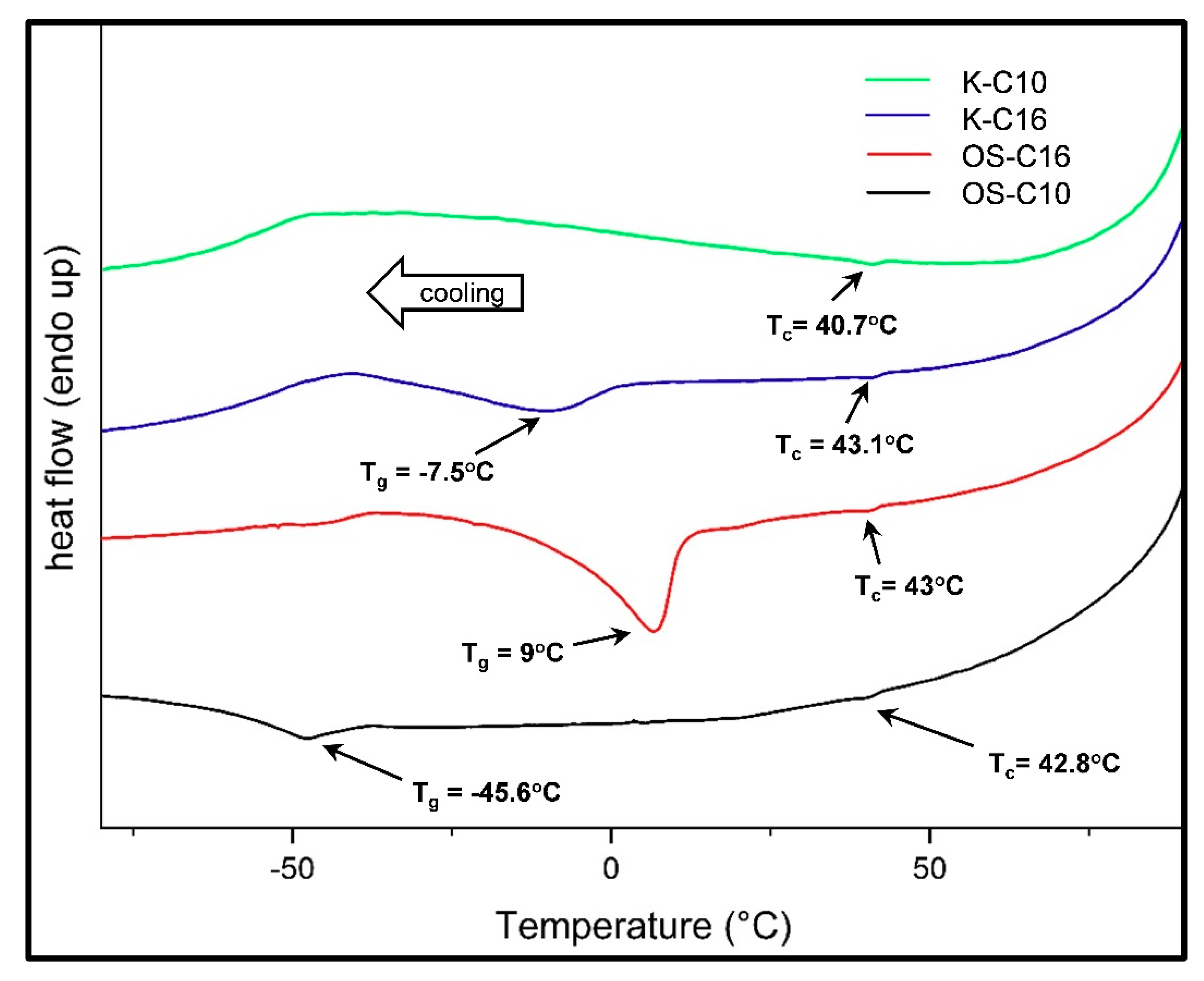
3.3. Thermal Characterization of Functionalized Lignins
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azadi, P.; Inderwildi, O.R.; Farnood, R.R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Jackson, D.; Trammel, N.E.; Hunt, M.A.; Naskar, A.K. Development of lignin-based polyurethane thermoplastics. RSC Adv. 2013, 3, 21832. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Hrůzová, K.; Matsakas, L.; Sand, A.; Rova, U.; Christakopoulos, P. Organosolv lignin hydrophobic micro- and nanoparticles as a low-carbon footprint biodegradable flotation collector in mineral flotation. Bioresour. Technol. 2020, 306, 123235. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Lopes, A.M.D.C.; Lukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Mu, L.; Wu, J.; Matsakas, L.; Chen, M.; Rova, U.; Christakopoulos, P.; Zhu, J.; Shi, Y. Two important factors of selecting lignin as efficient lubricating additives in poly (ethylene glycol): Hydrogen bond and molecular weight. Int. J. Boil. Macromol. 2019, 129, 564–570. [Google Scholar] [CrossRef]
- Beckham, G.T.; Johnson, C.W.; Karp, E.M.; Salvachúa, D.; Vardon, D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016, 42, 40–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsakas, L.; Karnaouri, A.; Cwirzen, A.; Rova, U.; Christakopoulos, P. Formation of Lignin Nanoparticles by Combining Organosolv Pretreatment of Birch Biomass and Homogenization Processes. Molecules 2018, 23, 1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent advances in organosolv fractionation: Towards biomass fractionation technology of the future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef] [PubMed]
- Kalogiannis, K.G.; Matsakas, L.; Aspden, J.; Lappas, A.; Rova, U.; Christakopoulos, P. Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation. Molecules 2018, 23, 1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasser, W.G. Classification of Lignin According to Chemical and Molecular Structure; American Chemical Society (ACS): Washington, DC, USA, 1999; Volume 742, pp. 216–238. [Google Scholar]
- Wang, C.; Kelley, S.S.; Venditti, R. Lignin-Based Thermoplastic Materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Robles, E.; Egüés, I.; Labidi, J. Lignin-ester derivatives as novel thermoplastic materials. RSC Adv. 2016, 6, 86909–86917. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Ashori, A.; Sheshmani, S. Hybrid composites made from recycled materials: Moisture absorption and thickness swelling behavior. Bioresour. Technol. 2010, 101, 4717–4720. [Google Scholar] [CrossRef]
- Laurichesse, S.; Averous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Lewis, H.F.; Brauns, F.E. Esters of Lignin Material. U.S. Patent Application No. 2,429,102, 14 October 1944. [Google Scholar]
- Glasser, W.G.; Jain, R.K. Lignin derivatives. I. Alkanoates. Holzforschung 1993, 47, 225–233. [Google Scholar] [CrossRef]
- Guo, Z.-X.; Gandini, A.; Pla, F. Polyesters from lignin. 1. The reaction of Kraft lignin with dicarboxylic acid chlorides. Polym. Int. 1992, 27, 17–22. [Google Scholar] [CrossRef]
- Guo, Z.-X.; Gandini, A. Polyesters from lignin—2. The copolyesterification of Kraft lignin and polyethylene glycols with dicarboxylic acid chlorides. Eur. Polym. J. 1991, 27, 1177–1180. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.-S.; Lin, W.-S. Preparation and application of dimer acid/lignin graft copolymer. BioResources 2011, 6, 2874–2884. [Google Scholar]
- Korich, A.L.; Fleming, A.B.; Walker, A.R.; Wang, J.; Tang, C.; Iovine, P.M. Chemical modification of organosolv lignin using boronic acid-containing reagents. Polymer 2012, 53, 87–93. [Google Scholar] [CrossRef]
- Han, Y.; Yuan, L.; Li, G.; Huang, L.; Qin, T.; Chu, F.; Tang, C. Renewable polymers from lignin via copper-free thermal click chemistry. Polymer 2016, 83, 92–100. [Google Scholar] [CrossRef]
- De Oliveira, W.; Glasser, W.G. Multiphase materials with lignin. 11. Starlike copolymers with caprolactone. Macromolecules 1994, 27, 5–11. [Google Scholar] [CrossRef]
- Chung, H.; Al-Khouja, A.; Washburn, N.R. Lignin-Based Graft CopolymersviaATRP and Click Chemistry. In Green Polymer Chemistry: Biocatalysis and Materials II; American Chemical Society (ACS): Washington, DC, USA, 2013; Volume 1144, pp. 373–391. [Google Scholar]
- Kim, Y.S.; Kadla, J.F. Preparation of a Thermoresponsive Lignin-Based Biomaterial through Atom Transfer Radical Polymerization. Biomacromolecules 2010, 11, 981–988. [Google Scholar] [CrossRef]
- Hilburg, S.L.; Elder, A.N.; Chung, H.; Ferebee, R.; Bockstaller, M.; Washburn, N.R. A universal route towards thermoplastic lignin composites with improved mechanical properties. Polymer 2014, 55, 995–1003. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Cui, C.; Argyropoulos, D.S. Toward Thermoplastic Lignin Polymers. Part 1. Selective Masking of Phenolic Hydroxyl Groups in Kraft Lignins via Methylation and Oxypropylation Chemistries. Ind. Eng. Chem. Res. 2012, 51, 16713–16720. [Google Scholar] [CrossRef]
- Glasser, W.G.; Barnett, C.A.; Rials, T.G.; Saraf, V.P. Engineering plastics from lignin II. Characterization of hydroxyalkyl lignin derivatives. J. Appl. Polym. Sci. 1984, 29, 1815–1830. [Google Scholar] [CrossRef]
- Kazzaz, A.E.; Feizi, Z.H.; Fatehi, P. Grafting strategies for hydroxy groups of lignin for producing materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef] [Green Version]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A novel hybrid organosolv: Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels 2018, 11, 160. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Rulli, F.; Crestini, C. Gel Permeation Chromatography in Determining Molecular Weights of Lignins: Critical Aspects Revisited for Improved Utility in the Development of Novel Materials. ACS Sustain. Chem. Eng. 2016, 4, 5167–5180. [Google Scholar] [CrossRef]
- Ren, W.; Pan, X.; Wang, G.; Cheng, W.; Liu, Y. Dodecylated lignin-g-PLA for effective toughening of PLA. Green Chem. 2016, 18, 5008–5014. [Google Scholar] [CrossRef]
- Hergert, H.L. Infrared Spectra of Lignin and Related Compounds. II. Conifer Lignin and Model Compounds 1,2. J. Org. Chem. 1960, 25, 405–413. [Google Scholar] [CrossRef]
- Faix, O. Classification of Lignins from Different Botanical Origins by FT-IR Spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.; Van Dam, J.E. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind. Crop. Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Derkacheva, O.Y.; Sukhov, D. Investigation of Lignins by FTIR Spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Gabov, K.; Gosselink, R.J.A.; Smeds, A.I.; Fardim, P. Characterization of Lignin Extracted from Birch Wood by a Modified Hydrotropic Process. J. Agric. Food Chem. 2014, 62, 10759–10767. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, L.; Wang, Z.; Laskar, D.D.; Swita, M.S.; Cort, J.R.; Yang, B. Characterization of lignin derived from water-only and dilute acid flowthrough pretreatment of poplar wood at elevated temperatures. Biotechnol. Biofuels 2015, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Todorciuc, T.; Cǎpraru, A.-M.; Kratochvílová, I.; Popa, V.I. Characterization of non-wood lignin and its hydoxymethylated derivatives by spectroscopy and self-assembling investigations. Cellul. Chem. Technol. 2009, 43, 399–408. [Google Scholar]
- Sammons, R.J.; Harper, D.P.; Labbé, N.; Bozell, J.J.; Elder, T.; Rials, T.G. Characterization of Organosolv Lignins using Thermal and FT-IR Spectroscopic Analysis. BioResources 2013, 8, 2752–2767. [Google Scholar] [CrossRef]
- Hergert, H.L. Infrared spectra. In Lignins: Occurrence, Formation, Structures and Reactions; Sarkanen, K.V., Hergert, H.L., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1971; pp. 267–297. [Google Scholar]
- Swapna, K.; Murthy, S.N.; Jyothi, M.T.; Nageswar, Y.V.D. Recyclable heterogeneous copper oxide on alumina catalyzed coupling of phenols and alcohols with aryl halides under ligand-free conditions. Org. Biomol. Chem. 2011, 9, 5978–5988. [Google Scholar] [CrossRef] [PubMed]
- Jammi, S.; Sakthivel, S.; Rout, L.; Mukherjee, T.; Mandal, S.; Mitra, R.; Saha, P.; Punniyamurthy, T. ChemInform Abstract: CuO Nanoparticles Catalyzed C-N, C-O, and C-S Cross-Coupling Reactions: Scope and Mechanism. ChemInform 2009, 40, 1971–1976. [Google Scholar] [CrossRef]
- Hult, E.-L.; Koivu, K.; Asikkala, J.; Ropponen, J.; Wrigstedt, P.; Sipilä, J.; Poppius-Levlin, K. Esterified lignin coating as water vapor and oxygen barrier for fiber-based packaging. Holzforschung 2013, 67, 899–905. [Google Scholar] [CrossRef]
- Chen, F.; Dai, H.; Dong, X.; Yang, J.; Zhong, M. Physical properties of lignin-based polypropylene blends. Polym. Compos. 2011, 32, 1019–1025. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shah, F.U. Thermal stability of choline based amino acid ionic liquids. J. Mol. Liq. 2018, 266, 597–602. [Google Scholar] [CrossRef]
- Durbeej, B.; Eriksson, L. A Density Functional Theory Study of Coniferyl Alcohol Intermonomeric Cross Linkages in Lignin—Three-Dimensional Structures, Stabilities and the Thermodynamic Control Hypothesis. Holzforschung 2003, 57, 150–164. [Google Scholar] [CrossRef]
- An, Y.-X.; Li, N.; Wu, H.; Lou, W.-Y.; Zong, M.-H. Changes in the Structure and the Thermal Properties of Kraft Lignin during Its Dissolution in Cholinium Ionic Liquids. ACS Sustain. Chem. Eng. 2015, 3, 2951–2958. [Google Scholar] [CrossRef]
- Cao, Z.; Galuska, L.; Qian, Z.; Zhang, S.; Huang, L.; Prine, N.; Li, T.; He, Y.; Hong, K.; Gu, X. The effect of side-chain branch position on the thermal properties of poly(3-alkylthiophenes). Polym. Chem. 2020, 11, 517–526. [Google Scholar] [CrossRef]
- Prasad, S.; Jiang, Z.; Sinha, S.K.; Dhinojwala, A. Partial Crystallinity in Alkyl Side Chain Polymers Dictates Surface Freezing. Phys. Rev. Lett. 2008, 101, 065505. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.T. Crystallinity in isotactic polyolefins with unbranched side chains. Makromol. Chem. 1964, 71, 1–32. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Reid, V.M.C.; McEwen, I.J. Effect of side chain length on the glass transition of copolymers from styrene with n -alkyl citraconimides and with n -alkyl itaconimides. Br. Polym. J. 1990, 23, 353–357. [Google Scholar] [CrossRef]
- Mesfun, S.; Matsakas, L.; Rova, U.; Christakopoulos, P. Technoeconomic Assessment of Hybrid Organosolv–Steam Explosion Pretreatment of Woody Biomass. Energies 2019, 12, 4206. [Google Scholar] [CrossRef] [Green Version]

| Lignin | Total Phenol (mmol g−1) | Carboxyl (mmol g−1) | Aliphatic (mmol g−1) |
|---|---|---|---|
| Organosolv | 2.50 | 0.19 | 3.00 |
| Kraft | 3.92 | 0.35 | 2.18 |
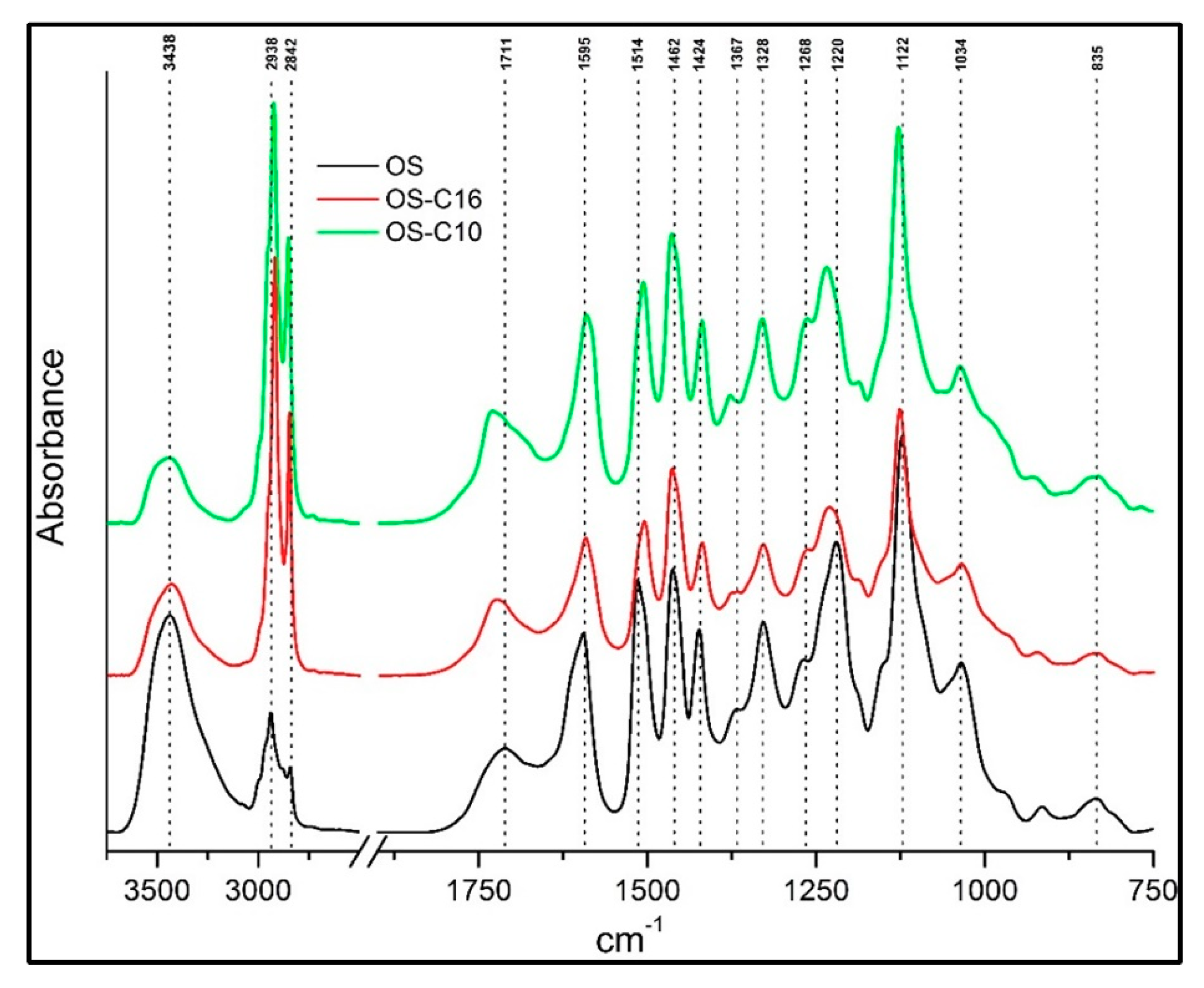
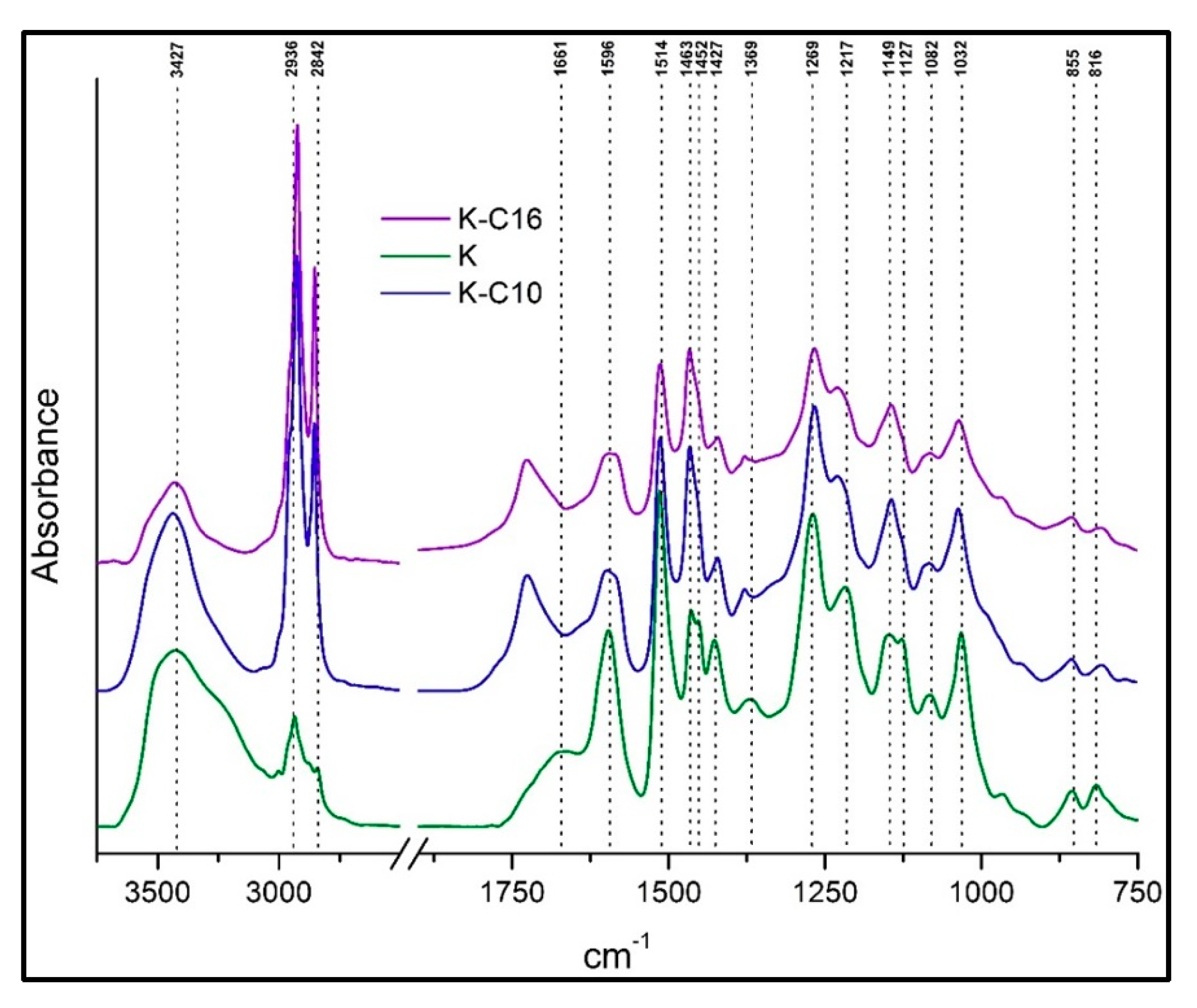
| Wave Number (cm−1) | IR Band Assignments |
|---|---|
| 3427–3442 | O-H stretching in aliphatic and phenolic -OH |
| 2924–2938 | C-H stretching in methyl groups |
| 2842–2854 | C-H stretch in methylene groups |
| 1711–1730 | C=O stretching in unconjugated ketones and carboxyl groups; saturated esters |
| 1661 | Stretching of C=O conjugated to aromatic rings (conjugated carbonyl) |
| 1590–1598 | Aromatic skeletal ring vibration (S > G) + C=O stretch |
| 1506–1514 | Aromatic skeletal ring vibrations (G > S) |
| 1452–1466 | C-H asymmetric deformations in methyl and methylene groups |
| 1419–1427 | Aromatic skeletal ring vibrations |
| 1367–1378 | Aliphatic C-H symmetric deformation in methyl (not methoxyl) + O-H deformation in phenols |
| 1328–1330 | S ring breathing vibration + G ring substituted in position 5 |
| 1264–1269 | G ring breathing vibration and C-O stretching |
| 1217–1235 | C-O stretching in phenols and ethers |
| 1122–1149 | C-H stretching in G ring and Aromatic C–H in plane deformation (S) |
| 1082 | C-O stretch of secondary alcohols and aliphatic ethers |
| 1034 | Aromatic C-H in-plane deformations in G units + C-O deformations in primary alcohols |
| 835/855 | Aromatic C-H out-of-plane deformation (only in GS and H lignin types) |
| Lignin | Number Average Molecular Weight, Mn (Da) | Weight Average Molecular Weight, Mw (Da) | Polydispersity Index |
|---|---|---|---|
| OS | 1757 | 4603 | 2.62 |
| OS-C10 | 1859 | 3719 | 2.00 |
| OS-C16 | 2661 | 6219 | 2.34 |
| K | 1924 | 7759 | 4.03 |
| K-C10 | 3452 | 11,664 | 3.38 |
| K-C16 | 3493 | 10,925 | 3.13 |
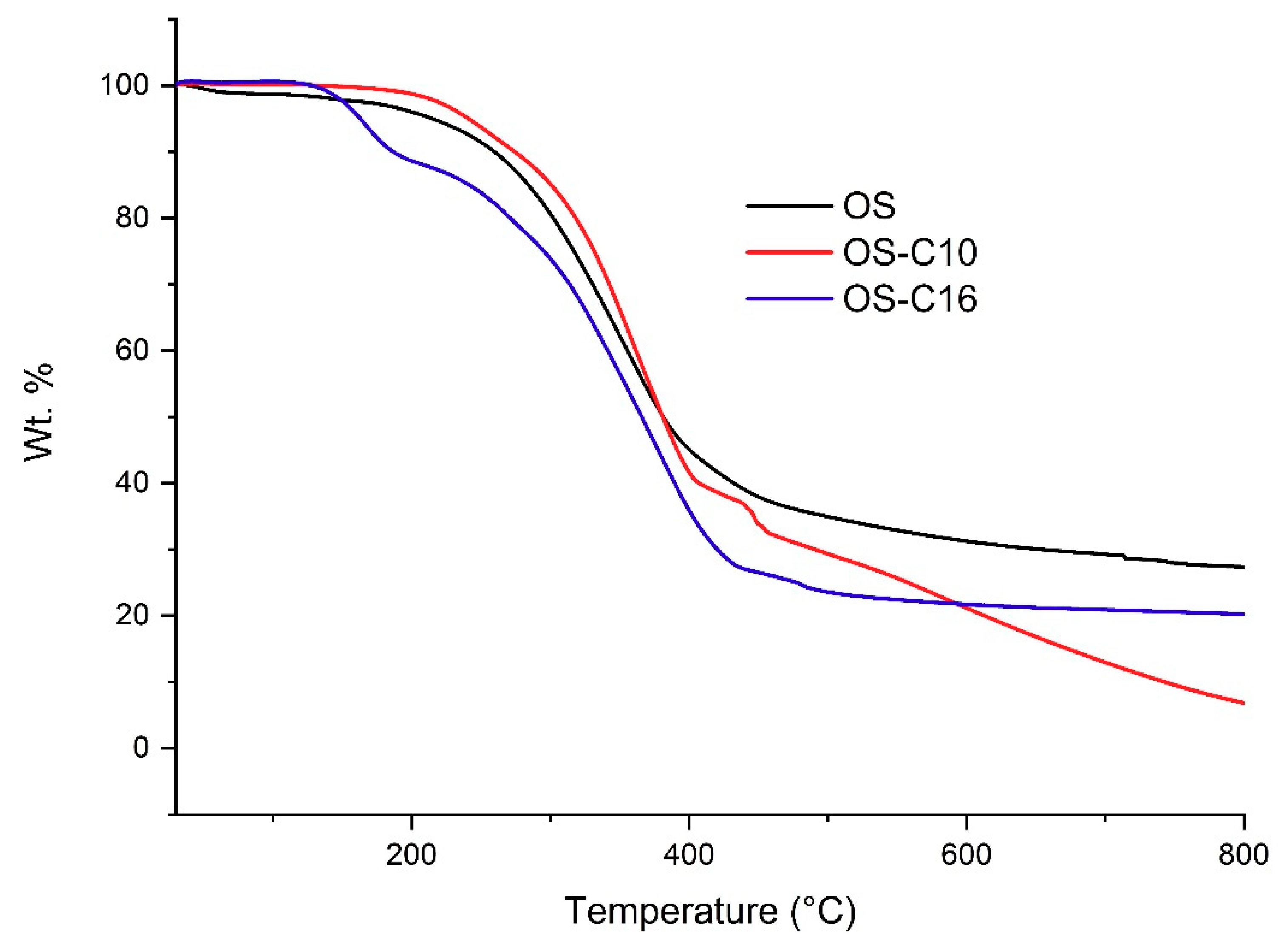
| Sample | T0.5% (°C) | T1% (°C) | T2% (°C) |
|---|---|---|---|
| OS | 50 | 65 | 143 |
| OS-C10 | 174 | 193 | 213 |
| OS-C16 | 135 | 140 | 148 |
| K | 54 | 66 | 133 |
| K-C16 | 148 | 156 | 168 |
| K-C10 | 75 | 88 | 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, S.; Matsakas, L.; Rova, U.; Christakopoulos, P. Melt Stable Functionalized Organosolv and Kraft Lignin Thermoplastic. Processes 2020, 8, 1108. https://doi.org/10.3390/pr8091108
Bhattacharyya S, Matsakas L, Rova U, Christakopoulos P. Melt Stable Functionalized Organosolv and Kraft Lignin Thermoplastic. Processes. 2020; 8(9):1108. https://doi.org/10.3390/pr8091108
Chicago/Turabian StyleBhattacharyya, Shubhankar, Leonidas Matsakas, Ulrika Rova, and Paul Christakopoulos. 2020. "Melt Stable Functionalized Organosolv and Kraft Lignin Thermoplastic" Processes 8, no. 9: 1108. https://doi.org/10.3390/pr8091108





