Advances in Cork Use in Adsorption Applications: An Overview of the Last Decade of Research
Abstract
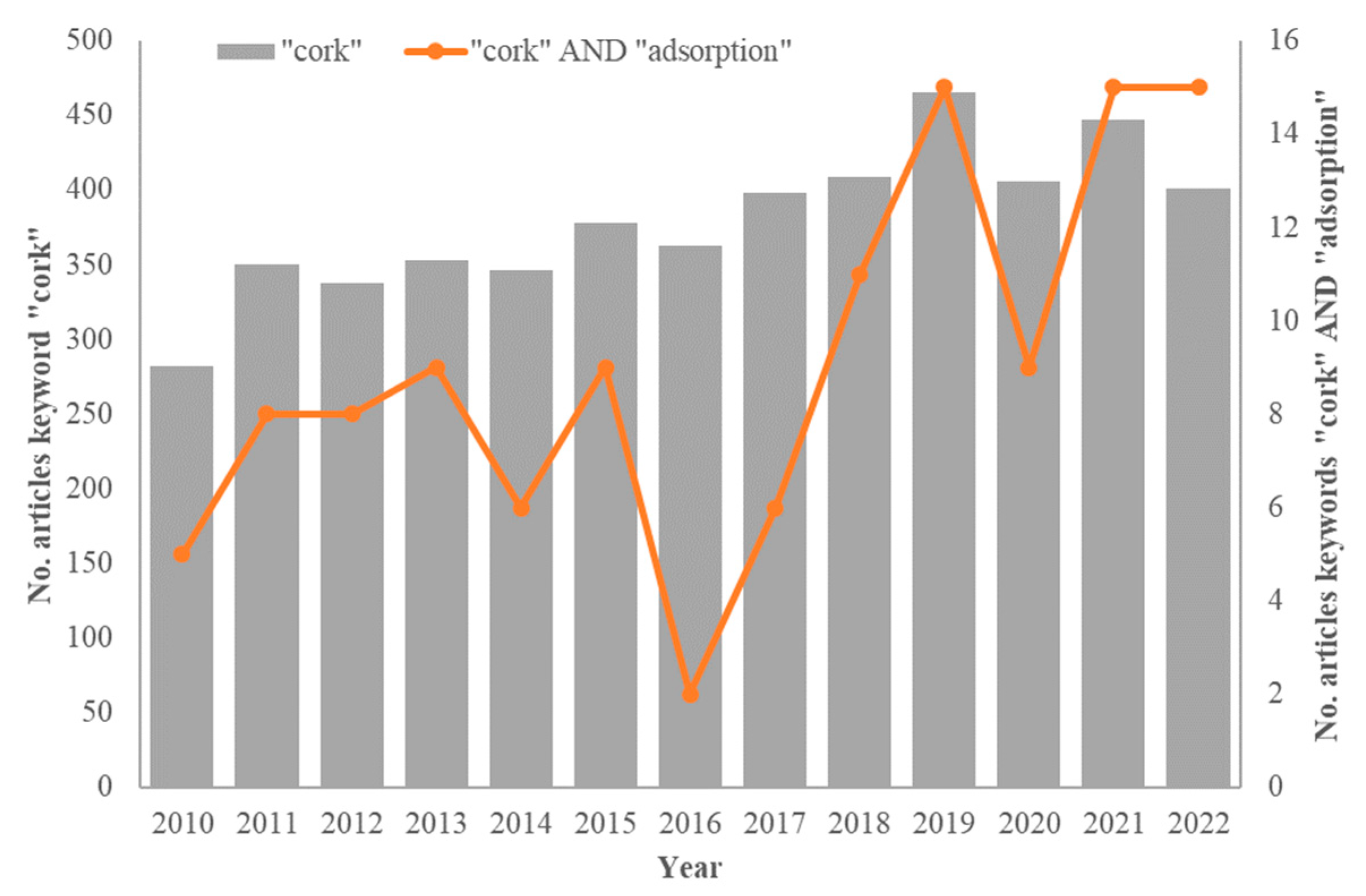
:1. Introduction
2. Brief Description of Cork Industrial Processes and Implications on Material Quality
3. Novel Insights into the Properties of Cork and Cork Powder
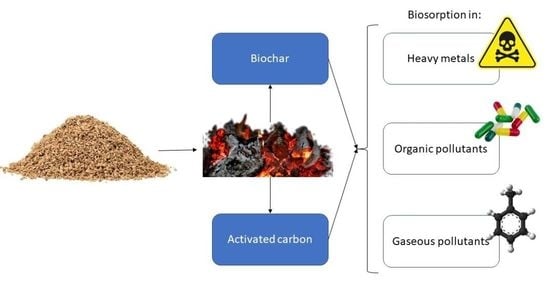
4. Production of Activated Carbons and Biochars
- Attempting the valorization of cork biomass from different origins (particularly cork powder);
- Reducing production costs for the valorization of waste biomass—mainly through the generation of biochars, skipping the activation step;
- Producing activated carbons with larger surface area and pore volume, honing their characteristics for their application.
4.1. Valorization of Different Types of Cork Biomass
4.2. Production of Cork Biochars
- a first stage up to 200 °C, in which no chemical reactions were apparent and only moisture loss was observed;
- a second stage from 200 to 430 °C, which corresponded to the degradation of the main chemical components and the largest mass loss;
- a later phase between 430 and 550 °C, identified as volatilization of residual lignin and unstable carbon.
4.3. Activation and Functionalization
5. Applied Biosorption with Cork-Based Materials
5.1. Biosorption of Metals
5.2. Biosorption of Organic Pollutants
| Source | Pollutant | Size (mm)/Activation Method | pH | Solid/Liquid Ratio (g·L−1) | Initial Concentration (mg·L−1) | Langmuir r2 | qmax (mg·g−1) |
|---|---|---|---|---|---|---|---|
| [47] | ofloxacin | 0.42–0.841 & >0.42 | 4 | 12.5 | 181–1806 | - | 31.1 |
| >0.42 | 9 | 37.9 | |||||
| 0.42–0.841 | 9 | 24.9 | |||||
| [50] | furosemide | - | - | 150 | 1–11 | 0.183 | 0.25 |
| [51] | phenol | <2 | 6 | 20 | 5–50 | 0.98 | 0.92 |
| 2-chlorophenol | 0.99 | 1.54 | |||||
| 2-nitrophenol | 0.99 | 5.09 | |||||
| 2,4-dichlorophenol | 0.94 | 6.24 | |||||
| pentachlorophenol | 10 | 0.95 | 5.31 | ||||
| [46] | methyl orange | <0.08 | 2 | 5 | 100 | 0.996 | 16.66 |
| [52] | fuchsin or basic violet 14 | 0.63–0.75 | 6 | 6.66 | 100 | 0.979 | 29.9 |
| [48] | carbamazepine | 3–4 | - | 100 | 1–35 | 0.878 | 0.37 |
| clofibric acid | 0.870 | 0.06 | |||||
| Ibuprofen | 0.876 | 0.32 | |||||
| [49] | chrysoidine G | >0.42 | 4 | 12.5 | - | - | 36.3 |
| >0.42 (in alginate) | 42.4 | ||||||
| 0.42–0.841 | 7 | 44.6 | |||||
| >0.42 | 57.3 | ||||||
| >0.42 (in alginate) | 61.5 | ||||||
| [53] | fluoxetine | <1 | 9 | 0.1–1.5 | 5 | 0.884 | 10 ± 3 |
| Biochar or activated carbon (AC) produced from cork | |||||||
| [25] | methylene blue | Two-step carbonization under N2 and KOH activation 3:1 w/w | - | 1 | 100–1800 | 1.000 | 806.4 |
| Two-step carbonization under N2 and KOH activation 4:1 w/w | 1.000 | 990.1 | |||||
| Two-step carbonization under N2 and KOH activation 5:1 w/w, 750 °C | 1.000 | 1059.8 | |||||
| [35] | methylene blue | <75 μm, activated with alkaline wastewater and carbonization under N2 | - | 2–3.5 | 10–700 | 0.902 | 333.33 |
| [34] | methylene blue | Two-step carbonization under N2 and KOH activation 5:1 w/w, 850 °C | - | 0.25 | 50–2500 | 0.997 | 1283.99 |
| rhodamine B | 0.982 | 4067.57 | |||||
| methyl orange | 0.992 | 2666.2 | |||||
| congo red | 0.997 | 8920.6 | |||||
| [19] | ibuprofen | steam activation, carbonization under N2 | 5 | 0.2–0.67 | 20–150 | 0.995 | 143.1 |
| KOH activation, carbonization under N2 | 0.993 | 174.4 | |||||
5.3. Biosorption of Gaseous Pollutants
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rives, J.; Fernandez-Rodriguez, I.; Rieradevall, J.; Gabarrell, X. Integrated environmental analysis of the main cork products in southern Europe (Catalonia–Spain). J. Clean. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Gil, L. New Cork-Based Materials and Applications. Materials 2015, 8, 625–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintor, A.M.A.; Ferreira, C.I.A.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Costa, A. How resilient is Quercus suber L. to cork harvesting? A review and identification of knowledge gaps. For. Ecol. Manag. 2012, 270, 257–272. [Google Scholar]
- APCOR, Cork as a Building Material Technical Manual. Available online: https://www.apcor.pt/wp-content/uploads/2015/07/Caderno_Tecnico_F_EN.pdf (accessed on 15 May 2023).
- Sierra-Pérez, J.; Boschmonart-Rives, J.; Dias, A.C.; Gabarrell, X. Environmental implications of the use of agglomerated cork as thermal insulation in buildings. J. Clean. Prod. 2016, 126, 97–107. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Bellat, J.-P.; Gougeon, R.D.; Karbowiak, T. Mechanical properties of agglomerated cork stoppers for sparkling wines: Influence of adhesive and cork particle size. Compos. Struct. 2018, 203, 789–796. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities, and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef] [Green Version]
- Pereira, H. Variability of the chemical composition of cork. Bioresources 2013, 8, 2246–2256. [Google Scholar] [CrossRef]
- Lagorce-Tachon, A.; Mairesse, F.; Karbowiak, T.; Gougeon, R.D.; Bellat, J.-P.; Sliwa, T.; Simon, J.-M. Contribution of image processing for analysing the cellular structure of cork. J. Chemom. 2018, 32, e2988. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Chanut, J.; Lagorce, A.; Winckler, P.; Wang, Z.; Verboven, P.; Nicolai, B.; Lherminier, J.; Ferret, E.; Gougeon, R.D.; et al. Four hundred years of cork imaging: New advances in the characterization of the cork structure. Sci. Rep. 2019, 9, 19682. [Google Scholar] [CrossRef] [Green Version]
- Lagorce-Tachon, A.; Karbowiak, T.; Loupiac, C.; Gaudry, A.; Ott, F.; Alba-Simionesco, C.; Gougeon, R.D.; Alcantara, V.; Mannes, D.; Kaestner, A.; et al. The cork viewed from the inside. J. Food Eng. 2014, 149, 214–221. [Google Scholar] [CrossRef]
- Berezovska, I.; Fettaka, H.; Salmon, T.; Toye, D.; Lodewyckx, P. Redistribution of a mixture of organic vapours inside an activated carbon filter. Chem. Eng. J. 2015, 280, 677–681. [Google Scholar] [CrossRef]
- Sharma, K.; Bilheux, H.Z.; Walker, L.M.H.; Voisin, S.; Mayes, R.T.; Kiggans, J.O., Jr.; Yiacoumi, S.; DePaoli, D.W.; Dai, S.; Tsouris, C. Neutron imaging of ion transport in mesoporous carbon materials. Phys. Chem. Chem. Phys. 2013, 15, 11740–11747. [Google Scholar] [CrossRef]
- Motte, J.-C.; Delenne, J.-Y.; Barron, C.; Dubreucq, É.; Mayer-Laigle, C. Elastic properties of packing of granulated cork: Effect of particle size. Ind. Crops Prod. 2017, 99, 126–134. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Shi, R.; Chen, H.; Du, Y.; Liu, B.; Zeng, Z.; Yin, Z.; Li, L. Natural Honeycomb-like structure cork carbon with hierarchical Micro-Mesopores and N-containing functional groups for VOCs adsorption. Appl. Surf. Sci. 2021, 565, 150550. [Google Scholar] [CrossRef]
- Atanes, E.; Nieto-Márquez, A.; Cambra, A.; Ruiz-Pérez, M.C.; Fernández-Martínez, F. Adsorption of SO2 onto waste cork powder-derived activated carbons. Chem. Eng. J. 2012, 211–212, 60–67. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, R.A.; Aroso, I.; Fernandes, E.M.; Pinto, M.L.; Reis, R.L.; Andrade, M.A.; Pires, J.; Silva, S.P.; Carvalho, A.P. Activated carbons prepared from industrial pre-treated cork: Sustainable adsorbents for pharmaceutical compounds removal. Chem. Eng. J. 2014, 253, 408–417. [Google Scholar] [CrossRef]
- Şen, A.U.; Nobre, C.; Durão, L.; Miranda, I.; Pereira, H.; Gonçalves, M. Low-temperature biochars from cork-rich and phloem-rich wastes: Fuel, leaching, and methylene blue adsorption properties. Biomass Convers. Biorefinery 2020, 12, 3899–3909. [Google Scholar] [CrossRef]
- Nobre, C.; Şen, A.; Durão, L.; Miranda, I.; Pereira, H.; Gonçalves, M. Low-temperature pyrolysis products of waste cork and lignocellulosic biomass: Product characterization. Biomass Convers. Biorefinery 2021, 13, 2267–2277. [Google Scholar] [CrossRef]
- Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lv, J.; Zhao, R.; Chen, X. Effect of Air Oxidation on Texture, Surface Properties and Dye Adsorption of Wood-Derived Porous Carbon Materials. Materials 2019, 12, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Lai, Z.; Mu, J.; Chu, D.; Zang, X. Converting industrial waste cork to biochar as Cu(II) adsorbent via slow pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Elsayed, I.; Song, X.; Shmulsky, R.; Hassan, E.B. Microporous carbon nanoflakes derived from biomass cork waste for CO2 capture. Sci. Total Environ. 2020, 748, 142465. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Luo, C.; Zhang, J.; Cao, X.; Liu, J.; Mu, J. Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J. Hazard. Mater. 2021, 416, 125896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chu, D.; Luo, C.; Lai, Z.; Shang, S.; Rahimi, S.; Mu, J. Transformation mechanism from cork into honeycomb–like biochar with rich hierarchical pore structure during slow pyrolysis. Ind. Crops Prod. 2022, 181, 114827. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Bouafif, H.; Neculita, C.M.; Koubaa, A. Influence of Pyro-Gasification and Activation Conditions on the Porosity of Activated Biochars: A Literature Review. Waste Biomass Valorization 2020, 11, 5079–5098. [Google Scholar] [CrossRef]
- Chun, Y.; Lee, S.K.; Yoo, H.Y.; Kim, S.W. Recent advancements in biochar production according to feedstock classification, pyrolysis conditions, and applications: A review. Bioresources 2021, 16, 6512–6547. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Ong, P.Y.; Klemeš, J.J.; Li, C.; Gao, Y. Lignocellulosic Biomass and Food Waste for Biochar Production and Application: A Review. Chem. Eng. Trans. 2020, 81, 427–432. [Google Scholar]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-N.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Ochai-Ejeh, F.O.; Bello, A.; Dangbegnon, J.; Khaleed, A.A.; Madito, M.J.; Bazegar, F.; Manyala, N. High Electrochemical Performance of Hierarchical Porous Activated Carbon Derived from Lightweight Cork (Quercus suber). J. Mater. Sci. 2017, 52, 10600–10613. [Google Scholar] [CrossRef] [Green Version]
- Ochai-Ejeh, F.O.; Momodu, D.Y.; Madito, M.J.; Khaleed, A.A.; Oyedotun, K.O.; Ray, S.C.; Manyala, N. Nanostructured Porous Carbons with High Rate Cycling and Floating Performance for Supercapacitor Application. AIP Adv. 2018, 8, 55208. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Ledesma, B.; Laginhas, C. Removal of Amitriptyline from Aqueous Media Using Activated Carbons. Adsorpt. Sci. Technol. 2012, 30, 255–263. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, C.; Lai, Z.; Chen, S.; He, D.; Mu, J. Honeycomb-like Cork Activated Carbon with Ultra-High Adsorption Capacity for Anionic, Cationic and Mixed Dye: Preparation, Performance and Mechanism. Bioresour. Technol. 2022, 357, 127363. [Google Scholar] [CrossRef]
- Novais, R.M.; Caetano, A.P.F.; Seabra, M.P.; Labrincha, J.A.; Pullar, R.C. Extremely Fast and Efficient Methylene Blue Adsorption Using Eco-Friendly Cork and Paper Waste-Based Activated Carbon Adsorbents. J. Clean. Prod. 2018, 197, 1137–1147. [Google Scholar] [CrossRef]
- Krika, F.; Azzouz, N.; Ncibi, M.C. Adsorptive Removal of Cadmium from Aqueous Solution by Cork Biomass: Equilibrium, Dynamic and Thermodynamic Studies. Arab. J. Chem. 2016, 9, S1077–S1083. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.B.; Oliveira, J.R.; Rocha, L.S.; Tavares, D.S.; Silva, C.M.; Silva, S.P.; Hartog, N.; Duarte, A.C.; Pereira, E. Cork Stoppers as an Effective Sorbent for Water Treatment: The Removal of Mercury at Environmentally Relevant Concentrations and Conditions. Environ. Sci. Pollut. Res. 2014, 21, 2108–2121. [Google Scholar] [CrossRef]
- Şen, A.U.; Olivella, M.À.; Fiol, N.; Miranda, I.; Villaescusa, I.; Pereira, H. Removal of Chromium (VI) in Aqueous Environments Using Cork and Heat-Treated Cork Samples from Quercus cerris and Quercus suber. Bioresources 2012, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Pintor, A.M.A.; Vieira, B.R.C.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Arsenate and Arsenite Adsorption onto Iron-Coated Cork Granulates. Sci. Total Environ. 2018, 642, 1075–1089. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vieira, B.R.C.; Boaventura, R.A.R.; Botelho, C.M.S. Removal of Antimony from Water by Iron-Coated Cork Granulates. Sep. Purif. Technol. 2020, 233, 116020. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Efficient Removal of Arsenic from Aqueous Solution by Continuous Adsorption onto Iron-Coated Cork Granulates. J. Hazard. Mater. 2022, 432, 128657. [Google Scholar] [CrossRef]
- Sfaksi, Z.; Azzouz, N.; Abdelwahab, A. Removal of Cr(VI) from Water by Cork Waste. Arab. J. Chem. 2014, 7, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Pintor, A.M.A.; Brandão, C.C.; Boaventura, R.A.R.; Botelho, C.M.S. Multicomponent Adsorption of Pentavalent As, Sb and P onto Iron-Coated Cork Granulates. J. Hazard. Mater. 2021, 406, 124339. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.M.A.; Vieira, B.R.C.; Brandão, C.C.; Boaventura, R.A.R.; Botelho, C.M.S. Complexation Mechanisms in Arsenic and Phosphorus Adsorption onto Iron-Coated Cork Granulates. J. Environ. Chem. Eng. 2020, 8, 104184. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Coelho, J.F.R.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Multi-cycle regeneration of arsenic-loaded iron-coated cork granulates for water treatment. J. Water Process Eng. 2022, 50, 103291. [Google Scholar] [CrossRef]
- Krika, F.; Benlahbib, O.E.F. Removal of Methyl Orange from Aqueous Solution via Adsorption on Cork as a Natural and Low-Coast Adsorbent: Equilibrium, Kinetic and Thermodynamic Study of Removal Process. Desalin. Water Treat. 2015, 53, 3711–3723. [Google Scholar] [CrossRef]
- Crespo-Alonso, M.; Nurchi, V.M.; Biesuz, R.; Alberti, G.; Spano, N.; Pilo, M.I.; Sanna, G. Biomass against Emerging Pollution in Wastewater: Ability of Cork for the Removal of Ofloxacin from Aqueous Solutions at Different PH. J. Environ. Chem. Eng. 2013, 1, 1199–1204. [Google Scholar] [CrossRef]
- Dordio, A.V.; Gonçalves, P.; Texeira, D.; Candeias, A.J.; Castanheiro, J.E.; Pinto, A.P.; Carvalho, A.J.P. Pharmaceuticals Sorption Behaviour in Granulated Cork for the Selection of a Support Matrix for a Constructed Wetlands System. Int. J. Environ. Anal. Chem. 2011, 91, 615–631. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crespo-Alonso, M.; Biesuz, R.; Alberti, G.; Pilo, M.I.; Spano, N.; Sanna, G. Sorption of Chrysoidine by Row Cork and Cork Entrapped in Calcium Alginate Beads. Arab. J. Chem. 2014, 7, 133–138. [Google Scholar] [CrossRef]
- Machado, A.I.; Dordio, A.; Fragoso, R.; Leitão, A.E.; Duarte, E. Furosemide Removal in Constructed Wetlands: Comparative Efficiency of LECA and Cork Granulates as Support Matrix. J. Environ. Manag. 2017, 203, 422–428. [Google Scholar] [CrossRef]
- Mallek, M.; Chtourou, M.; Portillo, M.; Monclús, H.; Walha, K.; ben Salah, A.; Salvadó, V. Granulated Cork as Biosorbent for the Removal of Phenol Derivatives and Emerging Contaminants. J. Environ. Manag. 2018, 223, 576–585. [Google Scholar] [CrossRef]
- Olivella, M.À.; Fiol, N.; de la Torre, F.; Poch, J.; Villaescusa, I. Assessment of Vegetable Wastes for Basic Violet 14 Removal: Role of Sorbent Surface Chemistry and Porosity. Desalin. Water Treat. 2015, 53, 2278–2288. [Google Scholar] [CrossRef]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-Based Biosorbents as Cost-Effective Alternatives to Commercial Adsorbents for the Retention of Fluoxetine from Water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef] [Green Version]
- Olivella, M.À.; Bazzicalupi, C.; Bianchi, A.; Fiol, N.; Villaescusa, I. New Insights into the Interactions between Cork Chemical Components and Pesticides. The Contribution of π–π Interactions, Hydrogen Bonding and Hydrophobic Effect. Chemosphere 2015, 119, 863–870. [Google Scholar] [CrossRef]

| Parameter (% Oven-Dry Cork) | Mean ± Stdev | %CV | |
|---|---|---|---|
| Extractives | Dichloromethane | 5.8 ± 0.8 | 13.8 |
| Ethanol | 5.9 ± 3.0 | 50.8 | |
| Water | 4.5 ± 1.6 | 35.6 | |
| Total | 16.2 ± 3.9 | 24.1 | |
| Suberin | Long chain lipids (LCL) | 41.0 ± 5.2 | 12.7 |
| Glycerol (Gly) | 3.8 ± 0.6 | 15.8 | |
| Ratio LCL: Gly | 11.3 ± 1.6 | 14.2 | |
| Total | 42.8 ± 6.2 | 14.5 | |
| Lignin | Klason lignin | 21.1 ± 3.3 | 15.6 |
| Acid soluble lignin | 0.9 ± 0.2 | 22.2 | |
| Total | 22.0 ± 3.3 | 15 |
| Source | Raw Material | Gas Flow | Heating Rate | Temperature Level | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|---|---|---|---|
| [18] | Cork powder (<0.17 mm) | N2, 100 mL·min−1 | 5 °C·min−1 | 750 °C, no hold | 7 | 0.01 |
| [23] | Cork granulates (0.25–0.45 mm) | N2, 300 mL·min−1 | 10 °C·min−1 | 450 °C, hold 30 min | 32 | 0.04 |
| 550 °C, hold 30 min | 220 | 0.23 | ||||
| 650 °C, hold 30 min | 322 | 0.24 | ||||
| 750 °C, hold 30 min | 393 | 0.24 | ||||
| 550 °C, hold 1 h | 245 | 0.24 | ||||
| 550 °C, hold 1.5 h | 266 | 0.24 | ||||
| 550 °C, hold 2 h | 275 | 0.24 | ||||
| [16] | Waste cork stoppers | N2 | 10 °C·min−1 | 600 °C, 2 h | 448 | 0.04 |
| [17] | Cork stoppers pulverized to <900 µm | N2, 100 mL·min−1 | 5 °C·min−1 | 800 °C, 1 h | 369 | 0.23 |
| [25] | Cork granulates (0.25–0.45 mm) | N2 | 550 °C, 1 h | 379 | 0.17 | |
| [26] | Cork granulates (0.25–0.45 mm) | N2, 100 mL·min−1 | 10 °C·min−1 | 150 °C, hold 90 min | 2 | 0.01 |
| 200 °C, hold 90 min | 2 | 0.01 | ||||
| 250 °C, hold 90 min | 3 | 0.01 | ||||
| 300 °C, hold 90 min | 3 | 0.01 | ||||
| 350 °C, hold 90 min | 4 | 0.01 | ||||
| 400 °C, hold 90 min | 5 | 0.01 | ||||
| 450 °C, hold 90 min | 31 | 0.03 | ||||
| 500 °C, hold 90 min | 210 | 0.12 | ||||
| 550 °C, hold 90 min | 489 | 0.27 |
| Source | Raw Material | Pretreatment | Activation Agent | Pyrolysis Conditions | SBET (m2/g) | Vp (cm3/g) |
|---|---|---|---|---|---|---|
| [33] | Cork granules | Carbonization under N2, 400 °C, 1 h | CO2 | 10 °C·min−1 to 800 °C, hold until burn-off 26% | 581 | 0.38 |
| 10 °C·min−1 to 800 °C, hold until burn-off 49% | 839 | 0.63 | ||||
| [18] | Cork powder (<0.17 mm) | CO2 | N2, 100 mL·min−1, 5 °C·min−1 to 750 °C, switch to CO2, hold 2 h | 76 | 0.06 | |
| KOH 1:1 w/w, impregnation | N2, 100 mL·min−1, 5 °C·min−1 to 750 °C, no hold | 584 | 0.33 | |||
| [19] | Regranulated cork (2.0–2.8 mm) | Hydrothermal carbonization, ~350 °C, 20 min (before study) | Steam | N2, 480 mL·min−1, 10 °C·min−1 to 800 °C, hold 1 h | 750 | 0.50 |
| Regranulated cork (0.5–1.0 mm) | KOH 1:1 w/w, impregnation | N2, 300 mL·min−1, 10 °C·min−1 to 700 °C, hold 1 h | 729 | 0.35 | ||
| N2, 300 mL·min−1, 10 °C·min−1 to 800 °C, hold 1 h | 948 | 0.47 | ||||
| KOH 2:1 w/w, impregnation | N2, 300 mL·min−1, 10 °C·min−1 to 700 °C, hold 1 h | 874 | 0.41 | |||
| K2CO3 1:1 w/w, impregnation | N2, 300 mL·min−1, 10 °C·min−1 to 700 °C, hold 1 h | 617 | 0.29 | |||
| N2, 300 mL·min−1, 10 °C·min−1 to 800 °C, hold 1 h | 907 | 0.42 | ||||
| K2CO3 2:1 w/w, impregnation | N2, 300 mL·min−1, 10 °C·min−1 to 700 °C, hold 1 h | 604 | 0.30 | |||
| [31] | Cork granules | KOH 1:1 w/w, impregnation | Ar, 300 mL·min−1, 800 °C, 2 h | 881 | 0.52 | |
| KOH 2:1 w/w, impregnation | 1082 | 0.66 | ||||
| KOH 3:1 w/w, impregnation | 916 | 0.54 | ||||
| [35] | Cork granulates (0.5–1.0 mm) | 10 M NaOH and alkaline wastewater, 50:50 v/v, impregnation 0.8 g/50 mL | N2, 5 °C·min−1 to 150 °C, 10 °C·min−1 to 900 °C, hold 30 min | 1670 | 1.14 | |
| [32] | Cork granules | 0·5 M H2SO4 + distilled water, hydrothermal carbonization at 160 °C for 2 h | KHCO3 1:1 | Ar, 850 °C, 2 h | 1057 | 0.64 |
| [22] | Cork granules from Quercus variabilis | Hydrothermal treatment at 180 °C, 5 h | N2, 100 mL·min−1, 800 °C, 1 h | 376 | 0.20 | |
| Hydrothermal treatment at 180 °C, 5 h; carbonization under N2, 100 mL·min−1 800 °C, 1 h | Air | 350 °C, 1 h | 404 | 0.23 | ||
| 400 °C, 1 h | 540 | 0.33 | ||||
| 450 °C, 1 h | 580 | 0.38 | ||||
| [24] | Cork powder (< 0.18 mm) | KOH 3:1 w/w, impregnation | 10 °C·min−1 to 700 °C, hold 1 h | 1231 | 0.54 | |
| Carbonization under N2, 10 °C·min−1 to 350 °C, 30 min | ZnCl2 3:1 w/w, impregnation | 10 °C·min−1 to 600 °C, hold 1 h | 1303 | 0.56 | ||
| KOH 3:1 w/w, impregnation | 10 °C·min−1 to 400 °C, hold 1 h | 470 | 0.25 | |||
| 10 °C·min−1 to 500 °C, hold 1 h | 1491 | 0.62 | ||||
| 10 °C·min−1 to 600 °C, hold 1 h | 1885 | 0.78 | ||||
| 10 °C·min−1 to 700 °C, hold 1 h | 2010 | 0.82 | ||||
| 10 °C·min−1 to 800 °C, hold 1 h | 1909 | 0.92 | ||||
| KOH 1:1 w/w, impregnation | 10 °C·min−1 to 700 °C, hold 1 h | 984 | 0.43 | |||
| KOH 2:1 w/w, impregnation | 1605 | 0.66 | ||||
| KOH 4:1 w/w, impregnation | 1949 | 0.84 | ||||
| KOH 5:1 w/w, impregnation | 2380 | 1.14 | ||||
| KOH 6:1 w/w, impregnation | 2379 | 1.29 | ||||
| [16] | Waste cork stoppers | Carbonization under N2, 10 °C·min−1 to 600 °C, 2 h | H2SO4 | 180 | 0.08 | |
| [17] | Cork stoppers pulverized to <900 µm | Carbonisation under N2, 100 mL·min−1, 5 °C·min−1 to 800 °C, 1 h | N2, 100 mL·min−1, 5 °C·min−1 to 900 °C, hold 1 h | 1149 | 0.96 | |
| NH3 | N2, 100 mL·min−1, 5 °C·min−1 to 700 °C, switch to NH3, hold 1 h | 558 | 0.36 | |||
| N2, 100 mL·min−1, 5 °C·min−1 to 800 °C, switch to NH3, hold 1 h | 1022 | 0.68 | ||||
| N2, 100 mL·min−1, 5 °C·min−1 to 900 °C, switch to NH3, hold 1 h | 2060 | 2.21 | ||||
| [25] | Cork granulates (0.25–0.45 mm) | Carbonization under N2, 550 °C, 1 h | KOH 3:1 w/w, solid mixing | N2, 750 °C, 2 h | 2567 | 1.16 |
| KOH 4:1 w/w, solid mixing | 2707 | 1.28 | ||||
| KOH 5:1 w/w, solid mixing | 2865 | 1.43 | ||||
| [34] | Cork powder | Carbonization under N2, 300 mL·min−1, 10 °C·min−1, 550 °C, 1 h | KOH 5:1 w/w, solid mixing | 650 °C, 2 h | 2422 | 1.09 |
| 750 °C, 2 h | 2948 | 1.37 | ||||
| 850 °C, 1 h | 3072 | 1.57 | ||||
| 850 °C, 1.5 h | 3246 | 1.81 | ||||
| 850 °C, 2 h | 3403 | 2.07 |
| Source | Pollutant | Size (mm)/Modification | pH | Solid/Liquid Ratio (g·L−1) | Initial Concentration (mg·L−1) | Langmuir r2 | qmax (mg·g−1) | |
|---|---|---|---|---|---|---|---|---|
| [36] | Cd(II) | <0.08 mm | 6 | 1 | 10–100 | 0.996 | 9.65 | (20 °C) |
| 0.996 | 12.48 | (30 °C) | ||||||
| 0.996 | 14.77 | (40 °C) | ||||||
| [38] | Cr(VI) | regranulated (300 °C steam heated), 0.25–0.42 mm | 2 | 6.67 | 25–1000 | 0.994 | 22.98 | |
| [39] | As(III) | iron-coated, 0.8–1.0 mm | 9 | 2.5 | 1–40 | 0.978 | 4.9 ± 0.3 | |
| [40] | Sb(III) | iron-coated, 0.8–1.0 mm | 6 | 2.5 | 1–40 | 0.953 | 5.8 ± 0.5 | |
| Sb(V) | 3 | 0.912 | 12 ± 2 | |||||
| [41] | As(V) | iron-coated, 0.5–1.0 mm | 3 | 2.5 | 1–40 | 0.997 | 5.8 ± 0.1 | (10 °C) |
| 0.996 | 6.2 ± 0.2 | (20 °C) | ||||||
| 0.999 | 6.9 ± 0.1 | (30 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, J.; Nunes da Silva, R.; Pintor, A. Advances in Cork Use in Adsorption Applications: An Overview of the Last Decade of Research. Separations 2023, 10, 390. https://doi.org/10.3390/separations10070390
Jesus J, Nunes da Silva R, Pintor A. Advances in Cork Use in Adsorption Applications: An Overview of the Last Decade of Research. Separations. 2023; 10(7):390. https://doi.org/10.3390/separations10070390
Chicago/Turabian StyleJesus, João, Raquel Nunes da Silva, and Ariana Pintor. 2023. "Advances in Cork Use in Adsorption Applications: An Overview of the Last Decade of Research" Separations 10, no. 7: 390. https://doi.org/10.3390/separations10070390