Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Biomass Carbonization
2.3. Surface Functionalization
2.4. Adsorption Test
2.5. Analysis
3. Results and Discussions
3.1. Carbonization and Functionalization Yield
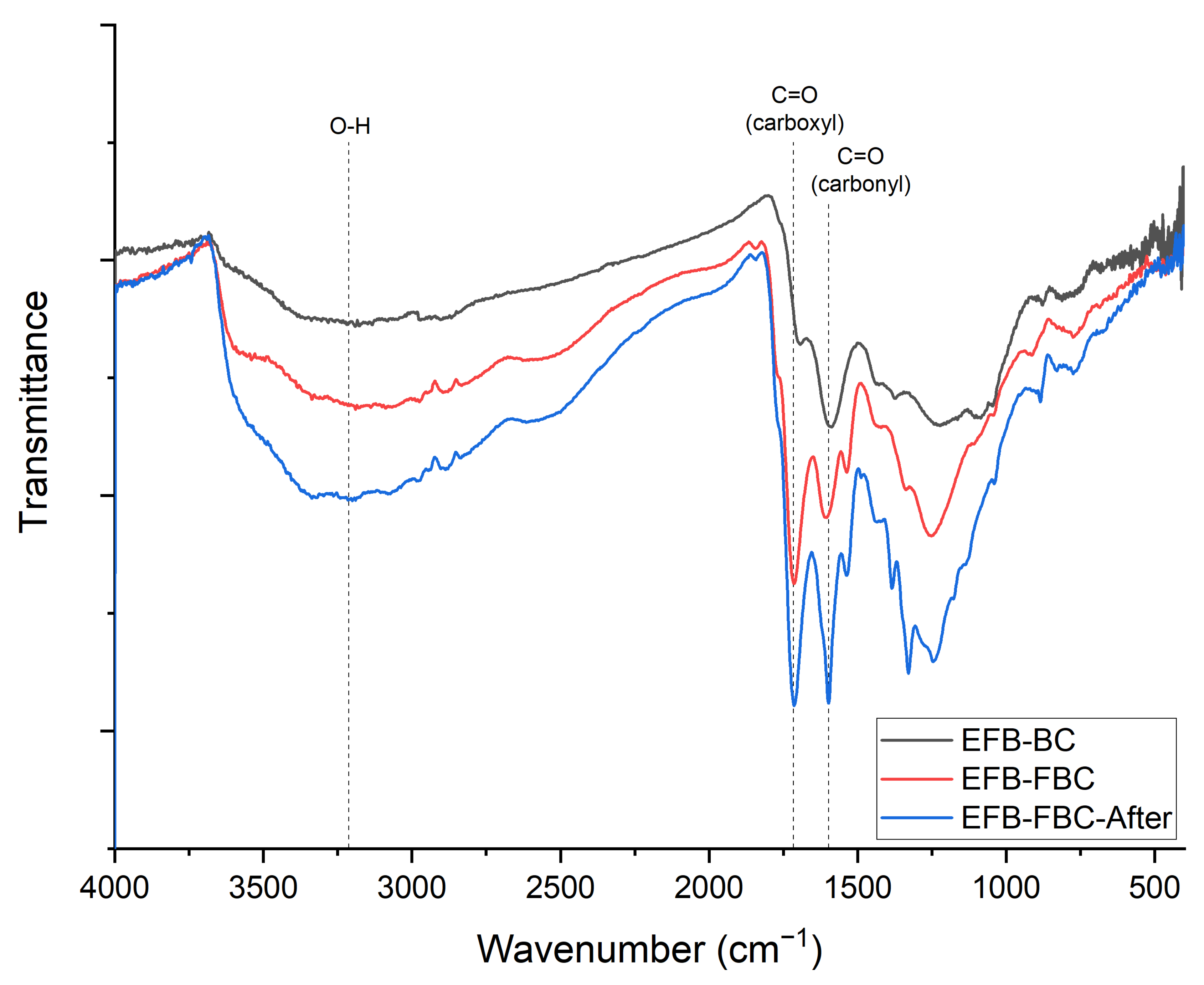
3.2. FTIR Analysis
3.3. Thermogravimetric Analysis
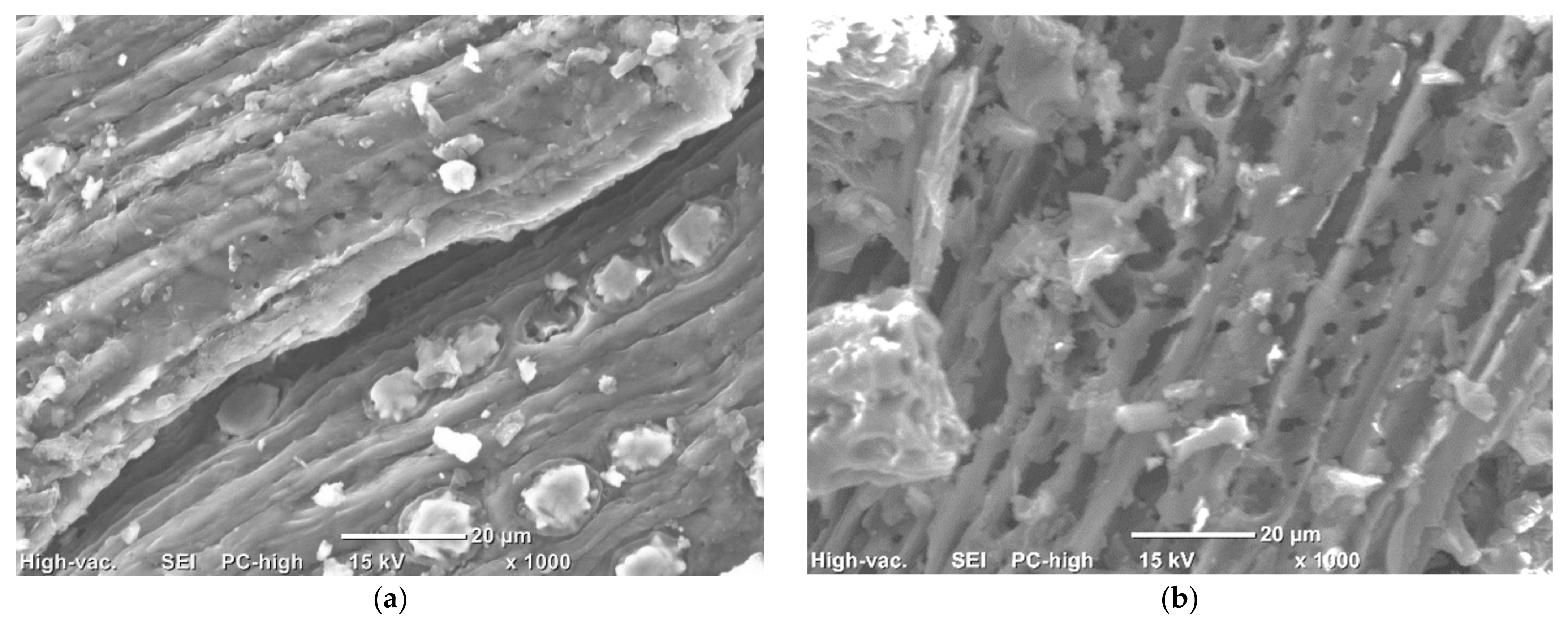
3.4. SEM Analysis
3.5. EDX Analysis
3.6. BET Surface Area
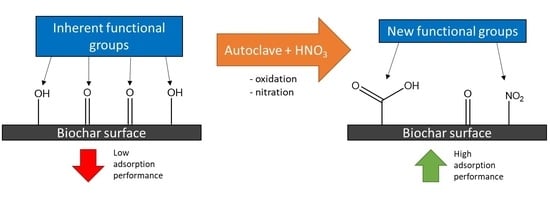
3.7. Mechanism of Hydrothermal Functionalization Using Nitric Acid
3.8. Adsorption Capacity, Isotherms, and Kinetic Study
3.9. MB Adsorption Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Jiang, S.; Nguyen, T.A.H.; Rudolph, V.; Yang, H.; Zhang, D.; Ok, Y.S.; Huang, L. Characterization of hard- and softwood biochars pyrolyzed at high temperature. Environ. Geochem. Health 2017, 39, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.A.; Yusup, S. A review on recent technological advancement in the activated carbon production from oil palm wastes. Chem. Eng. J. 2017, 314, 277–290. [Google Scholar] [CrossRef]
- Samsudin, M.H.; Hassan, M.A.; Idris, J.; Ramli, N.; Mohd Yusoff, M.Z.; Ibrahim, I.; Othman, M.R.; Mohd Ali, A.A.; Shirai, Y. A one-step self-sustained low temperature carbonization of coconut shell biomass produced a high specific surface area biochar-derived nano-adsorbent. Waste Manag. Res. 2019, 37. [Google Scholar] [CrossRef]
- Kim, J.A.; Vijayaraghavan, K.; Reddy, D.H.K.; Yun, Y.S. A phosphorus-enriched biochar fertilizer from bio-fermentation waste: A potential alternative source for phosphorus fertilizers. J. Clean. Prod. 2018, 196, 163–171. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; He, Y. Application and mechanism of sludge-based activated carbon for phenol and cyanide removal from bio-treated effluent of coking wastewater. Processes 2020, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chen, P.; Zhu, Y.G.; Cen, K.; Sun, G.X. Simultaneous adsorption and immobilization of As and Cd by birnessite-loaded biochar in water and soil. Env. Sci. Pollut. Res. 2019, 26, 8575–8584. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Malaysian Palm Oil Board. FFB Yield & Crude Palm Oil Yield of Oil Palm Estates 2019. Available online: http://bepi.mpob.gov.my/index.php/en/yield/yield-2019/yield-2019.html (accessed on 5 August 2020).
- Malaysian Palm Oil Board. Oil Palm Planted Area 2019. Available online: http://bepi.mpob.gov.my/index.php/en/area/area-2019/oil-palm-planted-area-as-at-dec-2019.html (accessed on 5 August 2020).
- Thoe, J.M.L.; Surugau, N.; Chong, H.L.H. Application of oil palm empty fruit bunch as adsorbent: A review. Trans. Sci. Technol. 2019, 6, 9–26. [Google Scholar]
- Bessou, C.; Verwilghen, A.; Beaudoin-Ollivier, L.; Marichal, R.; Ollivier, J.; Baron, V.; Bonneau, X.; Carron, M.P.; Snoeck, D.; Naim, M.; et al. Agroecological practices in oil palm plantations: Examples from the field. OCL Oilseeds FatsCrop. Lipids 2017, 24. [Google Scholar] [CrossRef] [Green Version]
- Yoshizaki, T.; Shirai, Y.; Hassan, M.A.; Baharuddin, A.S.; Raja Abdullah, N.M.; Sulaiman, A.; Busu, Z. Improved economic viability of integrated biogas energy and compost production for sustainable palm oil mill management. J. Clean. Prod. 2013, 44, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Idris, J.; Shirai, Y.; Andou, Y.; Mohd Ali, A.A.; Othman, M.R.; Ibrahim, I.; Hassan, M.A. Self-sustained carbonization of oil palm biomass produced an acceptable heating value charcoal with low gaseous emission. J. Clean. Prod. 2015, 89, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Idris, J.; Shirai, Y.; Andou, Y.; Mohd Ali, A.A.; Othman, M.R.; Ibrahim, I.; Yamamoto, A.; Yasuda, N.; Hassan, M.A. Successful scaling-up of self-sustained pyrolysis of oil palm biomass under pool-type reactor. Waste Manag. Res. 2016, 34, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Ibrahim, I.; Hassan, M.A.; Abd-Aziz, S.; Shirai, Y.; Andou, Y.; Othman, M.R.; Ali, A.A.M.; Zakaria, M.R. Reduction of residual pollutants from biologically treated palm oil mill effluent final discharge by steam activated bioadsorbent from oil palm biomass. J. Clean. Prod. 2017, 141, 122–127. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S.; Ahmad, M.M.; Mohamed, N.M.; Hameed, B.H. Activated carbon from the renewable agricultural residues using single step physical activation: A preliminary analysis. APCBEE Procedia 2012, 3, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Liou, T.-H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process. Technol. 2017, 160, 55–63. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess. Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Env. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Abdullah, M.O.; Lim, L.L.P.; Yeo, T.H.C. Surface modification and characterization of coconut shell-based activated carbon subjected to acidic and alkaline treatments. J. Appl. Sci. Process. Eng. 2017, 4, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 3rd ed.; Pearson Prentice Hall: Harlow, UK, 2008; ISBN 9780131755536. [Google Scholar]
- Lim Teik Zheng, A.; Phromsatit, T.; Boonyuen, S.; Andou, Y. Synthesis of silver nanoparticles/porphyrin/reduced graphene oxide hydrogel as dye adsorbent for wastewater treatment. FlatChem 2020, 23, 100174. [Google Scholar] [CrossRef]
- Idris, J.; Shirai, Y.; Anduo, Y.; Ali, A.A.M.; Othman, M.R.; Ibrahim, I.; Husen, R.; Hassan, M.A. Improved yield and higher heating value of biochar from oil palm biomass at low retention time under self-sustained carbonization. J. Clean. Prod. 2015, 104, 475–479. [Google Scholar] [CrossRef] [Green Version]
- Claoston, N.; Samsuri, A.W.; Ahmad Husni, M.H.; Mohd Amran, M.S. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Sritham, E.; Gunasekaran, S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Thermogravimetric, thermochemical, and infrared spectral characterization of feedstocks and biochar derived at different pyrolysis temperatures. Waste Manag. 2018, 78, 198–207. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Polovina, M.; Babić, B.; Kaluderović, B.; Dekanski, A. Surface characterization of oxidized activated carbon cloth. Carbon NY 1997, 35, 1047–1052. [Google Scholar] [CrossRef]
- Kasnejad, M.H.; Esfandiari, A.; Kaghazchi, T.; Asasian, N. Effect of pre-oxidation for introduction of nitrogen containing functional groups into the structure of activated carbons and its influence on Cu (II) adsorption. J. Taiwan Inst. Chem. Eng. 2012, 43, 736–740. [Google Scholar] [CrossRef]
- Yang, K.; Fox, J.T. Adsorption of humic acid by acid-modified granular activated carbon and powder activated carbon. J. Environ. Eng. 2018, 144, 04018104. [Google Scholar] [CrossRef]
- Li, B.; Li, K. Effect of nitric acid pre-oxidation concentration on pore structure and nitrogen/oxygen active decoration sites of ethylenediamine -modified biochar for mercury (II) adsorption and the possible mechanism. Chemosphere 2019, 220, 28–39. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Tang, J.; Zhang, C. Preparation of nitric acid modified powder activated carbon to remove trace amount of Ni (II) in aqueous solution. Water Sci. Technol. 2019, 80, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, J.; Shen, D.; Xiao, R.; Gu, S.; Zhao, M.; Liang, J. Removal of Pb (II) from water by the activated carbon modified by nitric acid under microwave heating. J. Colloid Interface Sci. 2016, 463, 118–127. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Li, K.; Jiang, Y.; Wang, X.; Bai, D.; Li, H.; Zheng, Z. Effect of nitric acid modification on the lead (II) adsorption of mesoporous biochars with different mesopore size distributions. Clean Technol. Environ. Policy 2016, 18, 797–805. [Google Scholar] [CrossRef]
- Lang, J.W.; Yan, X.B.; Liu, W.W.; Wang, R.T.; Xue, Q.J. Influence of nitric acid modification of ordered mesoporous carbon materials on their capacitive performances in different aqueous electrolytes. J. Power Sources 2012, 204, 220–229. [Google Scholar] [CrossRef]
- Thiemann, M.; Scheibler, E.; Wiegand, K.W. Nitric acid, nitrous acid, and nitrogen oxides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 177–223. [Google Scholar]
- Zhang, Y.; Xu, X.; Zhang, P.; Zhao, L.; Qiu, H.; Cao, X. Pyrolysis-temperature depended quinone and carbonyl groups as the electron accepting sites in barley grass derived biochar. Chemosphere 2019, 232, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Liljenberg, M.; Stenlid, J.H.; Brinck, T. Mechanism and regioselectivity of electrophilic aromatic nitration in solution: The validity of the transition state approach. J. Mol. Model. 2018, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Xu, S.; Wang, M.; Zhang, Y.; Xue, X. A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature. Bioresour. Technol. 2019, 282, 48–55. [Google Scholar] [CrossRef]
- Kah, M.; Sigmund, G.; Xiao, F.; Hofmann, T. Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res. 2017, 124, 673–692. [Google Scholar] [CrossRef]
- Lach, J.; Okoniewska, E.; Stepniak, L.; Ociepa-Kubicka, A. The influence of modification of activated carbon on adsorption of Ni (II) and Cd (II). Desalin. Water Treat. 2014, 52, 3979–3986. [Google Scholar] [CrossRef]



| N2 Adsorption | CO2 Adsorption | ||||
|---|---|---|---|---|---|
| aSBET (m2/g) | VT (cm3/g) | DP (nm) | amic (m2/g) | Vmic (cm3/g) | |
| EFB-BC | 3.7855 | 0.01836 | 19.401 | 3.44 | 0.1396 |
| EFB-FBC | 3.0499 | 0.01476 | 19.363 | 2.86 | 0.1312 |
| Carbon Material | Nitric Acid Concentration (v/v%) | Treatment Time (min) | Treatment Temperature (°C) | Reference |
|---|---|---|---|---|
| Biomass-based bagasse biochar | 10–25 | 360 | 60 | [39] |
| Coal-based activated carbon | 5–20 | 120 | 100 | [40] |
| Waste tea activated carbon | 20–80 | N/A a | 90 | [32] |
| Rice husk activated carbon | 42–71 | 20–50 | 90–150 | [41] |
| Activated carbon, carbon nanotube, and graphene oxide | N/A a | 30 | 140 | [42] |
| Carbon cloth | 68 | 60 | 90 | [36] |
| Bagasse activated carbon | 32.5 | 300 | 60 | [43] |
| Mesoporous carbon | 68 | 120 | 60 | [44] |
| Oil palm empty fruit bunch biochar | 6 | 60 | 132 | This study |
| qmax (mg/g) | Kads (L/mg) | R2 |
|---|---|---|
| 62.11 ± 0.14 | 0.9938 ± 0.0036 | 0.9991 |
| Model | Constants | |
|---|---|---|
| Pseudo-first order | Qe1 (mg/g) | 42.674 ± 0.316 |
| k1 (1/min) | 0.076 ± 0.006 | |
| R2 | 0.9402 | |
| Pseudo-second order | Qe2 (mg/g) | 45.662 ± 0.338 |
| k2 (g/mg.min) | 0.015 ± 0.003 | |
| R2 | 0.9666 | |
| Intraparticle diffusion | kp1 | 17.312 ± 0.140 |
| R21 | 0.9991 | |
| kp2 | 9.007 ± 0.073 | |
| R22 | 0.9901 | |
| kp3 | 7.817 ± 0.063 | |
| R23 | 0.9703 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, I.; Tsubota, T.; Hassan, M.A.; Andou, Y. Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process. Processes 2021, 9, 149. https://doi.org/10.3390/pr9010149
Ibrahim I, Tsubota T, Hassan MA, Andou Y. Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process. Processes. 2021; 9(1):149. https://doi.org/10.3390/pr9010149
Chicago/Turabian StyleIbrahim, Izzudin, Toshiki Tsubota, Mohd Ali Hassan, and Yoshito Andou. 2021. "Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process" Processes 9, no. 1: 149. https://doi.org/10.3390/pr9010149