Experimental Design and Multiple Response Optimization for the Extraction and Quantitation of Thirty-Four Priority Organic Micropollutants in Tomatoes through the QuEChERS Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standard Solutions
2.2. Instrumentation and Softwares
2.3. Tomato Samples and Pre-Treatment
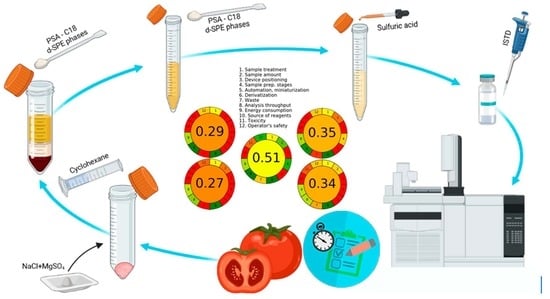
2.4. Analytical Protocol
2.4.1. Optimization of QuEChERS Extraction Parameters
2.4.2. Analysis of PAHs, Nitro-PAHs and PCBs and Recovery Evaluation
2.4.3. Protocol Validation
2.4.4. Optimized Protocol
3. Results and Discussion
3.1. Optimization of Extraction Protocol
3.1.1. Choice of Extraction Solvent
3.1.2. Optimization of Purification Conditions of Extract
Experimental Design
Multiple Response Optimization
3.2. Validation of the Analytical Protocol
3.2.1. Linearity
3.2.2. Method Detection and Quantitation Limits
3.2.3. Method Precision
3.2.4. Matrix Effect
3.3. Greennes Position of the Developed Method in the State of the Art
3.4. Real Sample Contamination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. The Global Goals, Goal 2: “Zero Hunger”. Available online: https://www.globalgoals.org/goals/2-zero-hunger/ (accessed on 20 January 2023).
- Food and Agriculture Organization of the United Nations. The State of Food Security and Nutrition in the World 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Droby, S. Microbial Food Contamination; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Darwish, A.; Ricci, M.; Zidane, F.; Vasquez, J.A.T.; Casu, M.R.; Lanteri, J.; Migliaccio, C.; Vipiana, F. Physical Contamination Detection in Food Industry Using Microwave and Machine Learning. Electronics 2022, 11, 3115. [Google Scholar] [CrossRef]
- Garvey, M. Food pollution: A comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutrire 2019, 44, 1. [Google Scholar] [CrossRef]
- European Food Safety Authority. Chemical Contaminants in Food and Feed. Available online: https://www.efsa.europa.eu/en/topics/topic/chemical-contaminants-food-feed (accessed on 18 January 2023).
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Yaacob, T.Z.; Jaafar, H.S.; Rahman, F.A. An overview of halal food product contamination risks during transportation. Sci. Int. 2016, 28, 3183–3190. [Google Scholar]
- Nerin, C.; Aznar, M.; Carrizo, D. Food contamination during food process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental chemical contaminants in food: Review of a global problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916. [Google Scholar]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Aubry, C.; Manouchehri, N. Urban agriculture and health: Assessing risks and overseeing practices. Field Actions Sci. Reports. J. Field Actions 2019, 20, 108–111. [Google Scholar]
- García, M.G.; Fernández-López, C.; Polesel, F.; Trapp, S. Predicting the uptake of emerging organic contaminants in vegetables irrigated with treated wastewater–implications for food safety assessment. Environ. Res. 2019, 172, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rivoira, L.; Castiglioni, M.; Kettab, A.; Ouazzani, N.; Al-Karablieh, E.; Boujelben, N.; Fibbi, D.; Coppini, E.; Giordani, E.; Del Bubba, M. Impact of effluents from wastewater treatments reused for irrigation: Strawberry as case study. Environ. Eng. Manag. J. (EEMJ) 2019, 18, 2133–2143. [Google Scholar]
- Bruzzoniti, M.C.; Rivoira, L.; Castiglioni, M.; El Ghadraoui, A.; Ahmali, A.; El Mansour, T.E.H.; Mandi, L.; Ouazzani, N.; Del Bubba, M. Extraction of polycyclic aromatic hydrocarbons and polychlorinated biphenyls from urban and olive mill wastewaters intended for reuse in agricultural irrigation. J. AOAC Int. 2020, 103, 382–391. [Google Scholar] [CrossRef]
- Rivoira, L.; Castiglioni, M.; Nurra, N.; Battuello, M.; Sartor, R.M.; Favaro, L.; Bruzzoniti, M.C. Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Seawater, Sediment and Biota of Neritic Ecosystems: Occurrence and Partition Study in Southern Ligurian Sea. Appl. Sci. 2022, 12, 2564. [Google Scholar] [CrossRef]
- Xue, W.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Letz, G. The toxicology of PCB’s—An overview for clinicians. West. J. Med. 1983, 138, 534. [Google Scholar]
- Lee, Y.-Y.; Hsieh, Y.-K.; Huang, B.-W.; Mutuku, J.K.; Chang-Chien, G.-P.; Huang, S. An Overview: PAH and Nitro-PAH Emission from the Stationary Sources and their Transformations in the Atmosphere. Aerosol Air Qual. Res. 2022, 22, 220164. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581, 237–257. [Google Scholar] [CrossRef]
- Meudec, A.; Dussauze, J.; Deslandes, E.; Poupart, N. Evidence for bioaccumulation of PAHs within internal shoot tissues by a halophytic plant artificially exposed to petroleum-polluted sediments. Chemosphere 2006, 65, 474–481. [Google Scholar] [CrossRef]
- Castiglioni, M.; Onida, B.; Rivoira, L.; Del Bubba, M.; Ronchetti, S.; Bruzzoniti, M.C. Amino groups modified SBA-15 for dispersive-solid phase extraction in the analysis of micropollutants by QuEchERS approach. J. Chromatogr. A 2021, 1645, 462107. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; Rivoira, L.; Castiglioni, M.; Cagno, E.; Kettab, A.; Fibbi, D.; Del Bubba, M. Optimization and Validation of a Method Based on QuEChERS Extraction and Gas Chromatographic-Mass Spectrometric Analysis for the Determination of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Olive Fruits Irrigated with Treated Wastewaters. Separations 2022, 9, 82. [Google Scholar]
- Manios, Y.; Detopoulou, V.; Visioli, F.; Galli, C. Mediterranean diet as a nutrition education and dietary guide: Misconceptions and the neglected role of locally consumed foods and wild green plants. Local Mediterr. Food Plants Nutraceuticals 2006, 59, 154–170. [Google Scholar]
- Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Analytical determination of antioxidants in tomato: Typical components of the Mediterranean diet. J. Sep. Sci. 2007, 30, 452–461. [Google Scholar] [CrossRef]
- Čechura, L.; Žáková Kroupová, Z.; Samoggia, A. Drivers of Productivity Change in the Italian Tomato Food Value Chain. Agriculture 2021, 11, 996. [Google Scholar] [CrossRef]
- Elgueta, S.; Valenzuela, M.; Fuentes, M.; Meza, P.; Manzur, J.P.; Liu, S.; Zhao, G.; Correa, A. Pesticide residues and health risk assessment in tomatoes and lettuces from farms of metropolitan region Chile. Molecules 2020, 25, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehel, J.; Vöröskői, P.; Palkovics, A.; Szabó, C.; Darnay, L.; Budai, P.; Laczay, P.; Lányi, K. Farm to table: Residues of different pesticides in tomato and tomato juice–Food safety aspects. Acta Vet. Hung. 2022, 70, 236–244. [Google Scholar] [CrossRef]
- Paris, A.; Ledauphin, J.; Poinot, P.; Gaillard, J.-L. Polycyclic aromatic hydrocarbons in fruits and vegetables: Origin, analysis, and occurrence. Environ. Pollut. 2018, 234, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Al Nasir, F.; Batarseh, M.I. Agricultural reuse of reclaimed water and uptake of organic compounds: Pilot study at Mutah University wastewater treatment plant, Jordan. Chemosphere 2008, 72, 1203–1214. [Google Scholar] [CrossRef]
- Araromi, A.; Ayodele, O.; Azeez, M.; Olanipekun, E. Assessment of Trace Organics in Tomatoes from Selected Markets in Ado-Ekiti, Nigeria. J. Mater. Environ. Sci. 2020, 11, 2084–2094. [Google Scholar]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Camargo, M.C.R.; Toledo, M.C.l.F. Polycyclic aromatic hydrocarbons in Brazilian vegetables and fruits. Food Control 2003, 14, 49–53. [Google Scholar] [CrossRef]
- Ingrando, I.; Rivoira, L.; Castiglioni, M.; Tumiatti, V.; Lenzi, F.; Pagliano, A.; Bruzzoniti, M.C. Microwave-assisted extraction and gas chromatographic determination of thirty priority micropollutants in biowaste fraction derived from municipal solid waste for material recovery in the circular-economy approach. Talanta 2022, 241, 123268. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the term “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 2201–2205. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Speer, K.; Horstmann, P.; Steeg, E.; Kühn, T.; Montag, A. Zur Analytik von Polycyclen in Gemüseproben. Z. Lebensm. Unters. Forsch. 1990, 191, 442–448. [Google Scholar] [CrossRef]
- Azaiez, I.; Giusti, F.; Sagratini, G.; Mañes, J.; Fernández-Franzón, M. Multi-mycotoxins analysis in dried fruit by LC/MS/MS and a modified QuEChERS procedure. Food Anal. Methods 2014, 7, 935–945. [Google Scholar] [CrossRef]
- Tilahun, S.; Seo, M.H.; Hwang, I.G.; Kim, S.H.; Choi, H.R.; Jeong, C.S. Prediction of lycopene and β-carotene in tomatoes by portable chroma-meter and VIS/NIR spectra. Postharvest Biol. Technol. 2018, 136, 50–56. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Anarjan, N.; Tan, C.P.; Nehdi, I.A.; Ling, T.C. Colloidal astaxanthin: Preparation, characterisation and bioavailability evaluation. Food Chem. 2012, 135, 1303–1309. [Google Scholar] [CrossRef]
- Myong-Kyun, R.; Min-Hee, J.; Jin-Nam, M.; Woi-Sook, M.; Sun-Mee, P.; Jae-Suk, C. A simple method for the isolation of lycopene from Lycopersicon esculentum. Bot. Sci. 2013, 91, 187–192. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Rejczak, T.; Tuzimski, T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015, 13, 980–1010. [Google Scholar] [CrossRef]
- Shim, J.-H.; Rahman, M.M.; Zaky, A.A.; Lee, S.-J.; Jo, A.; Yun, S.-H.; Eun, J.-B.; Kim, J.-H.; Park, J.-W.; Oz, E. Simultaneous Determination of Pyridate, Quizalofop-ethyl, and Cyhalofop-butyl Residues in Agricultural Products Using Liquid Chromatography-Tandem Mass Spectrometry. Foods 2022, 11, 899. [Google Scholar] [CrossRef]
- Ahmad, A.; Chan, C.; Abd Shukor, S.; Mashitah, M. Adsorption kinetics and thermodynamics of β-carotene on silica-based adsorbent. Chem. Eng. J. 2009, 148, 378–384. [Google Scholar] [CrossRef]
- Nielsen, T.; Ramdahl, T.; Bjørseth, A. The fate of airborne polycyclic organic matter. Environ. Health Perspect. 1983, 47, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Ares, G. Mathematical and Statistical Methods in Food Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bruzzoniti, M.C.; Kobylinska, D.K.; Franko, M.; Sarzanini, C. Flow injection method for the determination of silver concentration in drinking water for spacecrafts. Anal. Chim. Acta 2010, 665, 69–73. [Google Scholar] [CrossRef]
- Rivoira, L.; Appendini, M.; Fiorilli, S.; Onida, B.; Del Bubba, M.; Bruzzoniti, M.C. Functionalized iron oxide/SBA-15 sorbent: Investigation of adsorption performance towards glyphosate herbicide. Environ. Sci. Pollut. Res. 2016, 23, 21682–21691. [Google Scholar] [CrossRef] [PubMed]
- Rivoira, L.; Studzińska, S.; Szultka-Młyńska, M.; Bruzzoniti, M.C.; Buszewski, B. New approaches for extraction and determination of betaine from Beta vulgaris samples by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5133–5141. [Google Scholar] [CrossRef]
- Lamoree, M.; Swart, K.; Senhorst, H.; van Hattum, B. Validation of the Acidic Sample Clean-Up Procedure for the DR-CALUX Assay; Vrije Universiteit: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Fernandes, V.C.; Domingues, V.F.; Mateus, N.; Delerue-Matos, C. Multiresidue pesticides analysis in soils using modified Q u EC h ERS with disposable pipette extraction and dispersive solid-phase extraction. J. Sep. Sci. 2013, 36, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Liu, T.; Yang, D.; Mao, J.; Zhang, X.; Dong, M. Carboxylated multiwalled carbon nanotubes as dispersive solid-Phase extraction sorbent to determine eighteen polychlorinated biphenyls in vegetable samples by gas chromatography-mass spectrometry. J. Anal. Methods Chem. 2019, 2019, 4264738. [Google Scholar] [CrossRef]




| Analyte | MW | m/z a | LogP b | Surrogate | MW | m/z a |
|---|---|---|---|---|---|---|
| Naphthalene (Naph) | 128 | 128 | 2.963 | |||
| Acenaphthene (AcPY) | 152 | 152 | 3.329 | |||
| Acenaphthylene (AcPh) | 154 | 152 | 3.526 | |||
| Fluorene (Flu) | 166 | 166 | 3.739 | |||
| Phenanthrene (Phe) | 178 | 178 | 3.952 | |||
| Anthracene (Ant) | 178 | 178 | 3.952 | |||
| Fluoranthene (Flth) | 202 | 202 | 4.284 | |||
| Pyrene (Pyr) | 202 | 202 | 4.284 | |||
| Benzo[a]anthracene (BaA) | 228 | 228 | 4.942 | BaA-d12 | 240 | 240 |
| Chrysene (Chr) | 228 | 228 | 4.942 | Chr-d12 | 240 | 240 |
| Benzo[b]fluoranthene (BbFl) | 252 | 252 | 5.273 | BbFl-d12 | 264 | 264 |
| Benzo[k]fluoranthene (BkFl) | 252 | 252 | 5.273 | BkFl-d12 | 264 | 264 |
| Benzo[a]pyrene (BaP) | 252 | 252 | 5.273 | BaP-d12 | 264 | 264 |
| Indeno [1,2,3-cd]pyrene (Ind) | 276 | 276 | 5.605 | Ind-d12 | 288 | 288 |
| Dibenz[a,h]anthracene (DBA) | 278 | 278 | 5.931 | DBA-d14 | 292 | 292 |
| Benzo[g,h,i]perylene (BP) | 276 | 276 | 5.605 | BP-d12 | 288 | 288 |
| PCB11 | 223 | 222 | 4.829 | |||
| PCB15 | 223 | 222 | 4.829 | |||
| PCB28 | 258 | 186 | 5.433 | 13C12-PCB28 | 269 | 268 |
| PCB52 | 292 | 292 | 6.037 | 13C12-PCB52 | 304 | 304 |
| PCB101 | 326 | 254 | 6.641 | |||
| PCB81 * | 292 | 292 | 6.037 | |||
| PCB118 * | 326 | 326 | 6.641 | 13C12-PCB118 | 338 | 338 |
| PCB123 * | 326 | 326 | 6.641 | |||
| PCB138 | 361 | 360 | 7.245 | |||
| PCB153 | 361 | 360 | 7.245 | 13C12-PCB153 | 373 | 372 |
| PCB167 * | 361 | 360 | 7.245 | |||
| PCB180 | 395 | 394 | 7.849 | 13C12-PCB180 | 407 | 406 |
| PCB169 * | 361 | 360 | 7.245 | |||
| PCB189 * | 395 | 394 | 7.849 | |||
| 1-Nitronaphthalene | 173 | 173 | 2.904 | |||
| 2-Nitrofluorene | 211 | 211 | 3.679 | |||
| 1-Nitropyrene | 247 | 247 | 4.224 | 1-nitropyrene-d9 | 256 | 256 |
| 6-Nitrobenzo[a]pyrene | 297 | 297 | 5.440 | |||
| Anthracene-d10 | 188 | 188 | 3.952 | |||
| 13C12-PCB70 | 304 | 304 | 6.037 |
| Experiment | Coded Variables | Factors | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | H2SO4 (μL) | PSA (mg) | C18 (mg) | |
| 1 | − | − | − | 9 | 10 | 10 |
| 2 | + | − | − | 18 | 10 | 10 |
| 3 | − | + | − | 9 | 150 | 10 |
| 4 | + | + | − | 18 | 150 | 10 |
| 5 | − | − | + | 9 | 10 | 150 |
| 6 | + | − | + | 18 | 10 | 150 |
| 7 | − | + | + | 9 | 150 | 150 |
| 8 | + | + | + | 18 | 150 | 150 |
| Surrogate/Experimental Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| BaA-d12 (%) | 202 | 115 | 121 | 129 | 88 | 66 | 100 | 85 |
| Chr-d12 (%) | 184 | 157 | 104 | 122 | 118 | 105 | 86 | 80 |
| BbFl-d12 (%) | 235 | 251 | 127 | 131 | 188 | 101 | 102 | 76 |
| BkFl-d12 (%) | 228 | 274 | 148 | 139 | 282 | 168 | 108 | 122 |
| BaP-d12 (%) | 34 | 50 | 54 | 43 | 32 | 18 | 66 | 16 |
| Ind-d12 (%) | 188 | 189 | 80 | 145 | 129 | 89 | 80 | 64 |
| DBA-d14 (%) | 200 | 258 | 91 | 99 | 138 | 17 | 82 | 16 |
| BP-d12 (%) | 93 | 69 | 72 | 81 | 49 | 67 | 60 | 57 |
| 1-Nitropyrene-d9 (%) | 178 | 84 | 193 | 196 | 106 | 45 | 60 | 50 |
| 13C12-PCB28 (%) | 114 | 109 | 98 | 101 | 119 | 97 | 86 | 92 |
| 13C12-PCB52 (%) | 114 | 120 | 100 | 103 | 113 | 98 | 91 | 95 |
| 13C12-PCB118 (%) | 128 | 126 | 119 | 116 | 121 | 125 | 105 | 123 |
| 13C12-PCB153 (%) | 129 | 129 | 111 | 125 | 136 | 118 | 107 | 114 |
| 13C12-PCB180 (%) | 144 | 184 | 125 | 143 | 151 | 141 | 110 | 129 |
| Surrogate | MW | LogP | a1 | a2 | a3 |
|---|---|---|---|---|---|
| BaA-d12 | 240 | 4.942 | −7.3 | −0.745 | −0.752 |
| Chr-d12 | 240 | 4.942 | −1.9 | −0.642 | −0.339 |
| 1-nitropyrene-d9 | 256 | 4.224 | −1.24 | −0.095 | −0.304 |
| BbFl-d12 | 264 | 5.273 | −0.3 | −1.005 | 0.004 |
| BkFl-d12 | 264 | 5.273 | 0.09 | −1.08 | 0.52 |
| BaP-d12 | 264 | 5.273 | 1.105 | 0.3538 | 0.2258 |
| Ind-d12 | 288 | 5.605 | 1.17 | −0.952 | 0.08 |
| DBA-d14 | 292 | 5.931 | 3.25 | −0.925 | 0.252 |
| BP-d12 | 288 | 5.605 | 4.24 | −0.095 | −0.304 |
| 13C12-PCB28 | 269 | 5.433 | −1.07 | −0.262 | 0.051 |
| 13C12-PCB52 | 304 | 6.037 | 0.07 | −0.187 | 0.018 |
| 13C12-PCB118 | 338 | 6.641 | −0.585 | −0.1209 | −0.1592 |
| 13C12-PCB153 | 373 | 7.245 | −0.299 | −0.2655 | 0.1009 |
| 13C12-PCB180 | 407 | 7.849 | 0.1 | −0.236 | 0.133 |
| Analyte | MDL | MQL | Analyte | MDL | MQL |
|---|---|---|---|---|---|
| Naph | 0.6 | 1.9 | PCB11 | 3.6 | 11.0 |
| AcPY | 1.4 | 4.1 | PCB15 | 2.4 | 7.2 |
| AcPh | 1.1 | 3.2 | PCB28 | 3.9 | 11.8 |
| Flu | 0.7 | 2.2 | PCB52 | 6.3 | 19.1 |
| Phe | 2.2 | 6.5 | PCB101 | 1.9 | 5.7 |
| Ant | 0.9 | 2.7 | PCB81 | 2.8 | 8.4 |
| Flth | 2.0 | 6.1 | PCB118 | 1.7 | 5.2 |
| Pyr | 0.7 | 2.2 | PCB123 | 1.2 | 3.7 |
| BaA | 2.7 | 8.3 | PCB138 | 2.5 | 7.7 |
| Chr | 2.1 | 6.3 | PCB153 | 1.3 | 3.8 |
| BbFl | 1.7 | 5.1 | PCB167 | 2.4 | 7.2 |
| BkFl | 1.7 | 5.2 | PCB180 | 2.4 | 7.2 |
| BaP | 2.4 | 7.1 | PCB169 | 2.3 | 6.8 |
| Ind | 1.8 | 5.4 | PCB189 | 1.7 | 5.3 |
| DBA | 2.4 | 7.2 | |||
| BP | 2.6 | 8.0 | |||
| 1-Nitronaphthalene | 34.4 | 104 | |||
| 2-Nitrofluorene | 39.1 | 118 | |||
| 1-Nitropyrene | 27.9 | 84 | |||
| 6-Nitrobenzo[a]pyrene | 307 | 931 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivoira, L.; Del Bubba, M.; Cecconi, G.; Castiglioni, M.; Testa, V.; Isola, M.; Bruzzoniti, M.C. Experimental Design and Multiple Response Optimization for the Extraction and Quantitation of Thirty-Four Priority Organic Micropollutants in Tomatoes through the QuEChERS Approach. Separations 2023, 10, 174. https://doi.org/10.3390/separations10030174
Rivoira L, Del Bubba M, Cecconi G, Castiglioni M, Testa V, Isola M, Bruzzoniti MC. Experimental Design and Multiple Response Optimization for the Extraction and Quantitation of Thirty-Four Priority Organic Micropollutants in Tomatoes through the QuEChERS Approach. Separations. 2023; 10(3):174. https://doi.org/10.3390/separations10030174
Chicago/Turabian StyleRivoira, Luca, Massimo Del Bubba, Giasmin Cecconi, Michele Castiglioni, Valentina Testa, Mattia Isola, and Maria Concetta Bruzzoniti. 2023. "Experimental Design and Multiple Response Optimization for the Extraction and Quantitation of Thirty-Four Priority Organic Micropollutants in Tomatoes through the QuEChERS Approach" Separations 10, no. 3: 174. https://doi.org/10.3390/separations10030174