Synthesis of Silicon Hybrid Phenolic Resins with High Si-Content and Nanoscale Phase Separation Structure
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Phenolic Resins (PF)
2.3. Synthesis of Silicon Resins (SR)
2.4. Preparation of Hybrid Resins and Hybrid Composites
2.5. Characterization
3. Results and Discussion
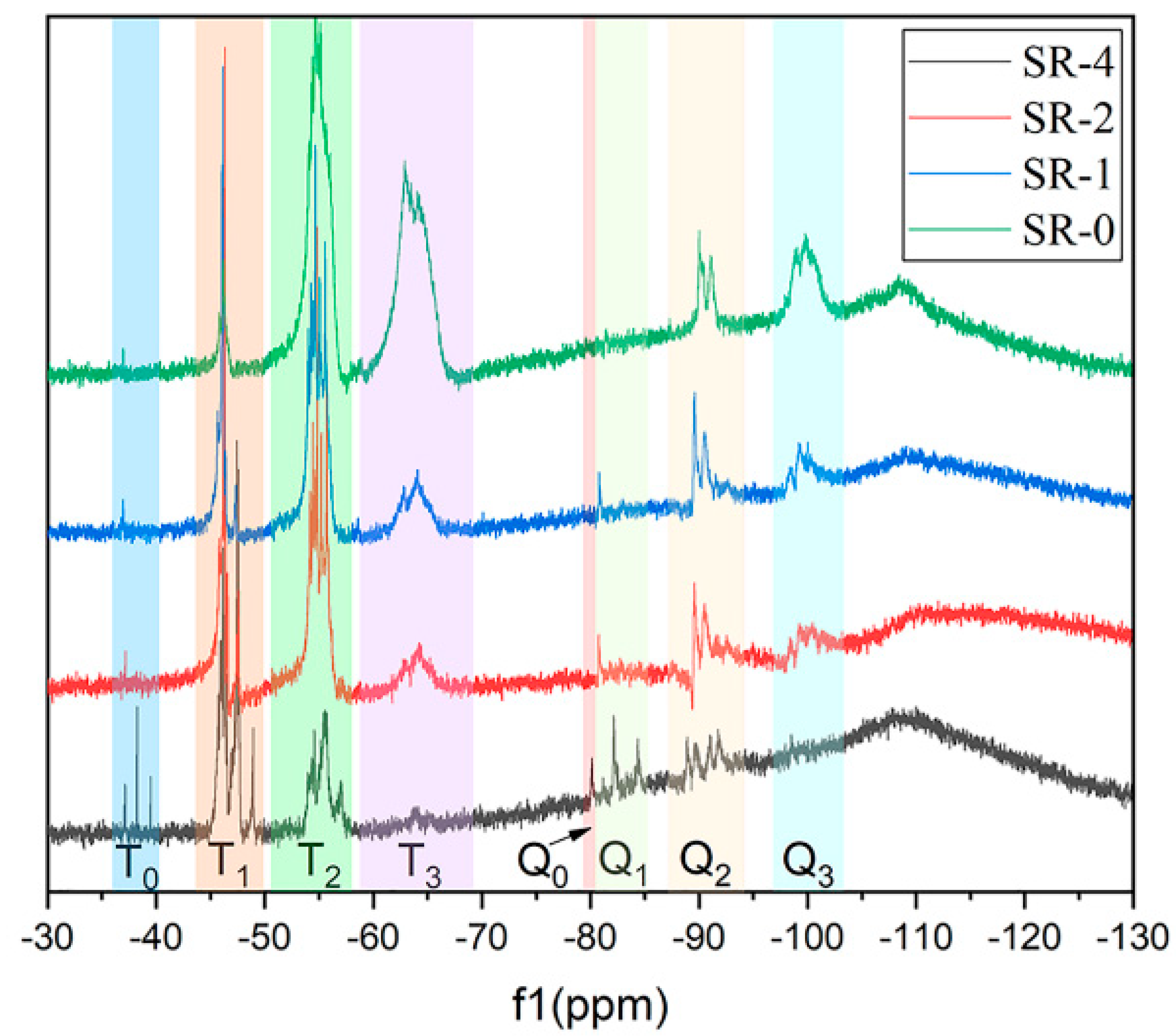
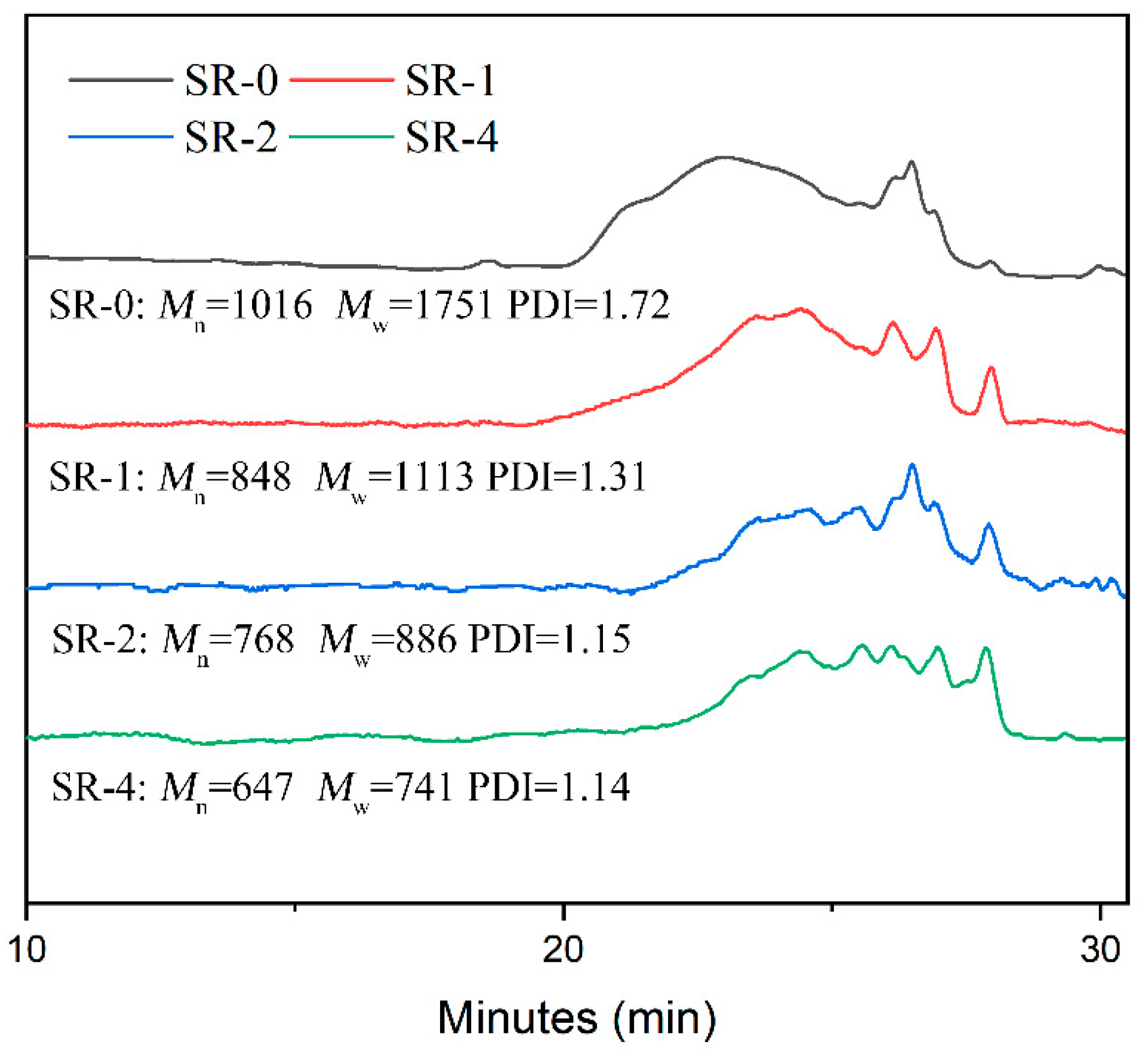
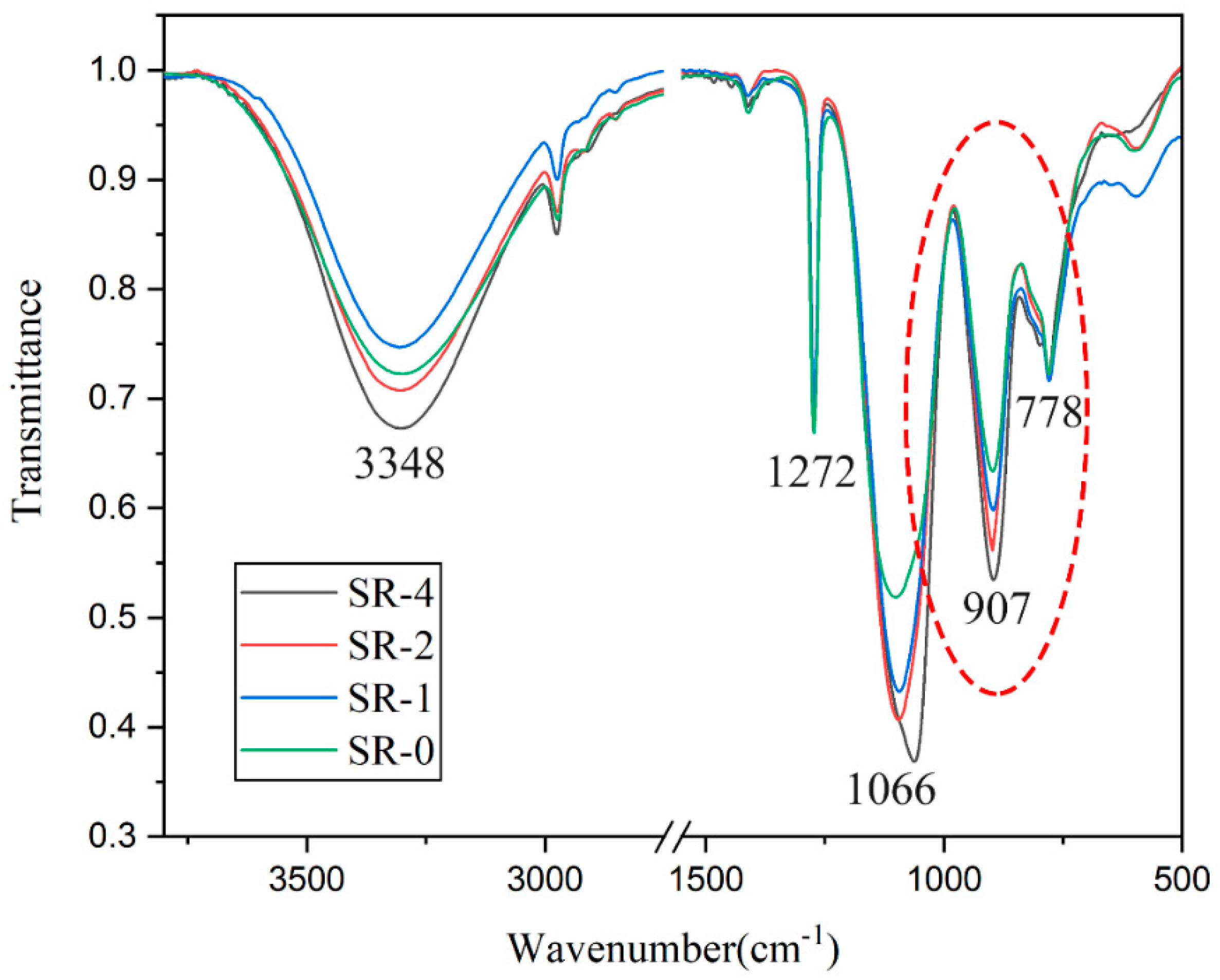
3.1. Structure Characterization of the SR Resins
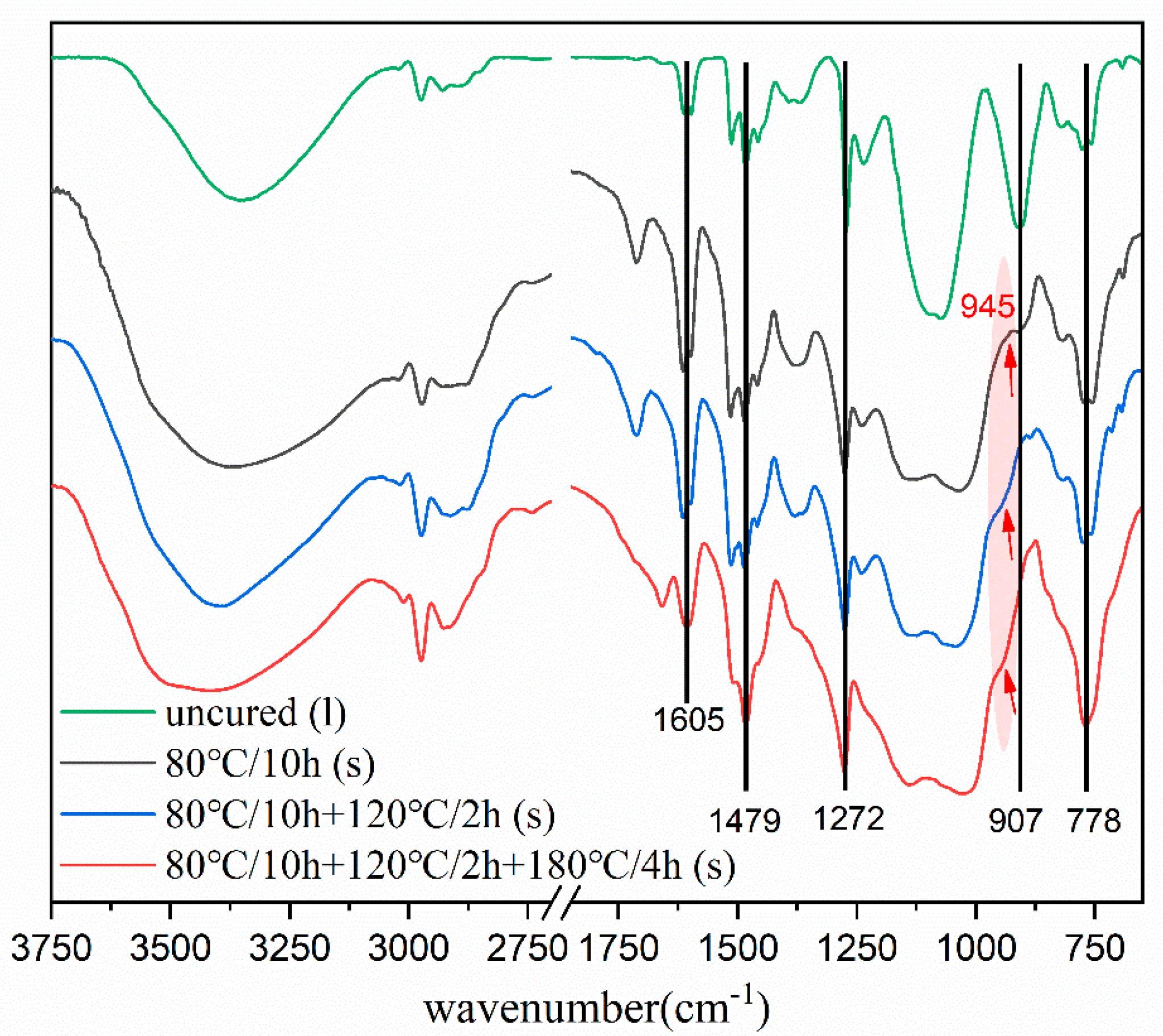
3.2. Curing Process of SPF Hybrid Resin
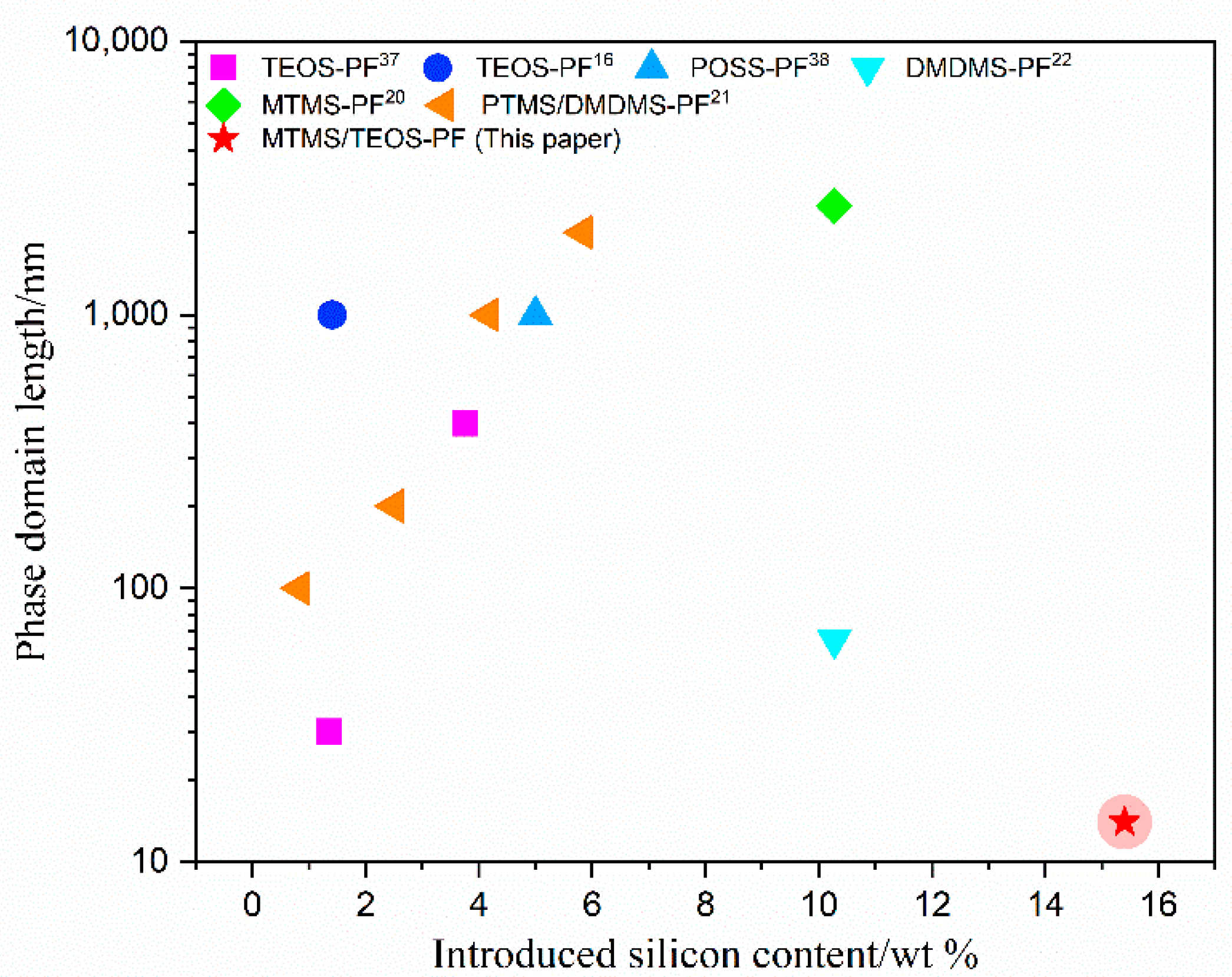
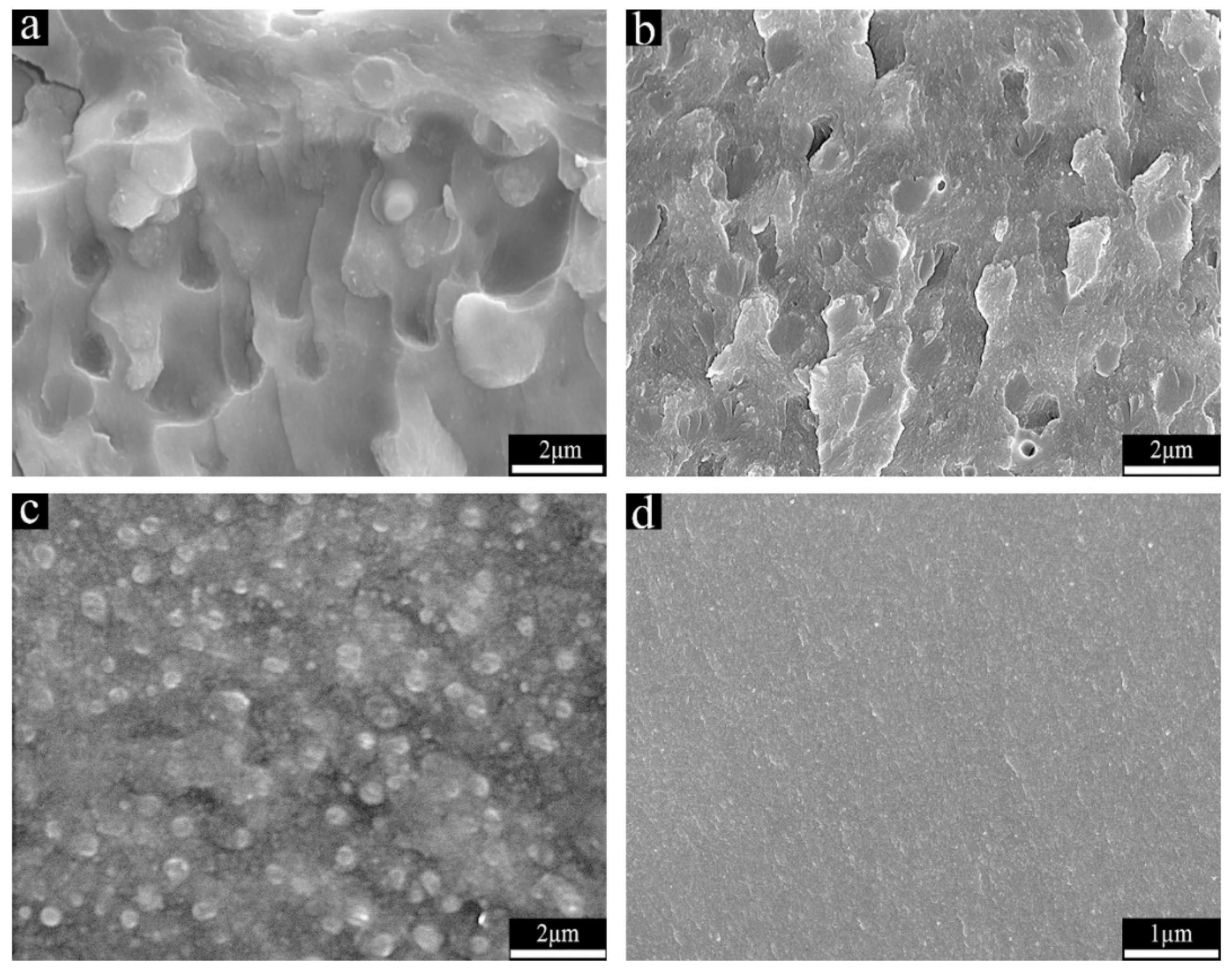
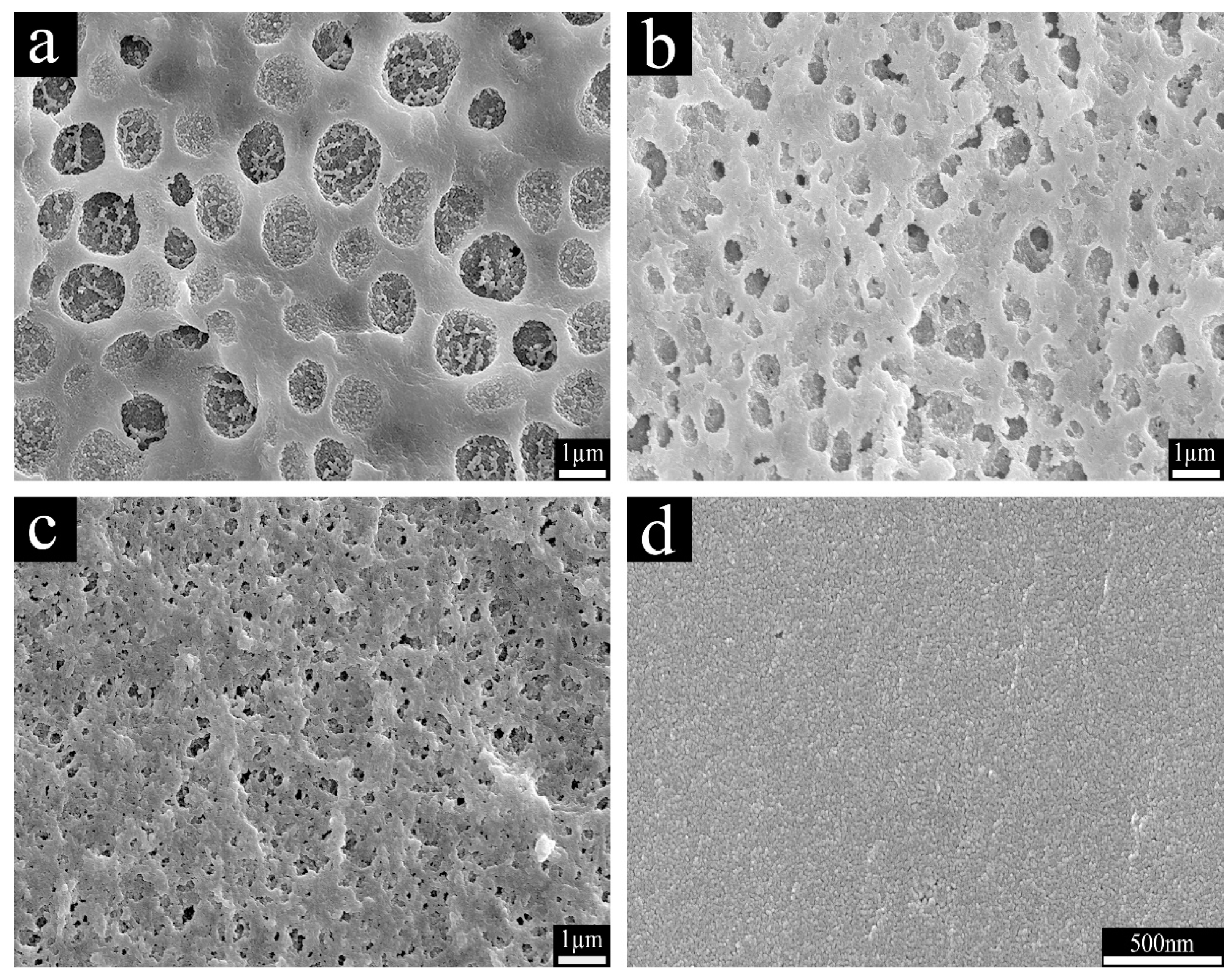
3.3. The Morphology of the Cured Hybrid Resins
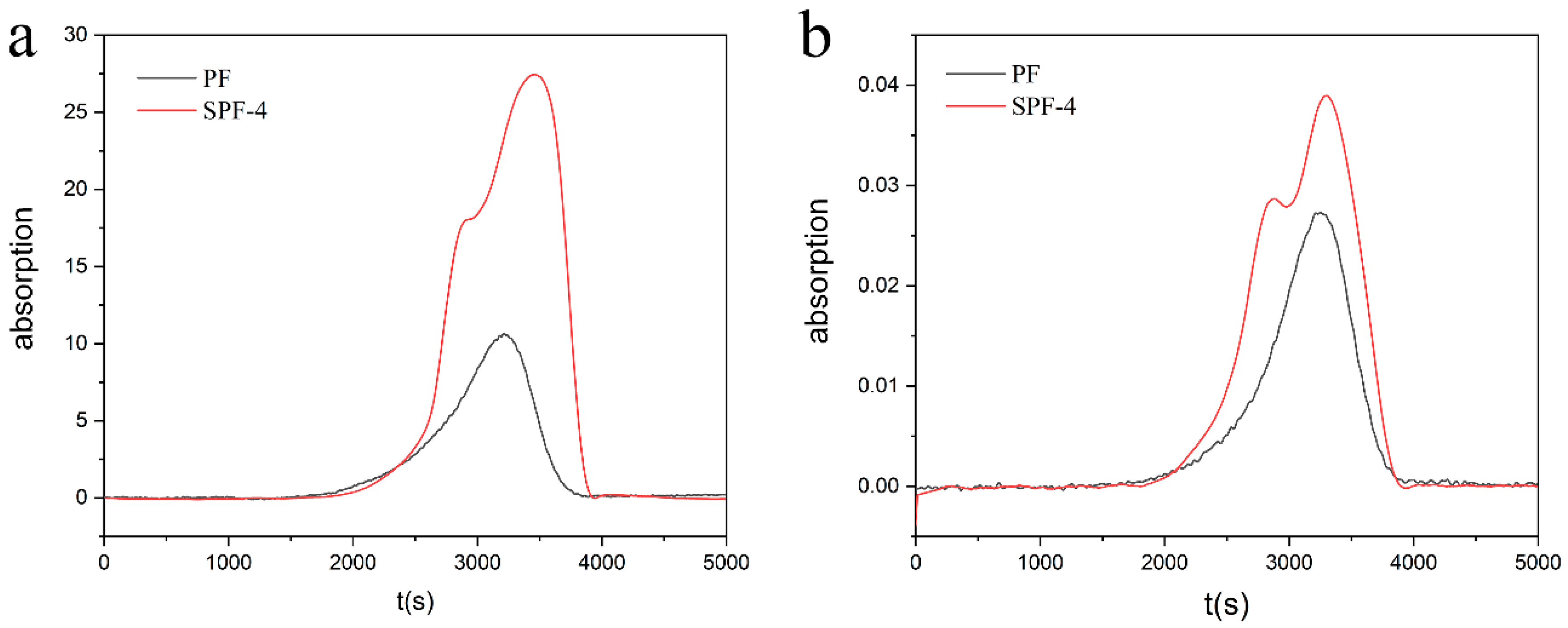
3.4. Thermal Stability of the SPF Resins
3.5. Ablative Property and Flame Retardancy of the SPF Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pilato, L. Phenolic resins: 100 years and still going strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar] [CrossRef]
- Shudo, Y.; Izumi, A.; Hagita, K.; Nakao, T.; Shibayama, M. Structure-mechanical property relationships in crosslinked phenolic resin investigated by molecular dynamics simulation. Polymer 2017, 116, 506–514. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Bian, C.; Zhong, Y.; Jing, X. Effect of chemical structure and cross-link density on the heat resistance of phenolic resin. Polym. Degrad. Stab. 2015, 111, 239–246. [Google Scholar] [CrossRef]
- Wang, S.; Jing, X.; Wang, Y.; Si, J. High char yield of aryl boron-containing phenolic resins: The effect of phenylboronic acid on the thermal stability and carbonization of phenolic resins. Polym. Degrad. Stab. 2014, 99, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Xing, X.; Wang, Y.; Wang, W.; Jing, X. Influence of poly (dihydroxybiphenyl borate) on the curing behaviour and thermal pyrolysis mechanism of phenolic resin. Polym. Degrad. Stab. 2017, 144, 378–391. [Google Scholar]
- Yun, J.; Chen, L.; Zhang, X.; Zhao, H.; Wen, Z.; Zhang, C. The effects of silicon and ferrocene on the char formation of modified novolac resin with high char yield. Polym. Degrad. Stab. 2017, 139, 97–106. [Google Scholar] [CrossRef]
- Zhong, Y.; Jing, X.; Wang, S.; Jia, Q.-X. Behavior investigation of phenolic hydroxyl groups during the pyrolysis of cured phenolic resin via molecular dynamics simulation. Polym. Degrad. Stab. 2016, 125, 97–104. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Zhang, B.; Luo, Z.; Li, H.; Zhao, T. Structure and improved thermal stability of phenolic resin containing silicon and boron elements. Polym. Degrad. Stab. 2016, 133, 321–329. [Google Scholar] [CrossRef]
- Yu, Z.L.; Yang, N.; Apostolopoulou-Kalkavoura, V.; Qin, B.; Ma, Z.Y.; Xing, W.Y.; Qiao, C.; Bergstrom, L.; Antonietti, M.; Yu, S.H. Fire-retardant and thermally insulating phenolic-silica aerogels. Angew. Chem. Int. Ed. Engl. 2018, 57, 4538–4542. [Google Scholar] [CrossRef]
- Yun, J.; Chen, L.; Zhang, X.; Zhao, H.; Wen, Z.; Zhu, D. Synthesis and structure evolution of phenolic resin/silicone hybrid composites with improved thermal stability. J. Mater. Sci. 2018, 53, 14185–14203. [Google Scholar] [CrossRef]
- Mercado, L.A.; Galia, M.; Reina, J.A. Silicon-containing flame retardant epoxy resins: Synthesis, characterization and properties. Polym. Degrad. Stab. 2006, 91, 2588–2594. [Google Scholar] [CrossRef]
- Ahmad, S.; Gupta, A.P.; Sharmin, E.; Alam, M.; Pandey, S.K. Synthesis, characterization and development of high performance siloxane-modified epoxy paints. Prog. Org. Coat. 2005, 54, 248–255. [Google Scholar] [CrossRef]
- Matsumoto, K.; Oba, Y.; Nakajima, Y.; Shimada, S.; Sato, K. One-pot sequence-controlled synthesis of oligosiloxanes. Angew. Chem. Int. Ed. Engl. 2018, 57, 4637–4641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Huang, Y.; Liu, X.L.; Yu, Y.Z. Studies on the silicone resins cured with polymethylsilazanes at ambient temperature. J. Appl. Polym. Sci. 2003, 89, 1702–1707. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhan, M. The preparation and characterization of abrasion-resistant coatings on polycarbonate. J. Coat. Technol. Res. 2012, 10, 79–86. [Google Scholar] [CrossRef]
- Haraguchi, K.; Usami, Y.; Yamamura, K.; Matsumoto, S. Morphological investigation of hybrid materials composed of phenolic resin and silica prepared by in situ polymerization. Polymer 1998, 39, 6243–6250. [Google Scholar] [CrossRef]
- Yin, R.; Cheng, H.; Hong, C.; Zhang, X. Synthesis and characterization of novel phenolic resin/silicone hybrid aerogel composites with enhanced thermal, mechanical and ablative properties. Compos. Part. A Appl. Sci. Manuf. 2017, 101, 500–510. [Google Scholar] [CrossRef]
- Humcke-Bogner, R.; Liu, J.-C.; Chien, Y.W. Methods for determining partial solubility parameters of potential film-coating polymers. Int. J. Pharm. 1988, 42, 199–209. [Google Scholar] [CrossRef]
- Carraher, C.E., Jr. Carraher’s Polymer Chemistry, 9th ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Li, S.; Han, Y.; Chen, F.; Luo, Z.; Li, H.; Zhao, T. The effect of structure on thermal stability and anti-oxidation mechanism of silicone modified phenolic resin. Polym. Degrad. Stab. 2016, 124, 68–76. [Google Scholar] [CrossRef]
- Li, S.; Chen, F.; Han, Y.; Zhou, H.; Li, H.; Zhao, T. Enhanced compatibility and morphology evolution of the hybrids involving phenolic resin and silicone intermediate. Mater. Chem. Phys. 2015, 165, 25–33. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Li, Z.; Zhou, H.; Guo, Y.; Chen, F.; Zhao, T. Polysiloxane modified phenolic resin with co-continuous structure. Polymer 2017, 120, 217–222. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, K.; Zheng, S. Organic-inorganic hybrid nanocomposites involving novolac resin and polyhedral oligomeric silsesquioxane. React. Funct. Polym. 2007, 67, 627–635. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Medoro, A.; Arendt, V.; Randall, J.; McCorkle, L.; Shonkwiler, B. Elastic behavior of methyltrimethoxysilane based aerogels reinforced with tri-isocyanate. ACS Appl. Mater. Interfaces 2010, 2, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-L.; Ma, C.C.M. Synthesis, characterization, thermal properties and flame retardance of novel phenolic resin/silica nanocomposites. Polym. Degrad. Stab. 2004, 83, 207–214. [Google Scholar] [CrossRef]
- Vallejo, P.P.; López, B.L.; Murillo, E.A. Hyperbranched phenolic-alkyd resins with high solid content. Prog. Org. Coat. 2015, 87, 213–221. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Huang, J.; Cao, J.; Gérard, J.-F.; Dai, L. Nanostructure of reactive polyhedral oligomeric silsesquioxane-based block copolymer as modifier in an epoxy network. High. Perform. Polym. 2016, 29, 1148–1157. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, S.; Adeel, M.; Zheng, S. Formation of poss-poss interactions in polyurethanes: From synthesis, morphologies to shape memory properties of materials. Polymer 2019, 160, 82–92. [Google Scholar] [CrossRef]
- Furukawa, N.; Yuasa, M.; Kimura, Y. Characterization of polysiloxane-block-polyimides with silicate group in the polysiloxane segments. Polymer 1999, 40, 1853–1862. [Google Scholar] [CrossRef]
- Hu, D.; Zheng, S. Reaction-induced microphase separation in polybenzoxazine thermosets containing poly(N-vinyl pyrrolidone)-block-polystyrene diblock copolymer. Polymer 2010, 51, 6346–6354. [Google Scholar] [CrossRef]
- Haraguchi, K.; Usami, Y. The novel organic-inorganic hybrid materials composed of phenolic resin and silica. Chem. Lett. 1997, 26, 51–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, S.; Yoonessi, M.; Liang, K.; Pittman, C.U. Phenolic resin-trisilanolphenyl polyhedral oligomeric silsesquioxane (poss) hybrid nanocomposites: Structure and properties. Polymer 2006, 47, 2984–2996. [Google Scholar] [CrossRef]
- Jia, P.; Liu, H.C.; Liu, Q.; Cai, X.F. Thermal degradation mechanism and flame retardancy of Mq silicone/epoxy resin composites. Polym. Degrad. Stab. 2016, 134, 144–150. [Google Scholar] [CrossRef]
- Sakka, S.; Kamiya, K. The Sol-Gel transition in the hydrolysis of metal alkoxides in relation to the formation of glass fibers and films. J. Non-Cryst. Solids 1982, 48, 31–46. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, T. Field desorption mass spectrometry characterization for relative molecular mass and distribution of phenolic resin. Acta Polymerica Sinica 2012, 2, 103–110. [Google Scholar] [CrossRef]
- Parnell, A.J.; Cadby, A.J.; Mykhaylyk, O.O.; Dunbar, A.D.; Hopkinson, P.E.; Donald, A.M.; Jones, R.A. Nanoscale phase separation of P3ht Pcbm thick films as measured by small-angle X-ray scattering. Macromolecules 2011, 44, 6503–6508. [Google Scholar] [CrossRef]
- Ophir, Z.; Wilkes, G.L. Saxs analysis of a linear polyester and a linear polyether urethane—Interfacial thickness determination. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1469–1480. [Google Scholar] [CrossRef]
- Dušek, K. Are cured thermoset resins inhomogeneous? Angew. Makromol. Chem. 1996, 240, 1–15. [Google Scholar] [CrossRef]
- Bonati, A.; Rainieri, S.; Bochicchio, G.; Tessadri, B.; Giuliani, F. Characterization of thermal properties and combustion behaviour of asphalt mixtures in the cone calorimeter. Fire Saf. J. 2015, 74, 25–31. [Google Scholar] [CrossRef]











| Sample | N2 | Air | ||
|---|---|---|---|---|
| T5% a (°C) | Rmax b (%) | T5% a (°C) | Rmax b (%) | |
| PF | 254.7 | 59.7 | 291.6 | 0.0 |
| SPF-0 | 285.5 | 71.9 | 337.0 | 32.8 |
| SPF-1 | 353.1 | 73.1 | 386.2 | 33.7 |
| SPF-2 | 386.8 | 73.9 | 397.3 | 37.4 |
| SPF-4 | 405.3 | 74.5 | 401.3 | 38.0 |
| Element | SPF-4 Composite | PF Composite | ||
|---|---|---|---|---|
| Interior | Upper | Interior | Upper | |
| C | 20.30wt% | 18.96wt% | 43.19wt% | 26.78wt% |
| O | 42.10wt% | 43.93wt% | 24.44wt% | 39.06wt% |
| Si | 37.60wt% | 37.11wt% | 32.37wt% | 34.16wt% |
| PF | SPF-4 | |
|---|---|---|
| PeakHRR (kW·m−2) | 150.51 | 117.03 |
| MeanHRR (kW·m−2) | 50.79 | 38.14 |
| THR (MJ·m−2) | 21.86 | 17.57 |
| TSR (m2/m2) | 199.48 | 146.66 |
| TOC (g) | 14.28 | 11.40 |
| MeanMLR (g/s) | 0.020 | 0.013 |
| Residual (%) | 78.72 | 86.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Chen, F.; Li, S.; Du, Y.; Luo, Z.; Sun, Y.; Li, H.; Zhao, T. Synthesis of Silicon Hybrid Phenolic Resins with High Si-Content and Nanoscale Phase Separation Structure. Processes 2020, 8, 1129. https://doi.org/10.3390/pr8091129
Yuan W, Chen F, Li S, Du Y, Luo Z, Sun Y, Li H, Zhao T. Synthesis of Silicon Hybrid Phenolic Resins with High Si-Content and Nanoscale Phase Separation Structure. Processes. 2020; 8(9):1129. https://doi.org/10.3390/pr8091129
Chicago/Turabian StyleYuan, Wenjie, Fenghua Chen, Shan Li, Youpei Du, Zhenhua Luo, Yanan Sun, Hao Li, and Tong Zhao. 2020. "Synthesis of Silicon Hybrid Phenolic Resins with High Si-Content and Nanoscale Phase Separation Structure" Processes 8, no. 9: 1129. https://doi.org/10.3390/pr8091129





