Nanostructure-Based Oil–Water Separation: Mechanism and Status
Abstract
:1. Introduction
2. Difficulty in the Field of Oil/Water Separation
3. Membrane Used for Oil–Water Separation
3.1. Metal Base Film Material
3.2. Polymer Materials
3.3. Biomass-Based Membrane Materials
3.4. Inorganic Material
4. Absorbability
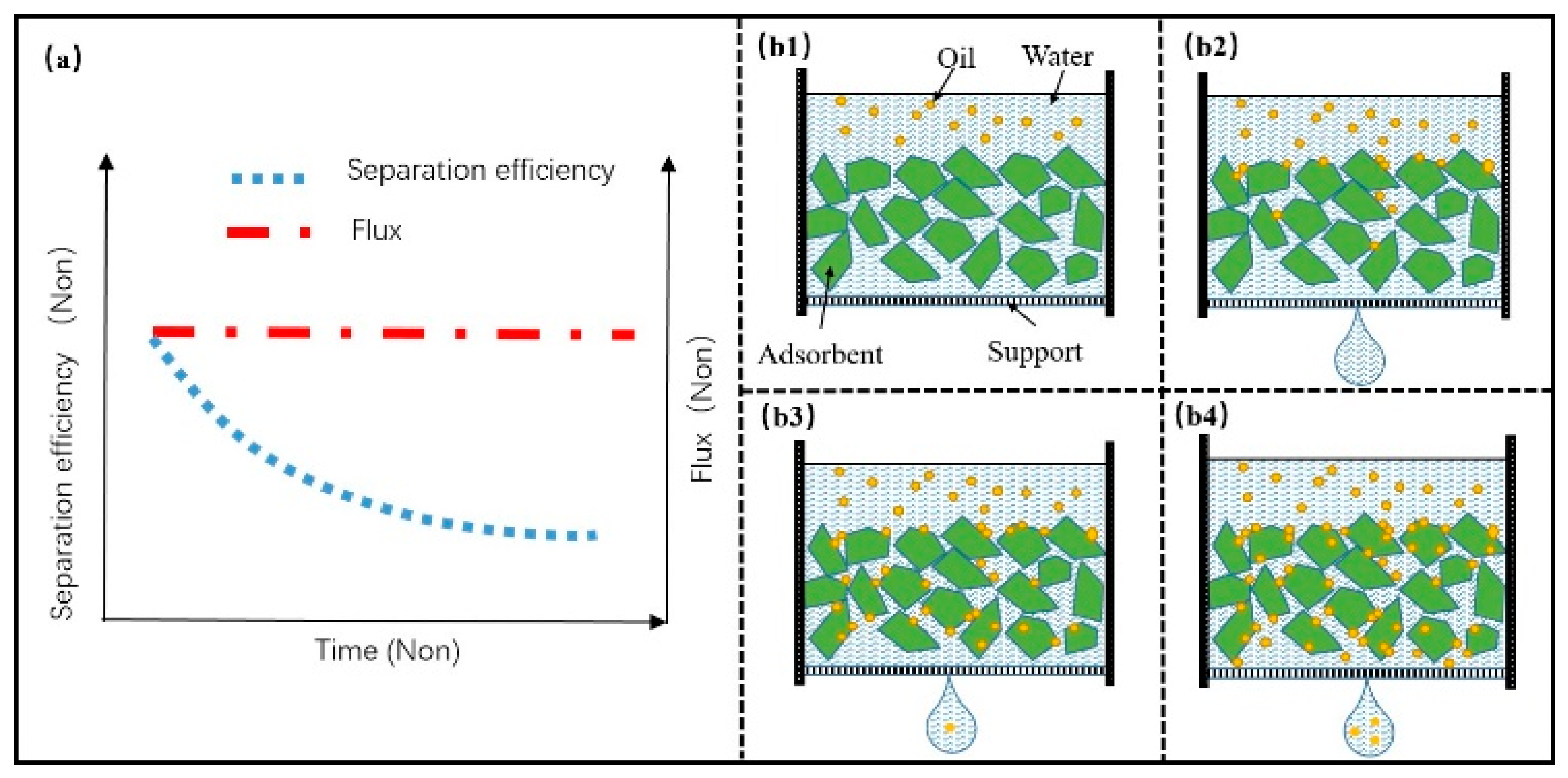
4.1. Adsorbing Porous Materials
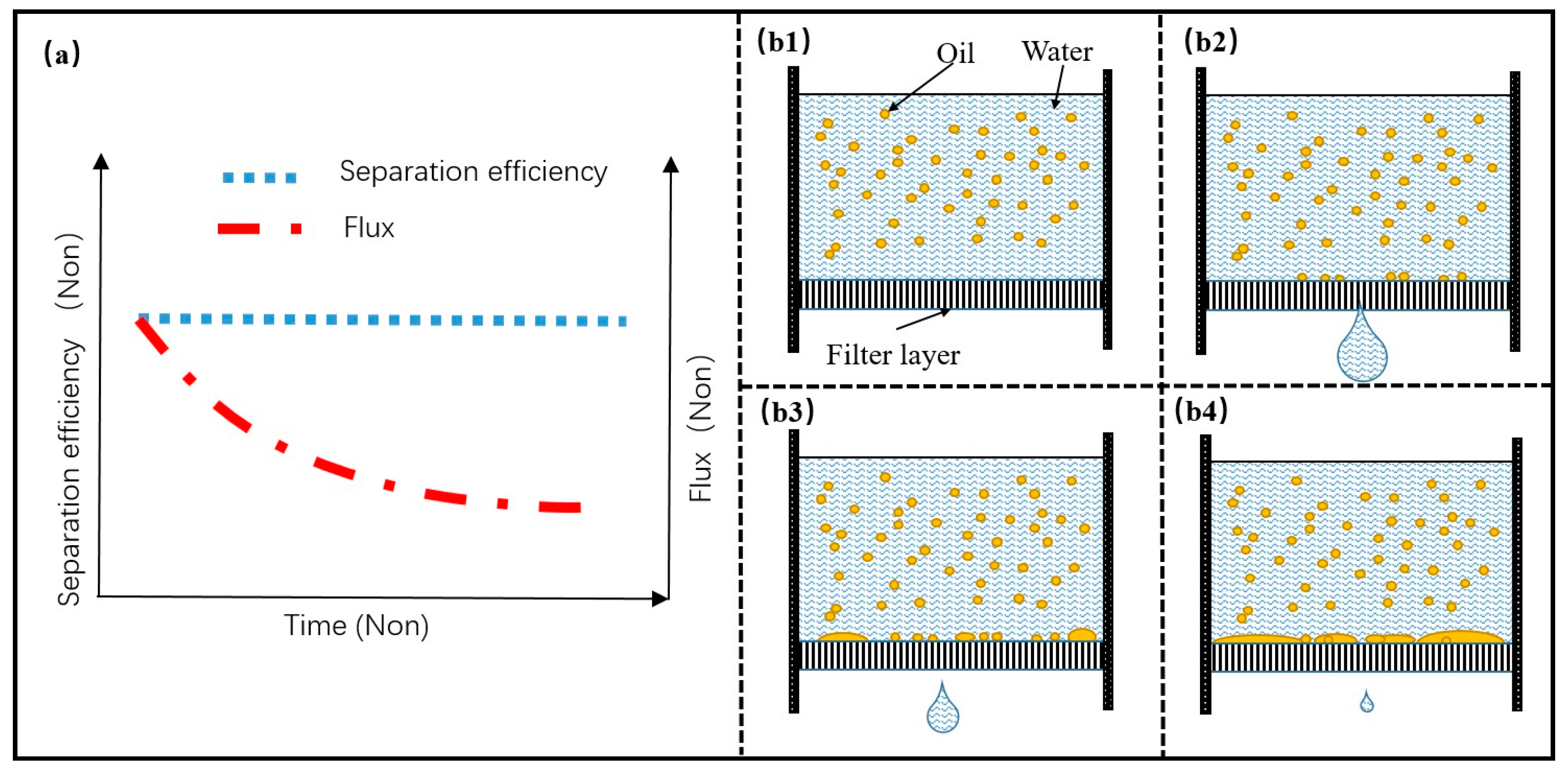
4.2. Filter Material Based on Adsorption Property
5. Obstruction
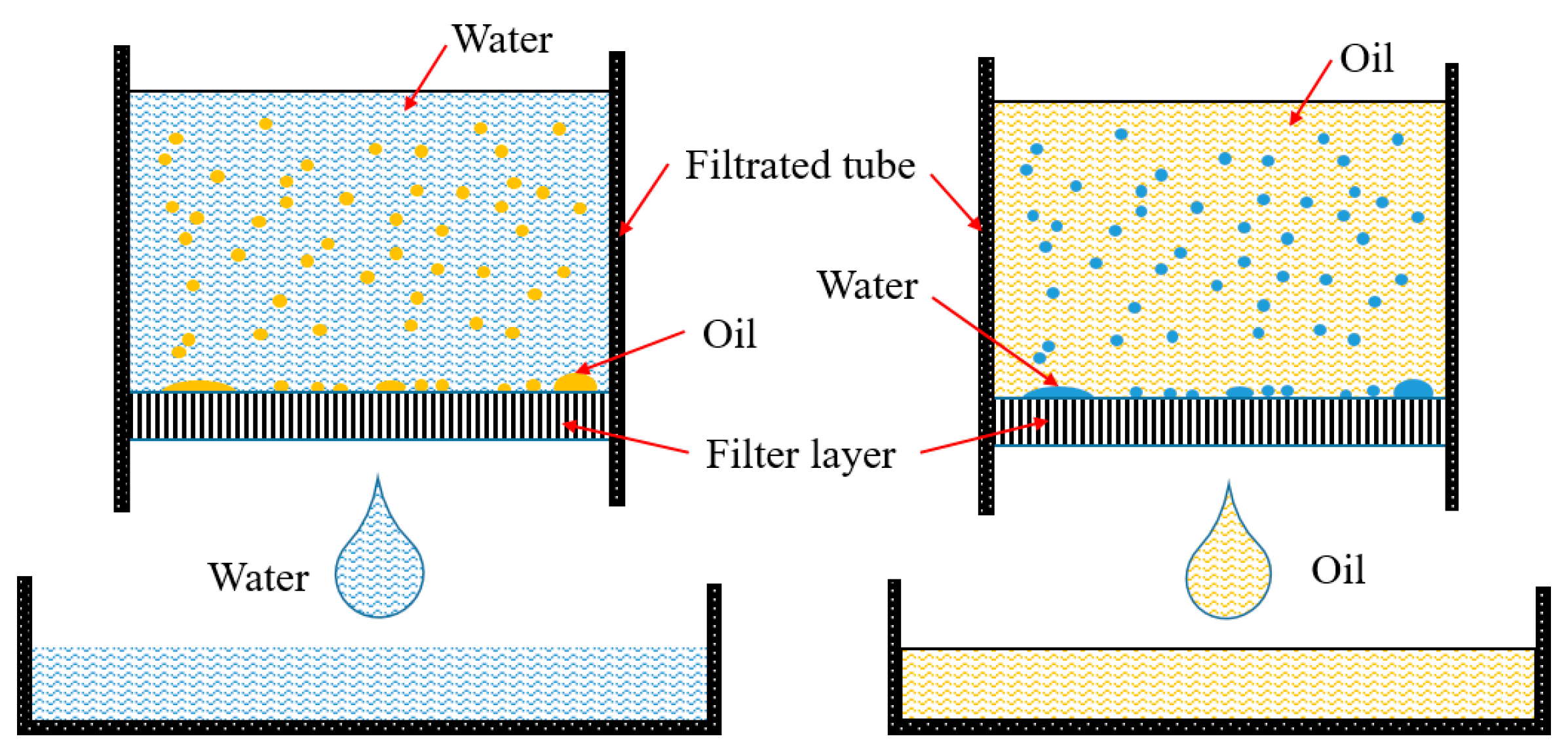
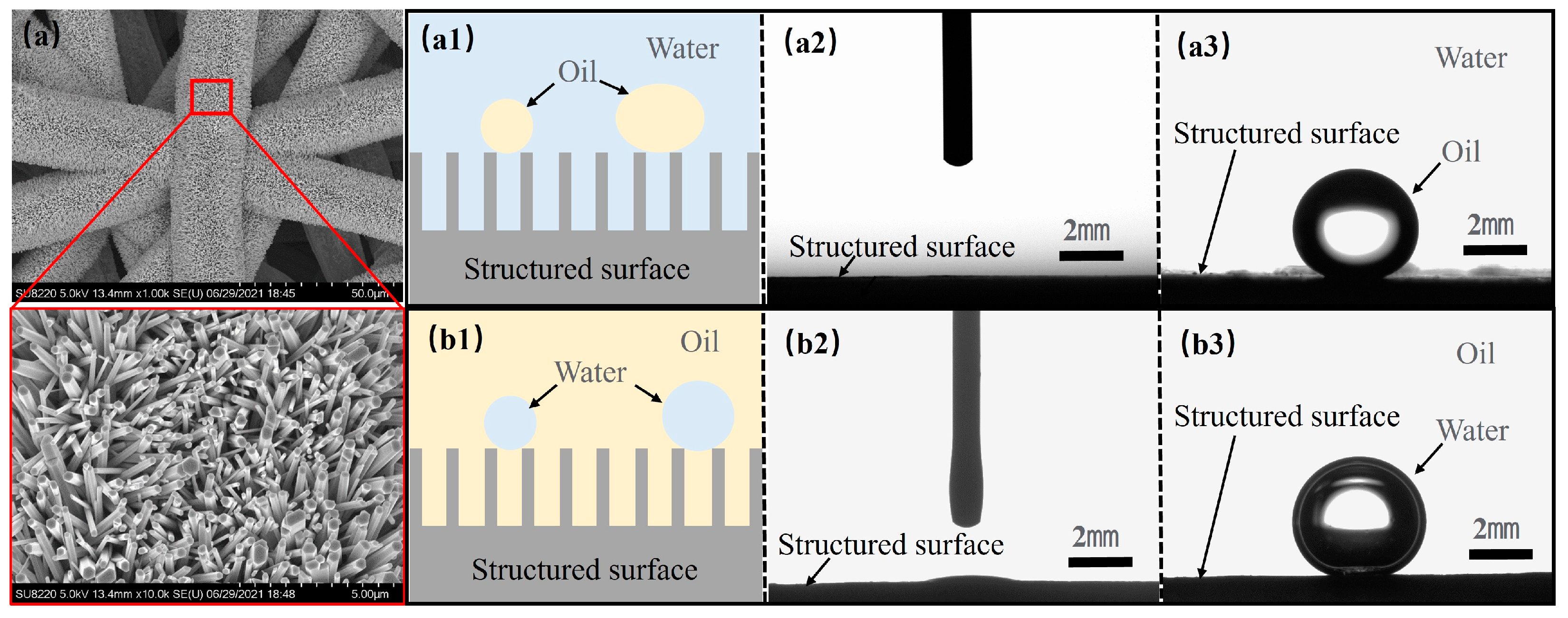
5.1. Oleophylic and Water-Repellent
5.2. Underwater Hydrophilic and Oil-Repellent
5.3. Mechanism and Failure Mode
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Wang, J.; Cui, M.; Su, R.; Huang, R. The preparation of antifouling and superhydrophobic polyurethane sponge by grafting Econea to diisocyanate monomer for durable continuous oil/water separation and cleanup of oil spills. Sep. Purif. Technol. 2023, 316, 123826. [Google Scholar] [CrossRef]
- Park, H.R.; Hwang, W.; Choi, D. Recent advances on Oil-water Separation Technology. Compos. Res. 2023, 36, 69–79. [Google Scholar]
- Chu, J.Q.; Lu, Y.; Gan, S.X.; Qi, Q.Y.; Jia, C.; Yao, J.; Zhao, X. A Superhydrophobic Trifluoromethyl-Containing Covalent Organic Framework Membrane for Efficient Oil/Water Separation. Macromol. Rapid Commun. 2023, 44, 2200641. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, F.; Guo, Z. Superwetting surfaces for filtration separation of high-viscosity raw petroleum/water mixtures. J. Mater. Chem. A 2022, 10, 14273–14292. [Google Scholar] [CrossRef]
- Gao, J.; Wang, J.; Cai, M.; Xu, Q.; Zhang, J.; Cao, X.; Zhang, J.; Chen, Y. Advanced superhydrophobic and multifunctional nanocellulose aerogels for oil/water separation: A review. Carbohydr. Polym. 2023, 300, 120242. [Google Scholar] [CrossRef]
- Sinha, S.; Mahmoud, K.A.; Das, S. Conditions for spontaneous oil–water separation with oil–water separators. RSC Adv. 2015, 5, 80184–80191. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Wang, D.; Zhou, S.; Sun, X.; Zhao, R.; Wang, G.; Yao, T.; Zhao, K.; Chen, R. A review of 3D superhydrophilic porous materials for oil/water separation. Sep. Purif. Technol. 2023, 326, 124847. [Google Scholar] [CrossRef]
- Wang, D.; Qiao, C.; Zhao, Z.; Huang, Y.; Gao, S.; Yang, D.; Liu, Q.; Zeng, H. Effect of charge density of reverse emulsion breaker on demulsification performance for steam-assisted gravity drainage (SAGD) emulsions under high temperature and high pressure. Energy Fuels 2020, 34, 13893–13902. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, X.; Li, J.; Wang, N. Bifunctional polyimide/ZIF8 composite nanofibrous membranes with controllable bilayer structure for bioprotective application and high-efficiency oil/water separation. J. Environ. Eng. 2023, 11, 110913. [Google Scholar] [CrossRef]
- Chen, M.; He, H.; Zhu, H.; Guo, W.; Chen, W.; Xue, F.; Wang, S.F. Prearation and properties of PH-responsive reversible-wettability biomass cellulose-based material for controllable oil/water separation. Carbohydr. Polym. 2019, 203, 246–255. [Google Scholar] [CrossRef]
- Li, J.; Du, F.; Zhao, Y.; Zhao, S.; Yu, H. Two–step fabrication of superhydrophobic surfaces with anti–adhesion. Opt. Laser Technol. 2019, 113, 273–280. [Google Scholar] [CrossRef]
- Li, J.; Bai, X.; Tang, X.; Zha, F.; Feng, H.; Qi, W. Underwater superoleophobic/underoil superhydrophobic corn cob coated meshes for on-demand oil/water separation. Sep. Purif. Technol. 2018, 195, 232–237. [Google Scholar] [CrossRef]
- Sun, S.; Xiao, Q.R.; Zhou, X.; Wei, Y.Y.; Shi, L.; Jiang, Y. A bio-based environment-friendly membrane with facile preparation process for oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 18–22. [Google Scholar] [CrossRef]
- Mi, T.; Cai, Y.; Wang, Q.; Habibul, N.; Ma, X.; Su, Z.; Wu, W. Synthesis of Fe3O4 nanocomposites for efficient separation of ultra-small oil droplets from hexadecane–water emulsions. RSC Adv. 2020, 10, 10309–10314. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Meng, B.W.; Hao, B.; Ma, P.C. Aggregation-induced demulsification triggered by the hydrophilic fabric for the separation of highly emulsified oil droplets from water. Aggregate 2022, 3, e131. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Chen, S.; Yu, Y.; Weng, D.; Mahmood, A.; Wang, G.; Wang, J. Preparation of underwater superoleophobic membranes via TiO2 electrostatic self-assembly for separation of stratified oil/water mixtures and emulsions. J. Membr. Sci. 2020, 602, 117976. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Wang, Y.; Xiao, H.; Huang, X.; Shi, B. Collagen fibers with tuned wetting properties for dual separation of oil-in-water and water-in-oil emulsion. J. Mater. Chem. A. 2020, 8, 24388–24392. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Wu, G.; Hen, S.-C.; Wang, Y.-Z. Highly-efficient, Rapid and continuous separation of surfactant-stabilized Oil/Water emulsions by selective under-liquid adhering emulsified droplets. J. Hazard. Mater. 2020, 400, 123132. [Google Scholar] [CrossRef]
- Babazadeh, H.; Foroutan, M. Investigation of the surfactant effects on oil-water separation on nano-crystalline titanium dioxide substrate using molecular dynamics simulation. Appl. Surf. Sci. 2019, 495, 143628. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Weng, D.; Chen, C.; Li, Z.; Wang, J. Tension gradient-driven rapid self-assembly method of large-area colloidal crystal film and its application in multifunctional structural color displays. J. Chem. Eng. J. 2022, 427, 130658. [Google Scholar] [CrossRef]
- Kuraimid, Z.K. Synthesis and evaluation of local de-emulsifiers with field assessment in Iraqi oil fields. Chem. Methodol. 2021, 5, 367–372. [Google Scholar]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T.; et al. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Sai, H.; Jin, Z.; Wang, Y.; Fu, R.; Wang, Y.; Ma, L. Facile and green route to fabricate bacterial cellulose membrane with superwettability for oil–water separation. Adv. Sustain. Syst. 2020, 4, 2000042. [Google Scholar] [CrossRef]
- Matsuno, A.; Junaid, Z.M.; Saito, T.; Dang, H.T.T.; Huyen, P.T.; Nga, T.T.V.; Kawamoto, K. Oil/water separation techniques using hydrophobized/oleophilized grains: A review of recent studies. GEOMATE J. 2021, 20, 28–34. [Google Scholar] [CrossRef]
- Ikhsan, S.N.W.; Yusof, N.; Aziz, F.; Ismail, A.F.; Jaafar, J.; Salleh, W.N.W.; Misdan, N. Superwetting materials for hydrophilic-oleophobic membrane in oily wastewater treatment. J. Environ. Manag. 2021, 290, 112565. [Google Scholar] [CrossRef]
- Yu, J.; Cao, C.; Pan, Y. Advances of adsorption and filtration techniques in separating highly viscous crude oil/water mixtures. Adv. Mater. Interfaces 2021, 8, 2100061. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, J.; Li, S.; Ge, M.; Teng, L.; Hen, Z.; Lai, Y. Advanced materials with special wettability toward intelligent oily wastewater remediation. ACS Appl. Mater. Interfaces 2020, 13, 67–87. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Zhang, X.; Tang, Y.; Zhou, X.; Zhang, K.; Chen, Z.; Lai, Y. Rational design of materials interface at nanoscale towards intelligent oil–water separation. Nanoscale Horiz. 2018, 3, 235–260. [Google Scholar] [CrossRef]
- El-Tabei, A.; El-Tabey, A.; El-Sharaky, E. Novel synthesized polymeric surfactants additives based on phenethylamine as an emulsion breaker for water droplet coalescence in naturally Egyptian crude oil emulsion. J. Mol. Liq. 2021, 338, 116779. [Google Scholar] [CrossRef]
- Ortiz, S.N.C.; Cabanzo, R.; Mejia-Ospino, E. Crude oil/water emulsion separation using graphene oxide and amine-modified graphene oxide particles. Fuel 2019, 240, 162–168. [Google Scholar] [CrossRef]
- Chang, H.; Zhang, Y.; Dandekar, A.; Ning, S.; Barnes, J.; Edwards, R.; Schulpen, W.; Cercone, D.P.; Ciferno, J. Experimental investigation on separation behavior of heavy-oil emulsion for polymer flooding on Alaska North Slope. J. SPE Prod. Oper. 2020, 35, 579–591. [Google Scholar]
- Victor-Oji, C.O.; Chukwu, U.J.; Akaranta, O. Evaluation of triethanolamine-cashew nutshell liquid derivatives as crude oil emulsion breakers. Appl. Petrochem. Res. 2021, 11, 209–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, X. Smart and durable pH-responsive superhydrophobic fabrics with switchable surface wettability for high-efficiency and complex oil/water separation. Giant 2023, 14, 100157. [Google Scholar] [CrossRef]
- Zhiheng, W.; Yimin, D.; Chengqian, F.; Ling, C.; Qi, L.; Yaqi, L.; Ling, C.; Bo, L.; Yue-Fei, Z.; Yan, L.; et al. A bio-inspired green method to fabricate pH-responsive sponge with switchable surface wettability for multitasking and effective oil-water separation. Appl. Surf. Sci. 2022, 602, 154192. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.S.; Tian, D.H.; Wu, H.; Lv, X.; Jiang, G.; Huang, Y.X. Tuning the membrane rejection behavior by surface wettability engineering for an effective water-in-oil emulsion separation. Chin. Chem. Lett. 2023, 17, 109090. [Google Scholar] [CrossRef]
- Si, Y.; Yu, C.; Dong, Z.; Jiang, L. Wetting and spreading: Fundamental theories to cutting-edge applications. Curr. Opin. Colloid Interface Sci. 2018, 36, 10–19. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.R.; Kim, H.J.; Lee, D.; Ha, S.; Lee, K.; Hwang, W. Self-cleaning mechanisms according to the wettability of the surface and deposition material. Appl. Surf. Sci. 2023, 626, 157197. [Google Scholar] [CrossRef]
- Lv, W.; Mei, Q.; Xiao, J.; Du, M.; Zheng, Q. 3D Multiscale Superhydrophilic Sponges with Delicately Designed Pore Size for Ultrafast Oil/Water Separation. Adv. Funct. Mater. 2017, 27, 1704293. [Google Scholar] [CrossRef]
- Wang, H.; Gao, F.; Ren, R.; Wang, Z.; Yue, R.; Wei, J.; Wang, X.; Kong, Z.; Zhang, H.; Zhang, X. Caffeic acid polymer rapidly modified sponge with excellent anti-oil-adhesion property and efficient separation of oil-in-water emulsions. J. Hazard. Mater. 2021, 404, 124197. [Google Scholar] [CrossRef]
- Su, C.; Yang, H.; Song, S.; Lu, B.; Chen, R. A magnetic superhydrophilic/oleophobic sponge for continuous oil-water separation. Chem. Eng. J. 2017, 309, 366–373. [Google Scholar] [CrossRef]
- Wu, L.; Luo, J.; Li, Y.; Zhang, W.; Wang, L.; Huang, X.; Xiao, W.; Tang, L.; Gao, J. Emulsion dipping based superhydrophobic, temperature tolerant, and multifunctional coatings for smart strain sensing applications. Compos. Sci. Technol. 2021, 216, 109045. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Biserova-Tahchieva, A.; Sansa-L’opez, V.; Llorca-Isern, N. Superhydrophobic PDMS coated 304 stainless-steel mesh for the removal of HDPE microplastics. Prog. Org. 2022, 170, 107009. [Google Scholar] [CrossRef]
- Luo, W.; Zuo, Y.; Zheng, Y.; Long, X.; Jiao, F. Double network cross-linked hydrogel coating membrane with photocatalytic self-cleaning performance for efficient oil-water separation. Prog. Org. 2023, 185, 107882. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Chen, M.; Xu, Z.; Li, L.; Zhou, Y. Metal mesh-based special wettability materials for oil-water separation: A review of the recent development. J. Pet. Sci. Eng. 2021, 205, 108889. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Gao, Y.; Yun, J. Fabrication of superhydrophobic copper meshes via simply soaking for oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128648. [Google Scholar] [CrossRef]
- Ji, S.; Li, W.; Lv, N.; Chen, X. P(MA-AMPS)/PVA@SiO2 hybrid hydrogel coated stainless steel mesh for effective separation of stabilized oil-in-water emulsions under gravity. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132283. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, Y.; Xie, Y.; Zhou, M.; Gu, Q.; Zhong, Z.; Xing, W. Silicon carbide microfiltration membranes for oil-water separation: Pore structure-dependent wettability matters. Water Res. 2022, 216, 118270. [Google Scholar] [CrossRef]
- Liu, G.; Cai, Y.; Yuan, H.; Zhang, J.; Zhang, Z.; Zhao, D. Mesh membranes coated with zirconium metal-organic framework nanosheets of optimized morphology for oil-water separation. J. Membr. Sci. 2023, 668, 121077. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Zhou, W.T.; Li, S.Y.; Chen, Z.B.; Stegmaier, T.; Aliabadi, M.; Han, Z.W.; Ren, L.Q. Multifunctional 3D GO/g-C3N4/TiO2 foam for oil-water separation and dye adsorption. Appl. Surf. Sci. 2021, 541, 148638. [Google Scholar] [CrossRef]
- Shen, S.; Li, H.; Shen, Y.; Bai, R.; Zhang, G. Modification of PVDF membrane by post-modified NH2-MIL-88B(Fe) showing improved permeability and oil/water separation performance. J. Environ. Eng. 2023, 11, 109621. [Google Scholar] [CrossRef]
- Xu, J.; Cui, J.; Sun, H.; Wu, Y.; Xue, C.; Xie, A.; Li, C. Facile preparation of hydrophilic PVDF membrane via tea polyphenols modification for efficient oil-water emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130639. [Google Scholar] [CrossRef]
- Gao, D.; Xin, B.; Newton, M.A.A. Preparation and characterization of electrospun PVDF/PVP/SiO2 nanofiber membrane for oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 13215. [Google Scholar] [CrossRef]
- Cao, C.; Wang, F.; Lu, M.; Zhou, Y. One-step preparation of functionalized superhydrophobic cotton fabric with self-cleaning and oil/water separation ability. Mater. Today Commun. 2023, 36, 106851. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Tao, F.; An, Y.; Zhong, Y.; Liu, Z.; Hu, Z.; Zhang, X.; Wang, X. An overview of biomass-based Oil/Water separation materials. Sep. Purif. Technol. 2023, 316, 123767. [Google Scholar] [CrossRef]
- Zhang, M.; Ning, H.; Shang, J.; Liu, F.; Peng, S. A robust superhydrophobic-superoleophilic PDMS/Al2O3/CM composite ceramic membrane: Stability, efficient emulsified oil/water separation, and anti-pollution performance. Sep. Purif. Technol. 2024, 328, 124864. [Google Scholar] [CrossRef]
- Luan, W.; Nie, C.; Chen, X.; Tang, Z.; Qiu, M.; Fan, Y. Effective construction of anti-fouling zwitterion-functionalized ceramic membranes for separation of oil-in-water emulsion based on PDA/PEI co-deposition. J. Environ. Eng. 2022, 10, 108396. [Google Scholar]
- Sutar, R.S.; Mane, M.S.; Latthe, S.S.; Pawar, P.; Kumbhar, S.S.; Nerle, U.V.; Mote, U.; Bhosale, J.; Kokare, B.; Sadasivuni, K.K.; et al. Oil–Water Separation by ZnO-Based Superhydrophobic PU Sponges. Macromol. Symp. 2020, 393, 2000036. [Google Scholar] [CrossRef]
- Ko, T.-J.; Hwang, J.-H.; Davis, D.; Shawkat, M.S.; Han, S.S.; Rodriguez, K.L.; Oh, K.H.; Lee, W.H.; Jung, Y. Superhydrophobic MoS2-based multifunctional sponge for recovery and detection of spilled oil. Curr. Appl. Phys. 2020, 20, 344–351. [Google Scholar] [CrossRef]
- Habibi, N.; Pourjavadi, A. Thermally conductive and superhydrophobic polyurethane sponge for solar-assisted separation of high-viscosity crude oil from water. ACS Appl. Mater. Interfaces 2022, 14, 7329–7339. [Google Scholar] [CrossRef]
- Tang, L.; Wang, G.; Zeng, Z.; Shen, L.; Zhu, L.; Zhang, Y.; Xue, Q. Three-dimensional adsorbent with pH induced superhydrophobic and superhydrophilic transformation for oil recycle and adsorbent regeneration. J. Colloid Interface Sci. 2020, 575, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, B.; Altemose, P.; Kowall, C.; Li, L. 3D-Printed Membranes with a Zwitterionic Hydrogeloating for More Robust Oil–Water Separation. Ind. Eng. Chem. Res. 2020, 59, 21058–21065. [Google Scholar] [CrossRef]
- Lv, P.; Yang, S.; Ma, P.-C. Bio-based oil gelling agent for effective removal of oil spills from the surface of water. Mater. Chem. Front. 2018, 2, 1784–1790. [Google Scholar] [CrossRef]
- Wang, B.; Feng, S.; Li, Z.; Chen, L.; Wang, C. The Use of Wasteoncrete as a Filter for Separating Water-in-oil Emulsions. In Proceedings of the 5th International Conference Onivil Engineering and Architecture, Hanoi, Vietnam, 16–18 December 2022; pp. 95–106. [Google Scholar]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. A novel hierarchical hollow SiO2@ MnO2 cubes reinforced elastic polyurethane foam for the highly efficient removal of oil from water. Chem. Eng. J. 2017, 327, 539–547. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Weng, D.; Li, X.; Li, Z.; Wang, J. Simulation study on the dynamic behaviors of water-in-oil emulsified droplets on coalescing fibers. Langmuir 2020, 36, 14872–14880. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Zhao, T.; Xu, Y.; Zhang, X.; Zeng, Y. The zein-based fiber membrane with switchable superwettability for on-demand oil/water separation. Sep. Purif. Technol. 2021, 263, 118393. [Google Scholar] [CrossRef]
- Huang, F.; Li, Q.; Ji, G.; Tu, J.; Ding, N.; Qu, Q.; Liu, G. Oil/water separation using a lauric acid-modified, superhydrophobic cellulose composite membrane. Mater. Chem. Phys. 2021, 266, 124493. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Guo, Z. Designing novel superwetting surfaces for high-efficiency oil–water separation: Design principles, opportunities, trends and challenges. J. Mater. Chem. A 2020, 8, 16831–16853. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Gao, Q.; Zhao, J.; Wang, Y.; Liu, C.; Shen, C.; Liu, X. Facile fabrication of durable superhydrophobic mesh via candle soot for oil-water separation. Prog. Org. Oat. 2019, 136, 105253. [Google Scholar] [CrossRef]
- Zeng, Q.; Ma, P.; Su, X.; Lai, D.; Lai, X.; Zeng, X.; Li, H. Facile fabrication of superhydrophobic and magnetic poly (lactic acid) nonwoven fabric for oil–water separation. Ind. Eng. Chem. Res. 2020, 59, 9127–9135. [Google Scholar] [CrossRef]
- Yao, X.; Hou, X.; Qi, G.; Zhang, R. Preparation of superhydrophobic polyimide fibrous membranes with controllable surface structure for high efficient oil-water emulsion and heavy oil separation. J. Environ. Chem. Eng. 2022, 10, 107470. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, X.; Zhang, L.; Hen, B.; Ding, T.; Ni, B.; Gao, G. Swelling poly (ionic liquid) supported by three-dimensional wire mesh for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 14347–14353. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.-Y.; Guan, J.; Zhang, M.; Yuan, P.-C.; Liu, L.-N.; Afzal, M.Z.; Wang, S.-G.; Sun, X.-F. Photoinduced superwetting membranes for separation of oil-in-water emulsions. Sep. Purif. Technol. 2020, 241, 116536. [Google Scholar] [CrossRef]
- Yin, Y.; Zhu, L.; Chang, X.; Xue, J.; Yu, S.; Li, X.; Xue, Q. Bioinspired Anti-Oil-Fouling Hierarchical Structured Membranes Decorated with Urchin-Like α-FeOOH Particles for Efficient Oil/Water Mixture andrude Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2020, 12, 50962–50970. [Google Scholar] [CrossRef]
- Hu, L.; Gao, S.; Ding, X.; Wang, D.; Jiang, J.; Jin, J.; Jiang, L. Photothermal-responsive single-walled carbon nanotube-based ultrathin membranes for on/off switchable separation of oil-in-water nanoemulsions. ACS Nano 2015, 9, 4835–4842. [Google Scholar] [CrossRef]
- Ge, J.; Jin, Q.; Zong, D.; Yu, J.; Ding, B. Biomimetic multilayer nanofibrous membranes with elaborated superwettability for effective purification of emulsified oily wastewater. ACS Appl. Mater. Interfaces 2018, 10, 16183–16192. [Google Scholar] [CrossRef]
- Zisman, W.A. Relation of the equilibrium contact angle to liquid and solid constitution. Adv. Chem. 1964, 43, 1–51. [Google Scholar]
- Liu, X.; Feng, S.; Wang, C.; Yan, D.; Chen, L.; Wang, B. Wettability Improvement in Oil-Water Separation by Nano-Pillar ZnO Texturing. Nanomaterials 2022, 12, 740. [Google Scholar] [CrossRef]
- Weng, C.; Chen, J.; Yang, J.; Zhou, M.; Jiang, B. Experimental Investigation and Molecular Dynamics Simulation on the Anti-Adhesion Behavior of Alkanethiols on Nickel Insert in Micro Injection Molding. Nanomaterials 2021, 11, 1834. [Google Scholar] [CrossRef]
- Bin, Z.; Yan, L.; Li, S.Y.; Kaya, C.; Stegmaier, T.; Aliabadi, M.; Han, Z.; Ren, L. Fabrication of superwetting Cu@Cu2O cubic film for oil/water emulsion separation and photocatalytic degradation. Appl. Surf. Sci. 2019, 496, 143580. [Google Scholar]
- Danikas, M.G.; Gubanski, S.M.; Wankowicz, J. Loss and recovery of hydrophobicity on RTV coating surfaces. IEEE Trans. Dielectr. Electr. Insul. 2002, 2, 506–507. [Google Scholar] [CrossRef]
- Hiroki, M.; Tsuguyori, O. Evaluation of anti-adhesion characteristics of diamond-like carbon film using high-frequency, linear-oscillation tribometer. Wear 2017, 386, 188–194. [Google Scholar]
- Ruoxi, W.; Xueting, Z.; Ning, J.; Lijuan, H.; Lifen, L.; Ongjie, G. Superwetting Oil/Water Separation Membraneonstructed from In Situ Assembled Metal−Phenolic Networks and Metal−Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 10000–10008. [Google Scholar]
- Baiping, L.; Ning, L. Versatile aluminum alloy surface with various wettability. Appl. Surf. Sci. 2015, 326, 168–173. [Google Scholar]
- Taolei, S.; Lin, F.; Xuefeng, G.; Lei, J. Bioinspired Surfaces with Special Wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar]
- Caha, I.; Alves, A.C.; Chirico, C.; Pinto, A.M.; Tsipas, S.; Gordo, E.; Toptan, F. A promising method to develop TiO2-based nanotubular surfaces on Ti-40Nb alloy with enhanced adhesion and improved tribocorrosion resistance. Appl. Surf. Sci. 2021, 542, 148658. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Kundwa, M.J.; Li, Z.; Wei, D. Evaluation of the adhesion characteristics of material composition for polyphosphoric acid and SBS modified bitumen based on surface free energy theory. Constr. Build. Mater. 2021, 266, 121022. [Google Scholar] [CrossRef]
- Syed, S.; Alhazzaa, M.I.; Asif, M. Treatment of oily water using hydrophobic nano-silica. Chem. Eng. J. 2011, 167, 99–103. [Google Scholar] [CrossRef]
- Pan, S.; Guo, R.; Björnmalm, M.; Richardson, J.J.; Li, L.; Peng, C.; Bertleff-Zieschang, N.; Xu, W.; Jiang, J.; Caruso, F. Coatings super-repellent to ultralow surface tension liquids. Nat. Mater. 2018, 17, 1040–1047. [Google Scholar] [CrossRef]
- Men, X.; Shi, X.; Ge, B.; Li, Y.; Zhu, X.; Li, Y.; Zhang, Z. Novel transparent, liquid-repellent smooth surfaces with mechanical durability. Chem. Eng. J. 2016, 296, 458–465. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef]
- Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J.; et al. Preventing mussel adhesion using lubricant-infused materials. Science 2017, 357, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Urata, C.; Sato, T.; England, M.W.; Hozumi, A. Self-Healing Superhydrophobic Materials Showing Quick Damage Recovery and Long-Term Durability. Langmuir 2017, 33, 9972–9978. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gancheva, T.; Favis, B.D.; Abidli, A.; Wang, J.; Park, B. Hydrophobic porous polypropylene with hierarchical structures for ultrafast and highly selective oil/water separation. ACS Appl. Mater. Interfaces 2021, 13, 16859–16868. [Google Scholar] [CrossRef]
- Schmidt, D.L.; Brady, R.F.; Lam, K.; Schmidt, D.C.; Chaudhury, M.K. Contact angle hysteresis, adhesion, and marine biofouling. Langmuir 2004, 20, 2830–2836. [Google Scholar] [CrossRef]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef]
- Wang, L.; McCarthy, T.J. Ovalently Attached Liquids: Instant Omniphobic Surfaces with Unprecedented Repellency. Angew. Chem. Int. Ed. Engl. 2016, 55, 244–248. [Google Scholar] [CrossRef]
- Hongyu, L.; Yongfeng, B.; Yanhu, Z.; Xiaojing, X.; Junyan, Z. Conversion of organic films into fluorine-containing onion carbon as anti-adhesion solid lubricants. Mater. Lett. 2018, 233, 310–313. [Google Scholar]
- Wang, S.; Jiang, L. Definition of Superhydrophobic States. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Huang, J.; Chen, Z.; Chen, G.; Lai, Y. Bioinspired Surfaces with Superamphiphobic Properties:oncepts, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1707415. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Xu, X.; Men, X.; Zhu, X.; Zhou, X. Superoleophobic textured aluminum surfaces. New J. Chem. 2011, 35, 2422–2426. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Jia, Z.; Xie, H.; Guan, Z. A study on the loss and recovery of hydrophobicity of RTV coating. Int. Symp. Electr. Insul. Mater. IEEE 2002, 30, 623–626. [Google Scholar]
- Li, B.; Qi, B.; Guo, Z.; Wang, D.; Jiao, T. Recent developments in the application of membrane separation technology and its challenges in oil-water separation: A review. Hemosphere 2023, 327, 138528. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, Y.; Wang, J.; He, B.; Yang, S.; Pan, K.; Liu, F. Carbonaceous microsphere/nanofiber composite superhydrophilic membrane with enhanced anti-adhesion property towards oil and anionic surfactant: Membrane fabrication and applications. Sep. Purif. Technol. 2020, 235, 116189. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Kim, H.J.; Jung, Y.J.; Son, S.H.; Choi, W.S. Compressible Separator and Catalyst for Simultaneous Separation and Purification of Emulsions and Aqueous Pollutants. ACS Omega 2023, 8, 40741–40753. [Google Scholar] [CrossRef]
- Raturi, P.; Yadav, K.; Singh, J.P. ZnO-Nanowires-Coated Smart Surface Mesh with Reversible Wettability for Efficient On-Demand Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 1, 6007–6013. [Google Scholar] [CrossRef]


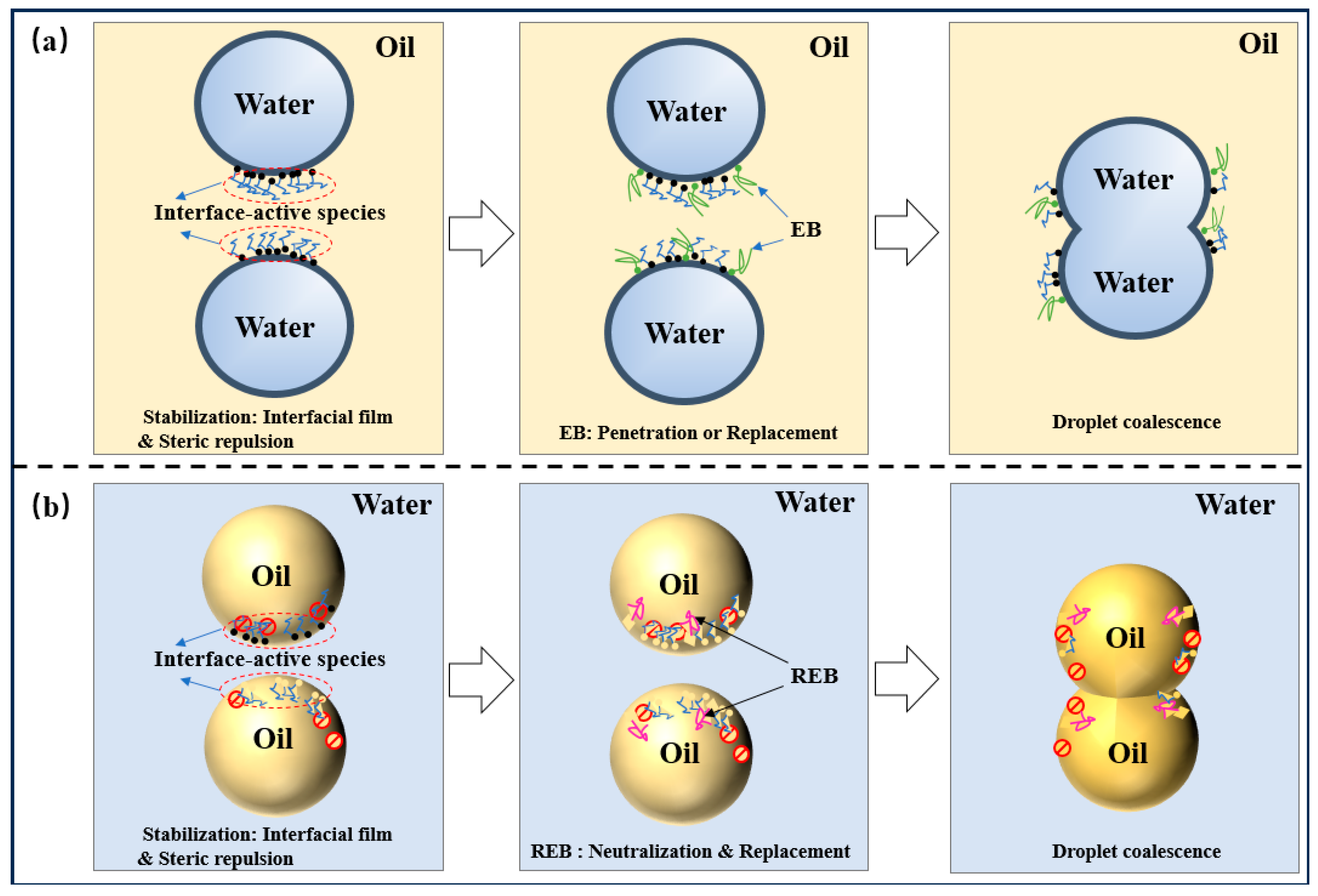
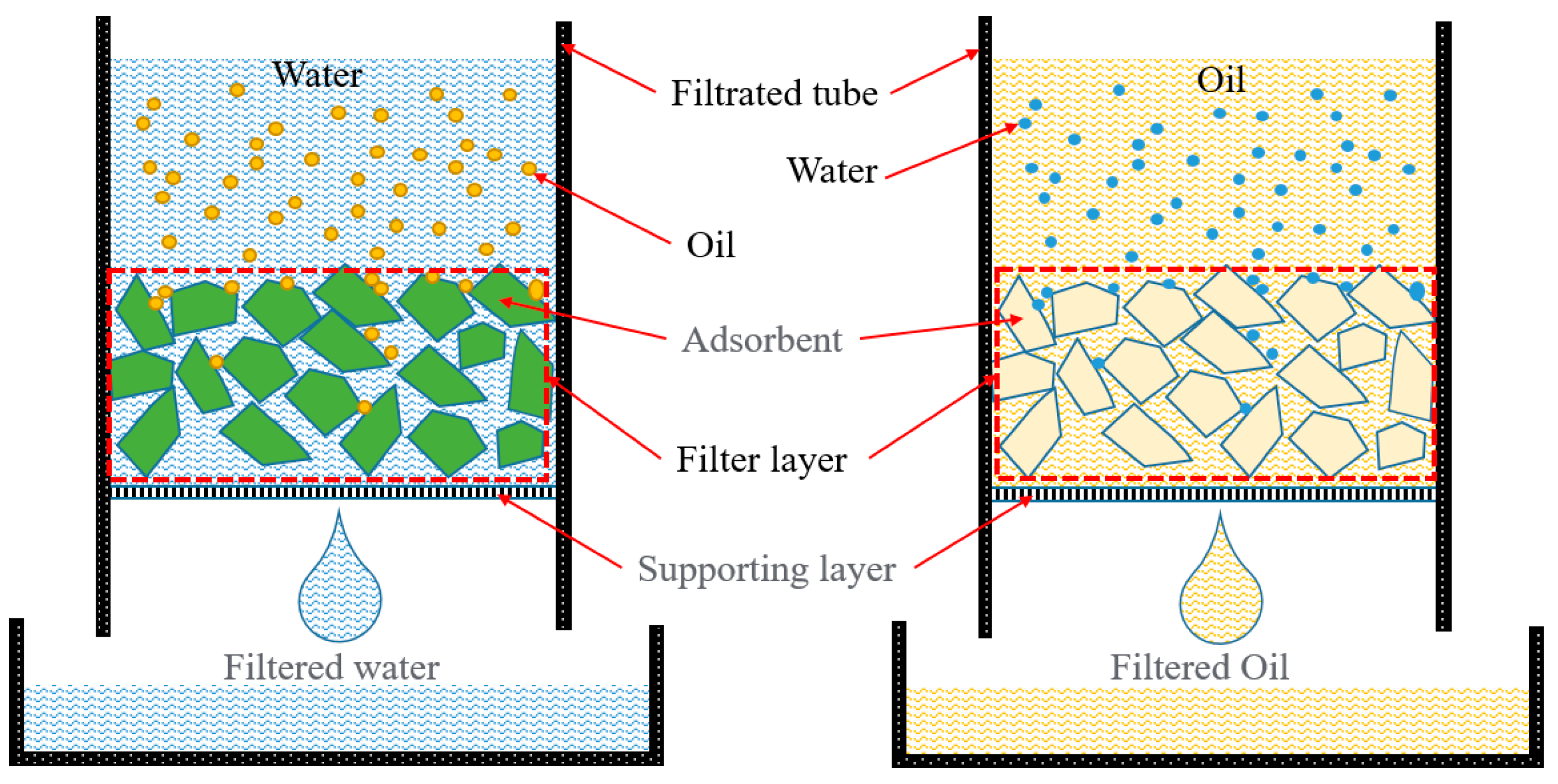
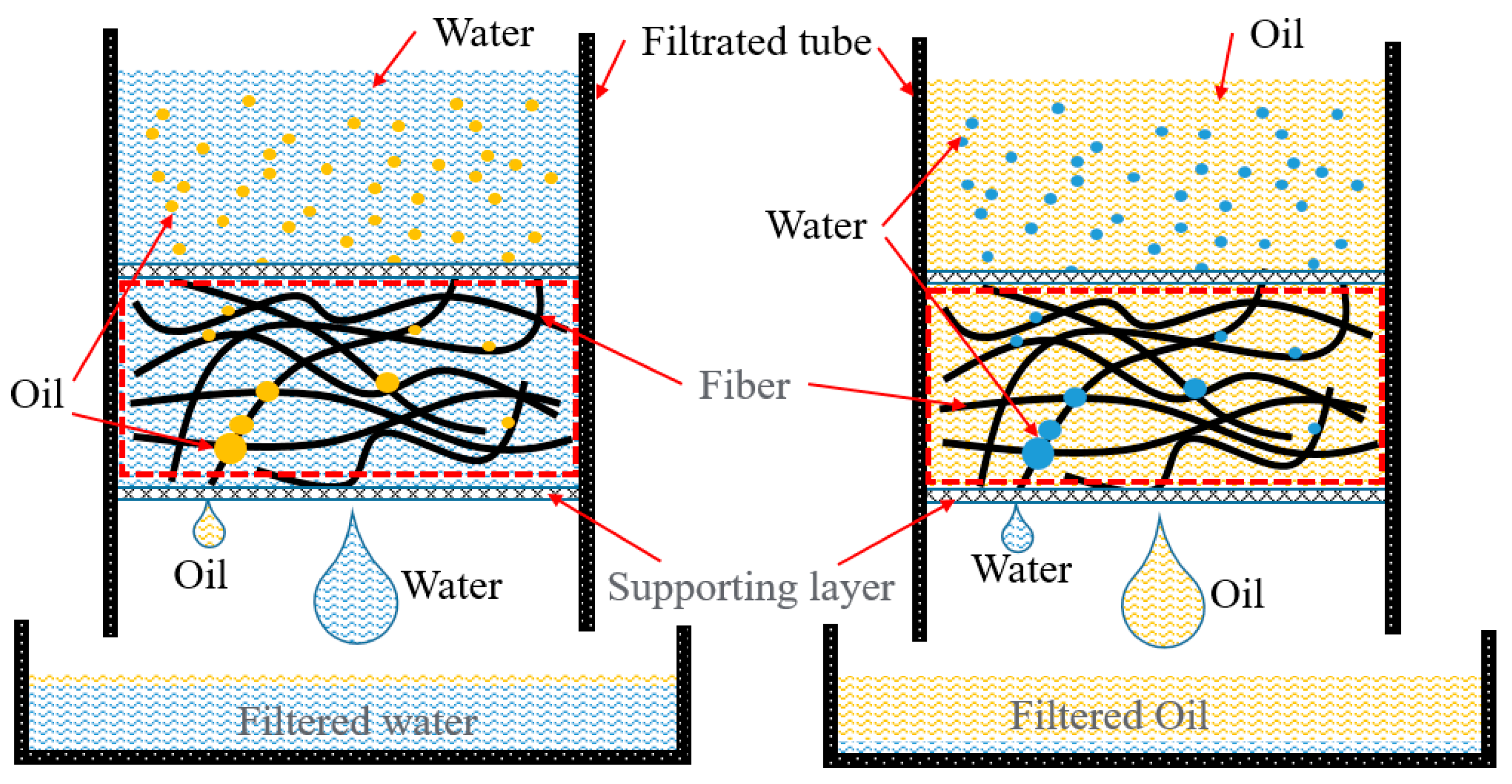
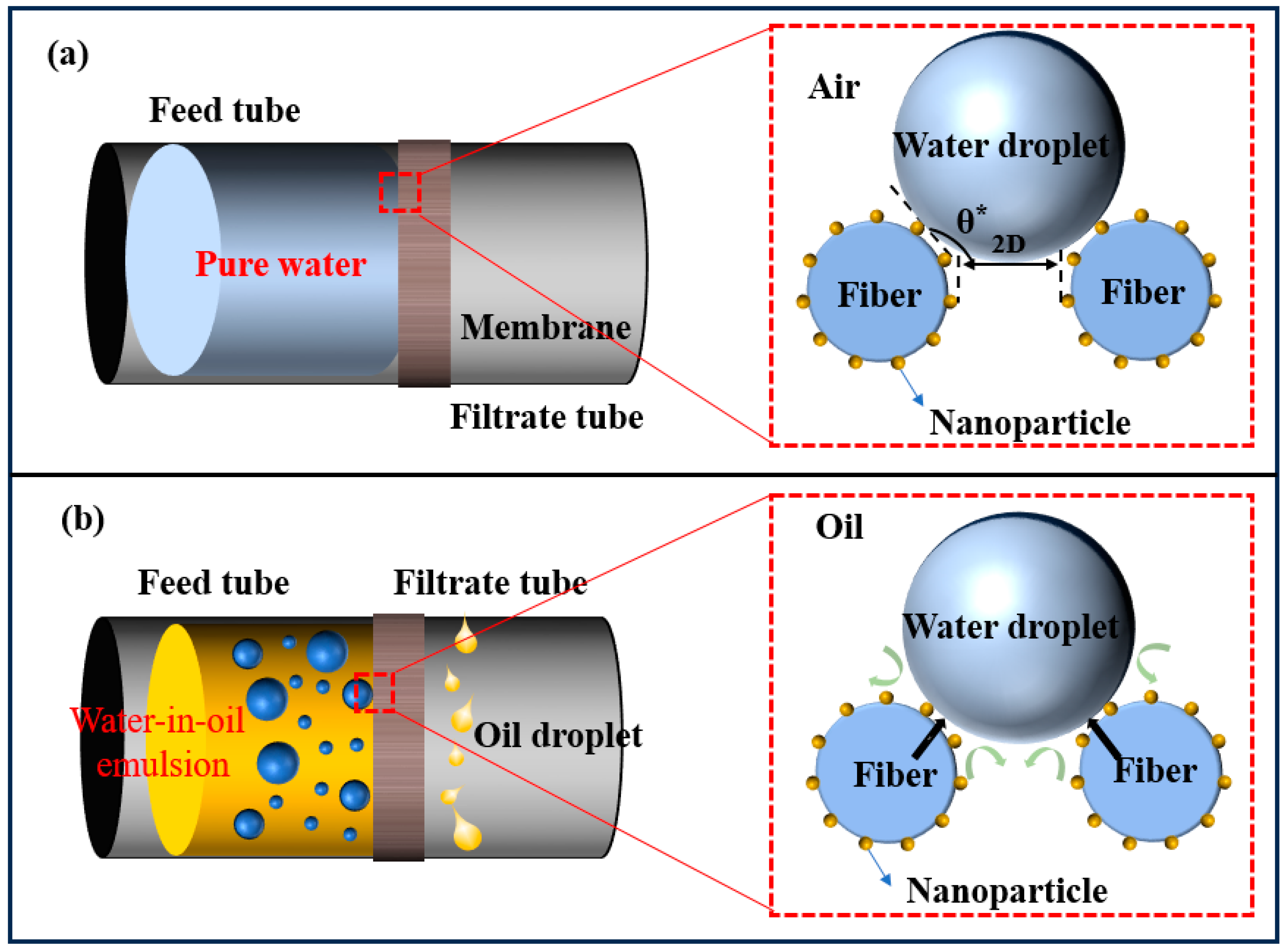

| Critical Material | Methods | Type of Solution for Oil–Water Separation | References |
|---|---|---|---|
| LDH–SiO2 | Electrospun and Co-deposited | Emulsified | 3D Multiscale Superhydrophilic Sponges with Delicately Designed Pore Size for Ultrafast Oil/Water Separation. Adv. Funct. Mater. 2017, 1704293 [40] |
| CA Polymer | Deposition | Emulsified | Caffeic acid polymer rapidly modified sponge with excellent anti-oil-adhesion property and efficient separation of oil-in-water emulsions. J. Hazard. Mater. 2021, 404, 124197 [41] |
| Fe3O4/CS-PFNA | One-step Dip-coating | Oil–water mixtures | A magnetic superhydrophilic–oleophobic sponge for continuous oil–water separation. Chem. Eng. J. 2017, 309, 366–373 [42] |
| PDMS | Coating | Oil–water mixtures | Emulsion dipping-based superhydrophobic, temperature-tolerant, and multifunctional coatings for smart strain sensing applications. Compos. Sci. Technol. 2021, 216, 109045 [43] |
| PDMS | Coating | Oil–water mixtures | Llorca-Isern. Superhydrophobic PDMS coated 304 stainless-steel mesh for the removal of HDPE microplastics. Prog. Org. Coat. 2022, 170, 107009 [44] |
| Gelatin—Tannic Acid—g-C3N4 (GE-CNTA) | Coating | Emulsified | Double network cross-linked hydrogel coating membrane with photocatalytic self-cleaning performance for efficient oil-water separation. Prog. Org. Coat. 2023, 185, 107882 [37] |
| Polyvinyl Alcohol | Coating | Emulsified | Aggregation-induced demulsification triggered by the hydrophilic fabric for the separation of highly emulsified oil droplets from water. Aggregate 2022, 3, e131 [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Feng, S.; Wang, C.; Liu, X.; Chen, L.; Yan, D. Nanostructure-Based Oil–Water Separation: Mechanism and Status. Separations 2023, 10, 569. https://doi.org/10.3390/separations10110569
Wang B, Feng S, Wang C, Liu X, Chen L, Yan D. Nanostructure-Based Oil–Water Separation: Mechanism and Status. Separations. 2023; 10(11):569. https://doi.org/10.3390/separations10110569
Chicago/Turabian StyleWang, Bao, Shaotong Feng, Caihua Wang, Xiaoyan Liu, Lei Chen, and Dayun Yan. 2023. "Nanostructure-Based Oil–Water Separation: Mechanism and Status" Separations 10, no. 11: 569. https://doi.org/10.3390/separations10110569






