Towards High Efficiency CO2 Utilization by Glow Discharge Plasma
Abstract
:1. Introduction
2. Materials and Methods
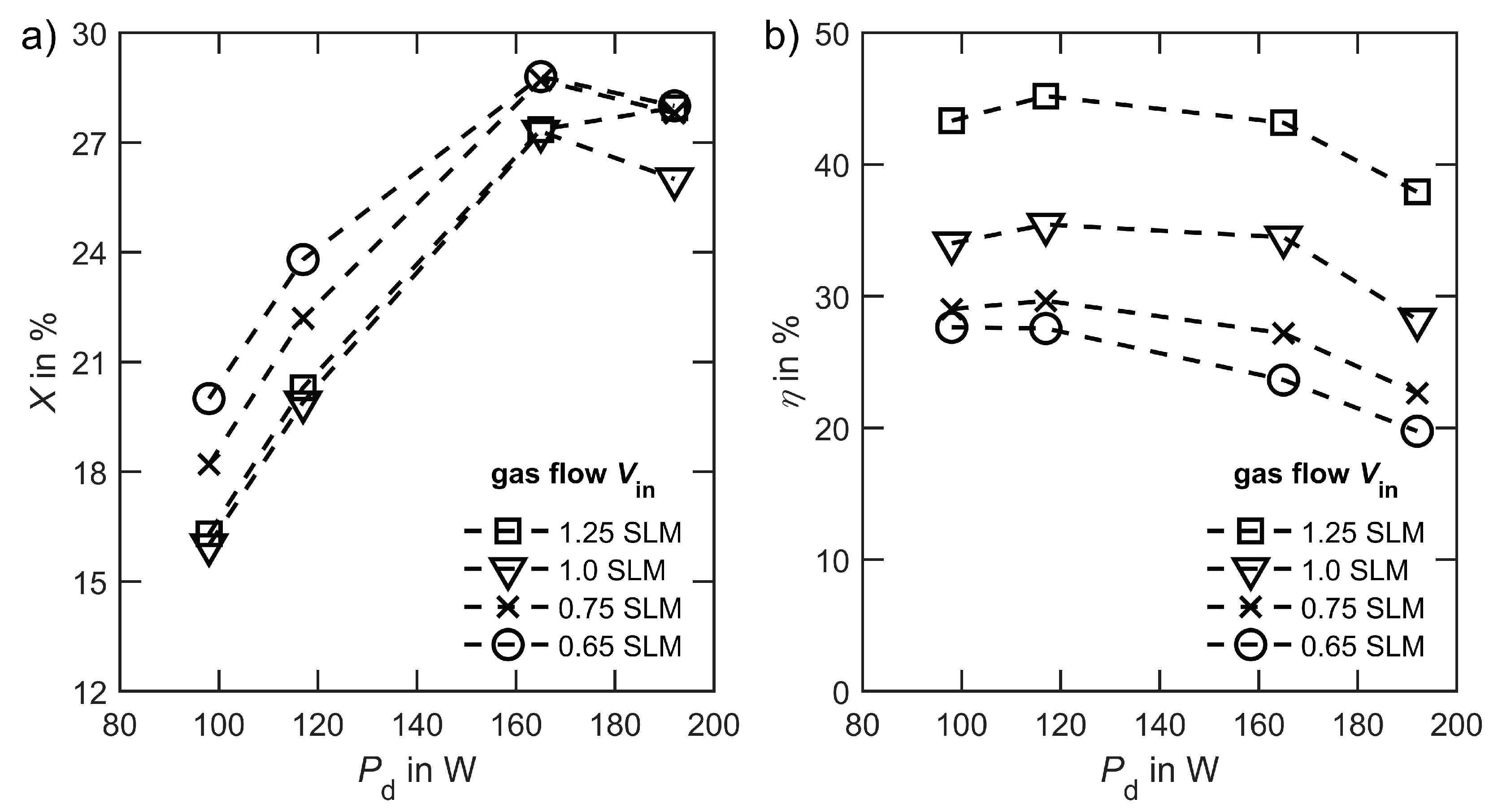
3. Results
4. Discussion
4.1. Performance of the Reactor
4.2. Comparison to Other Technologies
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Narayanan, V.A.; Yaashikaa, P.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Bogaerts, A.; Centi, G. Plasma technology for CO2 conversion: A personal perspective on prospects and gaps. Front. Energy Res. 2020, 8, 111. [Google Scholar] [CrossRef]
- Bogaerts, A.; Tu, X.; Whitehead, J.C.; Centi, G.; Lefferts, L.; Guaitella, O.; Azzolina-Jurry, F.; Kim, H.-H.; Murphy, A.B.; Schneider, W.F. The 2020 plasma catalysis roadmap. J. Phys. D Appl. Phys. 2020, 53.44, 443001. [Google Scholar] [CrossRef]
- Grim, R.G.; Huang, Z.; Guarnieri, M.T.; Ferrell, J.R.; Tao, L.; Schaidle, J.A. Transforming the carbon economy: Challenges and opportunities in the convergence of low-cost electricity and reductive CO2 utilization. Energy Environ. Sci. 2019, 13, 472–494. [Google Scholar] [CrossRef]
- Ozkan, A.; Dufour, T.; Silva, T.; Britun, N.; Snyders, R.; Reniers, F.; Bogaerts, A. DBD in burst mode: Solution for more efficient CO2 conversion? Plasma Sources Sci. Technol. 2016, 25, 055005. [Google Scholar] [CrossRef] [Green Version]
- Trenchev, G.; Bogaerts, A. Dual-vortex plasmatron: A novel plasma source for CO2 conversion. J. CO2 Util. 2020, 39, 101152. [Google Scholar] [CrossRef]
- Ramakers, M.; Medrano, J.A.; Trenchev, G.; Gallucci, F.; Bogaerts, A. Revealing the arc dynamics in a gliding arc plasmatron: A better insight to improve CO2 conversion. Plasma Sources Sci. Technol. 2017, 26, 125002. [Google Scholar] [CrossRef]
- Bharathi, R.; Sarathi, R.; Vinu, R. Development of a Swirl-Induced Rotating Glow Discharge Reactor for CO2 Conversion: Fluid Dynamics and Discharge Dynamics Studies. Energy Technol. 2020, 8.12, 2000535. [Google Scholar]
- Andreev, S.; Zakharov, V.; Ochkin, V.; Savinov, S. Plasma-chemical CO2 decomposition in a non-self-sustained discharge with a controlled electronic component of plasma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 3361–3369. [Google Scholar] [CrossRef]
- Bongers, W.; Bouwmeester, H.J.; Wolf, B.; Peeters, F.; Welzel, S.; Bekerom, D.V.D.; Harder, N.D.; Goede, A.; Graswinckel, M.; Groen, P.W.; et al. Plasma-driven dissociation of CO2 for fuel synthesis. Plasma Process. Polym. 2016, 14, e1600126. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Song, S.; Tom, C.P.; Xie, F. Carbon dioxide conversion in an atmospheric pressure microwave plasma reactor: Improving efficiencies by enhancing afterglow quenching. J. CO2 Util. 2019, 37, 240–247. [Google Scholar] [CrossRef]
- Renninger, S.; Lambarth, M.; Birke, K.P. High efficiency CO2-splitting in atmospheric pressure glow discharge. J. CO2 Util. 2020, 42, 101322. [Google Scholar] [CrossRef]
- Saifutdinov, A.I.; Timerkaev, B.A. Features of Transient Processes in DC Microdischarges in Molecular Gases: From a Glow Discharge to an Arc Discharge with a Unfree or Free Cathode Regime. JETP Lett. 2020, 112, 405–412. [Google Scholar] [CrossRef]
- Rabinovich, A.; Nirenberg, G.; Kocagoz, S.; Surace, M.; Sales, C.; Fridman, A. Scaling Up of Non-Thermal Gliding Arc Plasma Systems for Industrial Applications. Plasma Chem. Plasma Process. 2021, 1–16. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Li, X.; Huang, J.; Kong, X.; Xu, R.; Tu, X. Magnetically enhanced gliding arc discharge for CO2 activation. J. CO2 Util. 2019, 35, 28–37. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Li, X.; Wang, W.; Yan, J.; Tu, X. Warm plasma activation of CO2 in a rotating gliding arc discharge reactor. J. CO2 Util. 2018, 27, 472–479. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology—A novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46.19, 5805–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.C.; Lim, M.S.; Chun, Y.N. Reduction Characteristics of Carbon Dioxide Using a Plasmatron. Plasma Chem. Plasma Process. 2013, 34, 125–143. [Google Scholar] [CrossRef]
- Spencer, L.F.; Gallimore, A.D. Efficiency of CO2 Dissociation in a Radio-Frequency Discharge. Plasma Chem. Plasma Process. 2010, 31, 79–89. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Kutz, R.; Masel, R.I. CO2 Electrolysis to CO and O2 at High Selectivity, Stability and Efficiency Using Sustainion Membranes. J. Electrochem. Soc. 2018, 165, J3371–J3377. [Google Scholar] [CrossRef]
- Kaur, G.; Kulkarni, A.P.; Giddey, S. CO2 reduction in a solid oxide electrolysis cell with a ceramic composite cathode: Effect of load and thermal cycling. Int. J. Hydrogen Energy 2018, 43, 21769–21776. [Google Scholar] [CrossRef]
- Zonetti, C.P.; Letichevsky, S.; Gaspar, A.B.; Sousa-Aguiar, E.F.; Appel, L.G. The NixCe0.75Zr0.25−xO2 solid solution and the RWGS. Appl. Catal. A Gen. 2014, 475, 48–54. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renninger, S.; Rößner, P.; Stein, J.; Lambarth, M.; Birke, K.P. Towards High Efficiency CO2 Utilization by Glow Discharge Plasma. Processes 2021, 9, 2063. https://doi.org/10.3390/pr9112063
Renninger S, Rößner P, Stein J, Lambarth M, Birke KP. Towards High Efficiency CO2 Utilization by Glow Discharge Plasma. Processes. 2021; 9(11):2063. https://doi.org/10.3390/pr9112063
Chicago/Turabian StyleRenninger, Stephan, Paul Rößner, Jan Stein, Maike Lambarth, and Kai Peter Birke. 2021. "Towards High Efficiency CO2 Utilization by Glow Discharge Plasma" Processes 9, no. 11: 2063. https://doi.org/10.3390/pr9112063




