Fluoride Removal from Water Sources by Adsorption on MOFs
Abstract
:1. Introduction
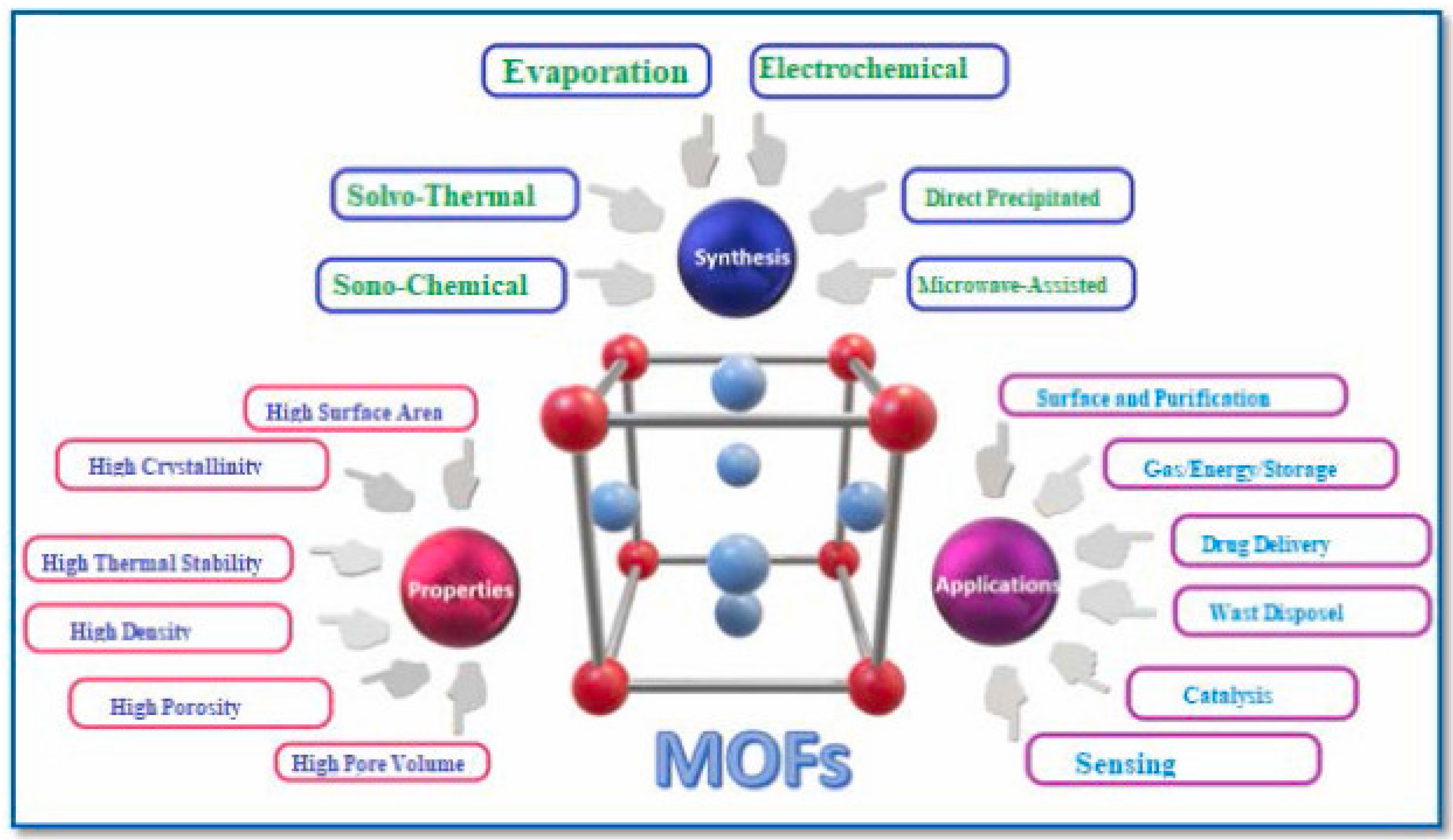
2. Metal–Organic Frameworks (MOFs)
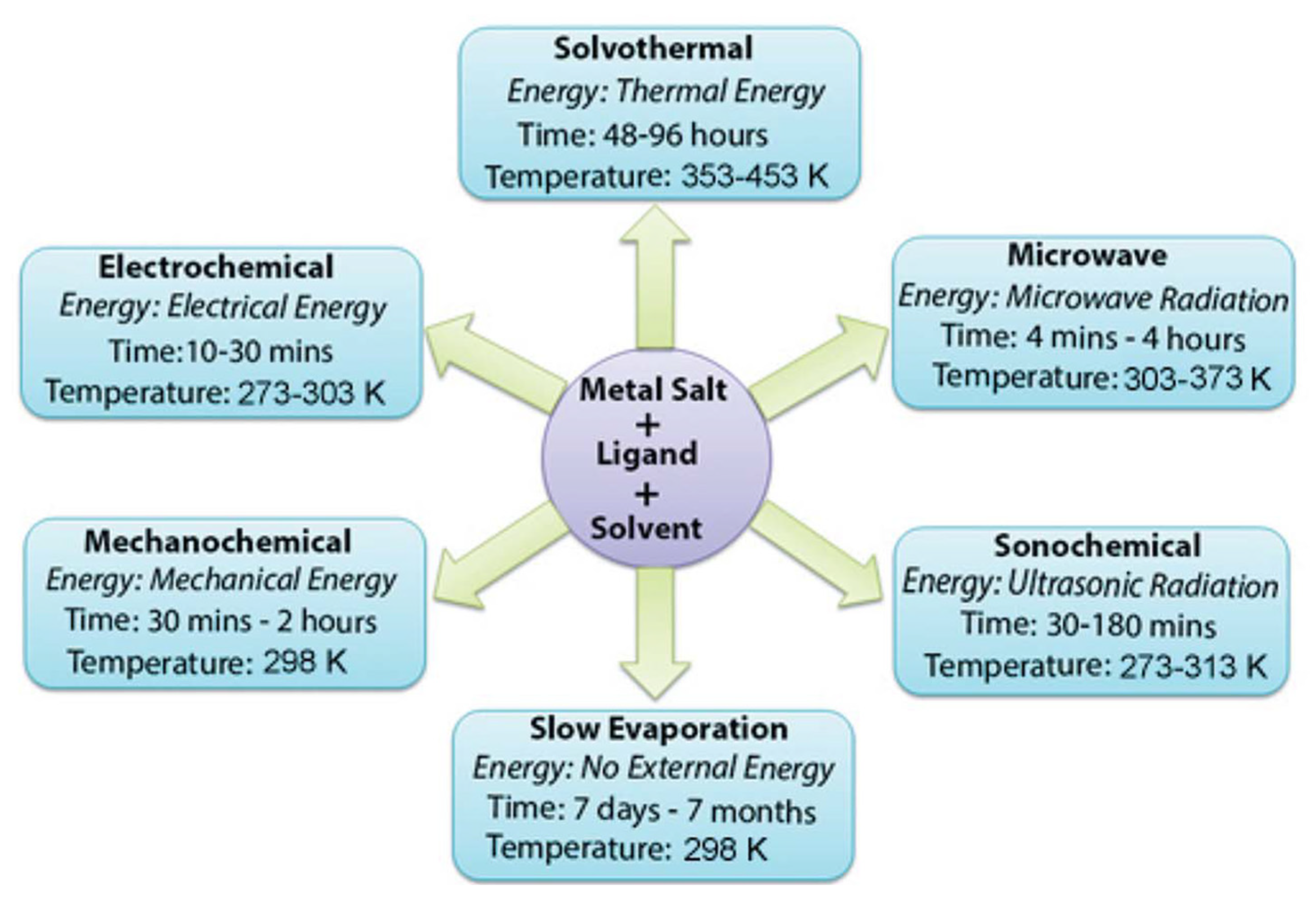
3. Main Synthesis Methods for MOFs
3.1. Typical Synthesis Methods
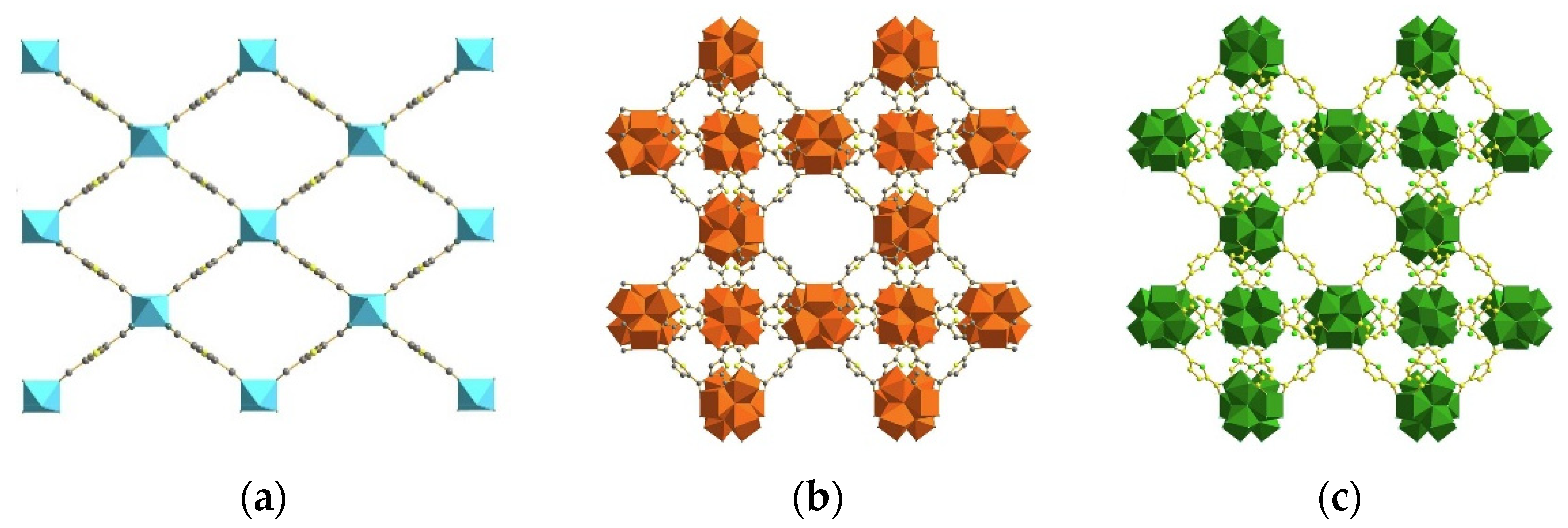
3.2. Reviewing Synthesis of MOFs Materials Applied for the Removal of Fluoride in Recent Literature
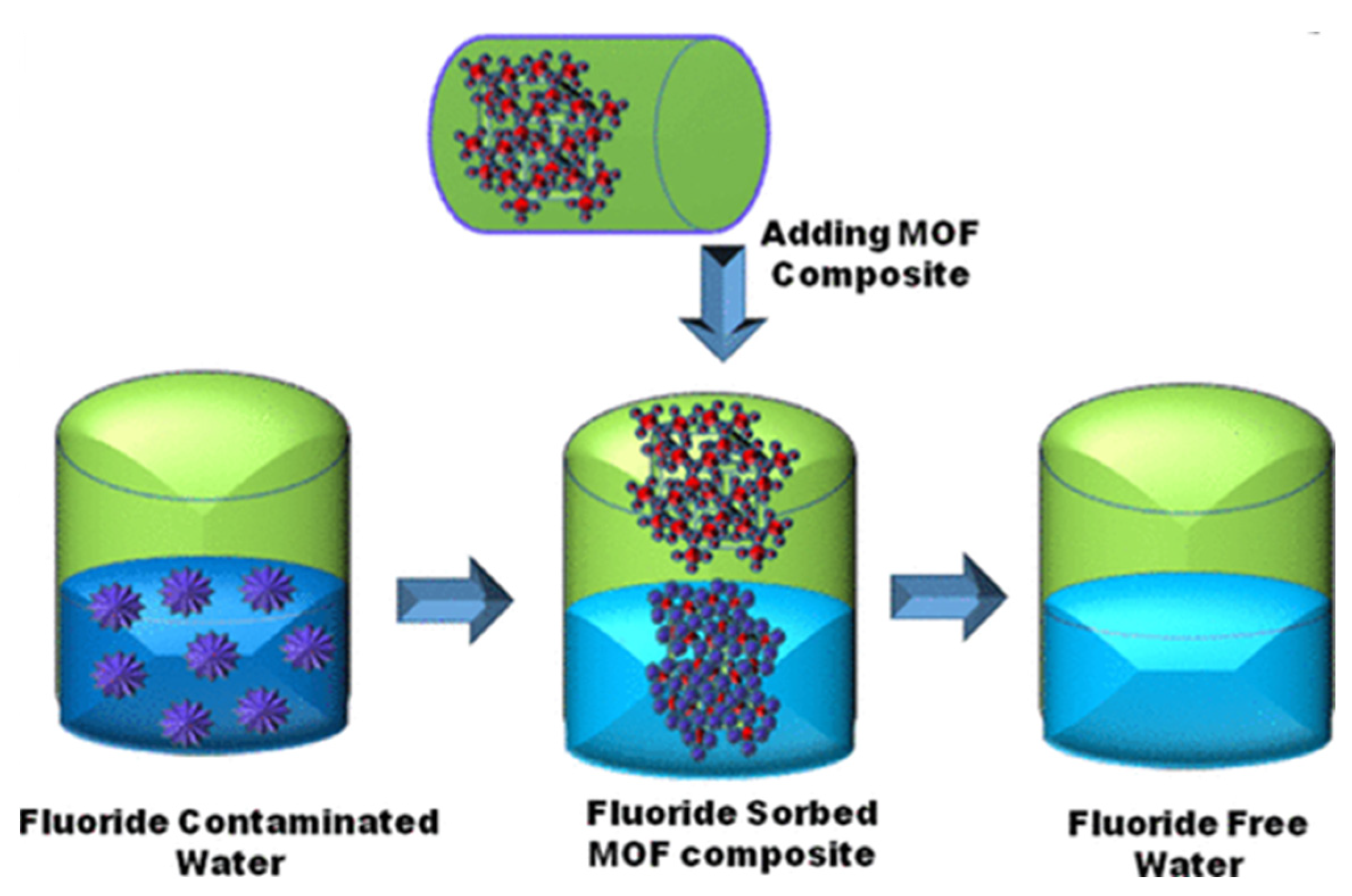
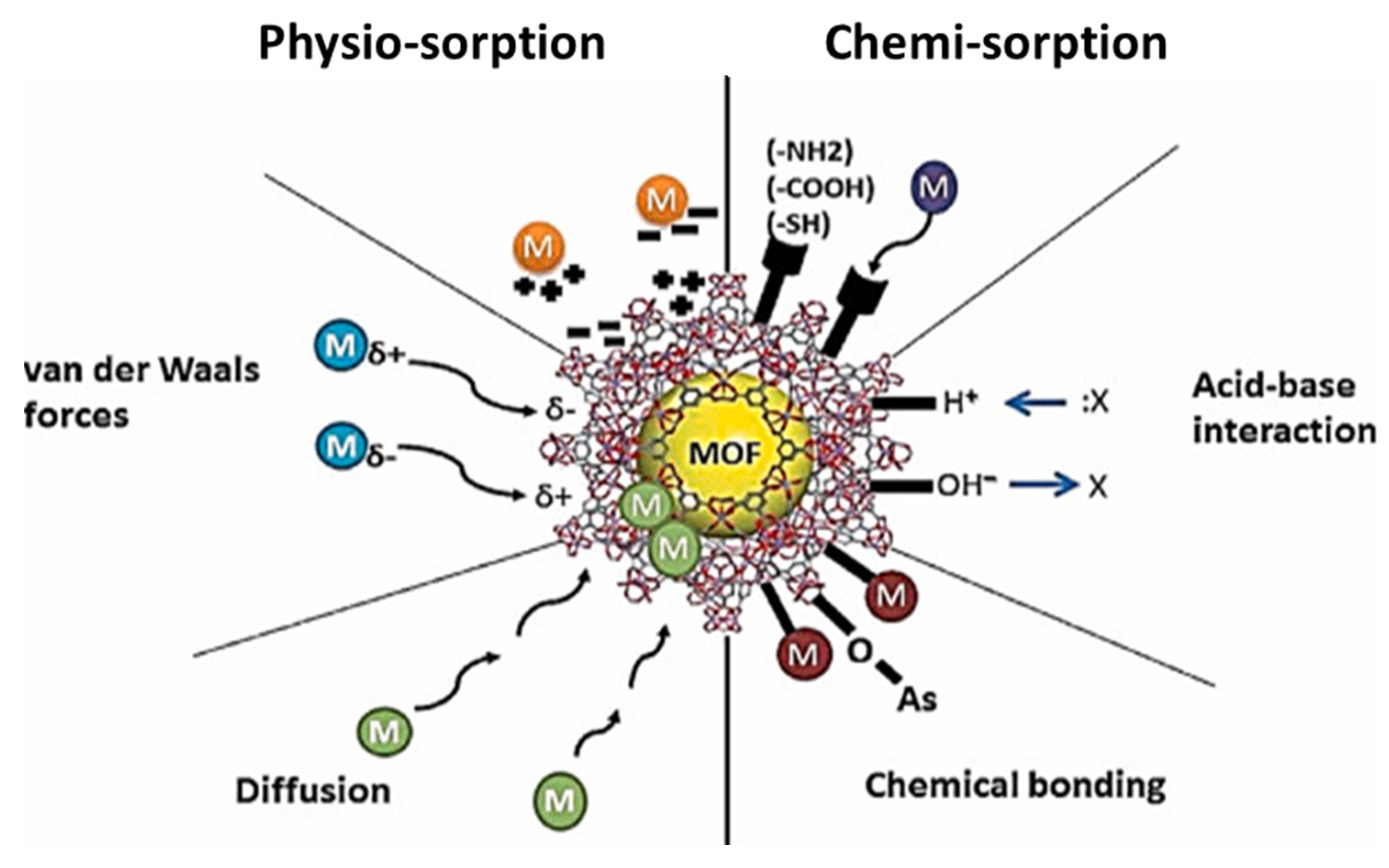
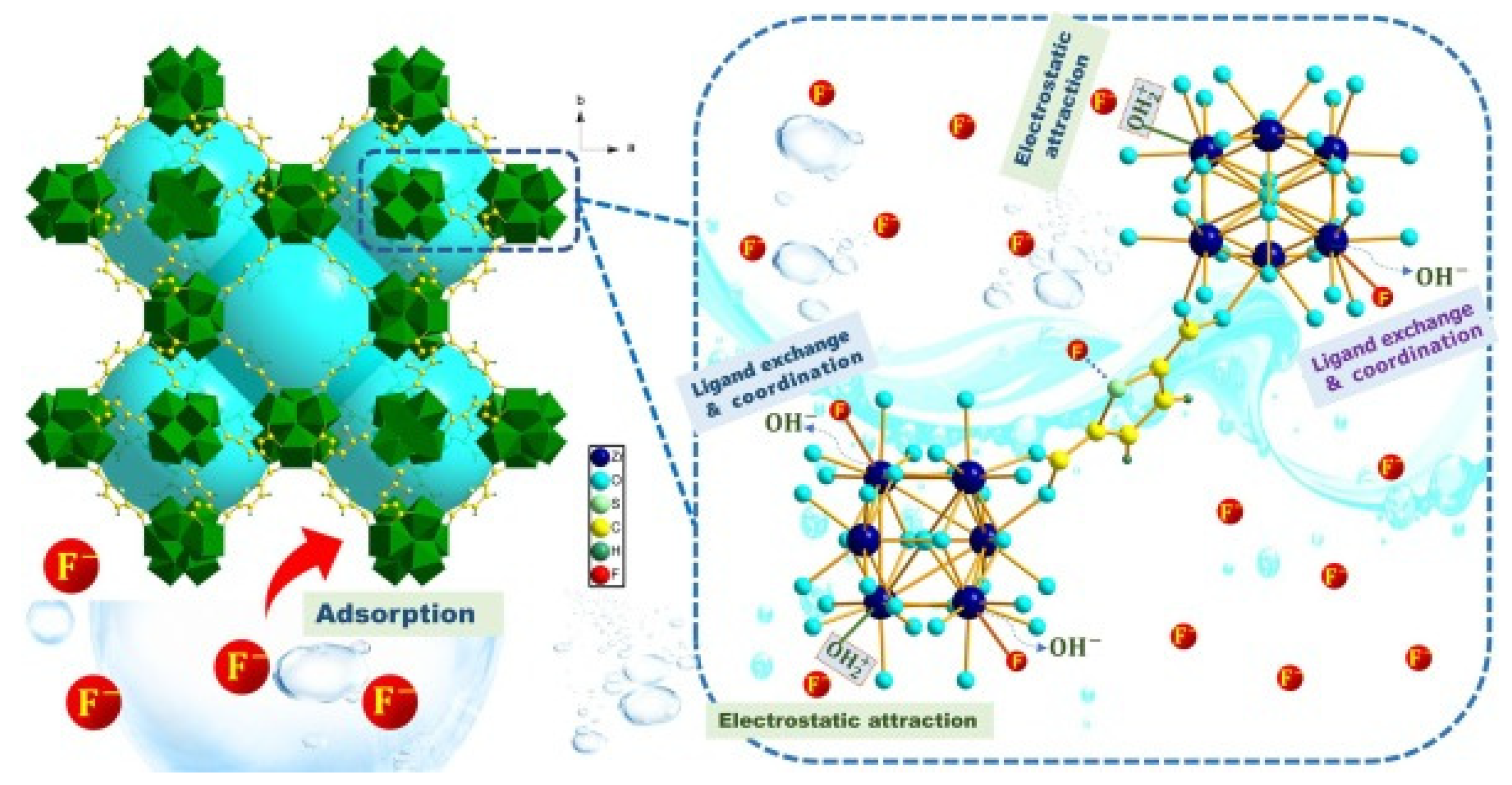
4. Application of MOFs for the Removal of Fluoride Anions
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, C.; Jin, J.; Wang, J.; Zhang, F.; Tian, Y.; Liu, C.; Zhang, F.; Cao, L.; Zhou, Y.; Han, Q. Metal–organic frameworks (MOFs) for the efficient removal of contaminants from water: Underlying mechanisms, recent advances, challenges, and future prospects. Coord. Chem. Rev. 2022, 468, 214595. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, C.; Xia, W.; Liang, Y.; Zeng, Y.; Zhao, X.; Xiong, W.; Cheng, M.; Wang, Z. Recent advances in metal–organic framework-based materials for removal of fluoride in water: Performance, mechanism, and potential practical application. Chem. Eng. J. 2022, 446, 137299. [Google Scholar] [CrossRef]
- Vishwas, H.; Chatterjee, R.; Majumder, C. Removal of fluoride from contaminated water by metal organic framework adsorbent—Review. J. Indian Chem. Soc. 2020, 97, 2854–2858. [Google Scholar]
- WHO. World Health Organization European Standards for Drinking-Water. Am. J. Med. Sci. 1970, 242, 56. [Google Scholar]
- Herschy, R.W. Water quality for drinking: WHO guidelines. Encycl. Earth Sci. Ser. 2012, 242, 876–883. [Google Scholar] [CrossRef]
- Singh, J.; Singh, P.; Singh, A. Fluoride ions vs. removal technologies: A study. Arab. J. Chem. 2016, 9, 815–824. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, J.; Jiang, W.; Kang, J.; Hu, Y.; Ngo, H.H.; Guo, W.; Liu, Y. Fluoride removal from water using a magnesia-pullulan composite in a continuous fixed-bed column. J. Environ. Manag. 2018, 206, 929–937. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Sun, N.; Sun, W.; Hu, Y.; Tang, H. Precipitation methods using calcium-containing ores for fluoride removal in wastewater. Minerals 2019, 9, 511. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Roy, A.; Sarkar, S.; Ghosh, S.; Majumdar, S.; Chakraborty, S.; Mandal, S.; Mukhopadhyay, A.; Bandyopadhyay, S. Combination technology of ceramic microfiltration and reverse osmosis for tannery wastewater recovery. Water Resour. Ind. 2013, 3, 48–62. [Google Scholar] [CrossRef]
- Mohamed, A.; Valadez Sanchez, E.P.; Bogdanova, E.; Bergfeldt, B.; Mahmood, A.; Ostvald, R.V.; Hashem, T. Efficient fluoride removal from aqueous solution using zirconium-based composite nanofiber membranes. Membranes 2021, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Plattner, J.; Naidu, G.; Wintgens, T.; Vigneswaran, S.; Kazner, C. Fluoride removal from groundwater using direct contact membrane distillation (DCMD) and vacuum enhanced DCMD (VEDCMD). Sep. Purif. Technol. 2017, 180, 125–132. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Meez, E.; Kyzas, G.Z.; Torretta, V.; Collivignarelli, M.C.; Caccamo, F.M.; Deliyanni, E.A.; Katsoyiannis, I.A. A Mini Review of Recent Findings in Cellulose-, Polymer- and Graphene-Based Membranes for Fluoride Removal from Drinking Water. C 2021, 7, 74. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Mitrakas, M.; Katsoyiannis, I.A.; Ernst, M.; Zouboulis, A.I. Fluoride removal from water by composite Al/Fe/Si/Mg pre-polymerized coagulants: Characterization and application. Chemosphere 2019, 231, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Tolkou, A.K.; Manousi, N.; Zachariadis, G.A.; Katsoyiannis, I.A.; Deliyanni, E.A. Recently developed adsorbing materials for fluoride removal from water and fluoride analytical determination techniques: A review. Sustainability 2021, 13, 7061. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Trikkaliotis, D.G.; Kyzas, G.Z.; Katsoyiannis, I.A.; Deliyanni, E.A. Simultaneous Removal of As(III) and Fluoride Ions from Water Using Manganese Oxide Supported on Graphene Nanostructures (GO-MnO2). Sustainability 2023, 15, 1179. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Tolkou, A.K.; Al Musawi, T.J.; Mengelizadeh, N.; Mohebi, S.; Balarak, D. Fluoride Removal from Water by Using Green Magnetic Activated Carbon Derived from Canola Stalks. Water Air Soil Pollut. 2022, 233, 424. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Application of composite pre-polymerized coagulants for the treatment of high-strength industrial wastewaters. Water 2020, 12, 1258. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Trikalioti, S.; Makrogianni, O.; Trikkaliotis, D.G.; Deliyanni, E.A.; Kyzas, G.Z.; Katsoyiannis, I.A. Magnesium modified activated carbons derived from coconut shells for the removal of fluoride from water. Sustain. Chem. Pharm. 2023, 31, 100898. [Google Scholar] [CrossRef]
- Damte, E.M. Removal of Fluoride From Water Using Granular Aluminium Hydroxide: Adsorption in a Fixed-Bed Column Removal of Fluoride From Water Using Granular Aluminium Hydroxide: Adsorption in a Fixed-Bed Column. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2006. [Google Scholar]
- Wan, S.; Lin, J.; Tao, W.; Yang, Y.; Li, Y.; He, F. Enhanced Fluoride Removal from Water by Nanoporous Biochar-Supported Magnesium Oxide. Ind. Eng. Chem. Res. 2019, 58, 9988–9996. [Google Scholar] [CrossRef]
- Zhou, N.; Guo, X.; Ye, C.; Yan, L.; Gu, W.; Wu, X.; Zhou, Q.; Yang, Y.; Wang, X.; Cheng, Q. Enhanced fluoride removal from drinking water in wide pH range using La/Fe/Al oxides loaded rice straw biochar. Water Supply 2022, 22, 779–794. [Google Scholar] [CrossRef]
- Xuebin, L.; Chunhui, L.; Jin, T.; Puli, Z.; Bin, Z.; Duo, B. Simultaneous removal of fluoride and arsenic in geothermal water in Tibet using modified yak dung biochar as an adsorbent. R. Soc. Open Sci. 2018, 5, 181266. [Google Scholar] [CrossRef]
- Xu, N.; Li, S.; Li, W.; Liu, Z. Removal of Fluoride by Graphene Oxide/Alumina Nanocomposite: Adsorbent Preparation, Characterization, Adsorption Performance and Mechanisms. ChemistrySelect 2020, 5, 1818–1828. [Google Scholar] [CrossRef]
- Singh, N.; Kumari, S.; Goyal, N.; Khan, S. Al2O3/GO cellulose based 3D-hydrogel for efficient fluoride removal from water. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100444. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Zhang, Z.; Wu, D. Removal of fluoride from water using aluminum hydroxide-loaded zeolite synthesized from coal fly ash. J. Hazard. Mater. 2022, 421, 126817. [Google Scholar] [CrossRef] [PubMed]
- Geethamani, C.K.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V. Alkali-treated fly ash for the removal of fluoride from aqueous solutions. Desalin. Water Treat. 2014, 52, 3466–3476. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Aminabhavi, T.M.; Arjmand, M. Simultaneous detection and removal of fluoride from water using smart metal-organic framework-based adsorbents. Coord. Chem. Rev. 2021, 445, 214037. [Google Scholar] [CrossRef]
- Ma, A.; Ke, F.; Jiang, J.; Yuan, Q.; Luo, Z.; Liu, J.; Kumar, A. Two lanthanide-based metal-organic frameworks for highly efficient adsorption and removal of fluoride ions from water. CrystEngComm 2017, 19, 2172–2177. [Google Scholar] [CrossRef]
- Ma, D.; Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and highly selective separation of CO2/C2H2on a thulium-organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Liu, S.; Li, F.; Li, T.; Cao, W. High-performance ZnIn2S4/Ni(dmgH)2 for photocatalytic hydrogen evolution: Ion exchange construction, photocorrosion mitigation, and efficiency enhancement by photochromic effect. J. Colloid Interface Sci. 2023, 642, 100–111. [Google Scholar] [CrossRef]
- Singh, N.; Dhillon, A.; Kumar, D. Metal-organic frameworks for adsorption of fluoride for groundwater treatment. Groundw. Sustain. Dev. 2023, 23, 100967. [Google Scholar] [CrossRef]
- Karmakar, S.; Bhattacharjee, S.; De, S. Experimental and modeling of fluoride removal using aluminum fumarate (AlFu) metal organic framework incorporated cellulose acetate phthalate mixed matrix membrane. J. Environ. Chem. Eng. 2017, 5, 6087–6097. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Zhong, H.; Chen, M.; Zhou, C.; Xiong, W.; Shao, B.; Song, B. Heterogeneous Fenton-like catalyst for treatment of rhamnolipid-solubilized hexadecane wastewater. Chemosphere 2019, 236, 124387. [Google Scholar] [CrossRef] [PubMed]
- Rego, R.M.; Kuriya, G.; Kurkuri, M.D.; Kigga, M. MOF based engineered materials in water remediation: Recent trends. J. Hazard. Mater. 2021, 403, 123605. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, D.; Cheng, M.; Liu, Z.; Lai, C.; Zhang, C.; Zhou, C.; Xiong, W.; Qin, L.; Shao, B.; et al. Metal sulfide/MOF-based composites as visible-light-driven photocatalysts for enhanced hydrogen production from water splitting. Coord. Chem. Rev. 2020, 409, 213220. [Google Scholar] [CrossRef]
- Karmakar, S.; Dechnik, J.; Janiak, C.; De, S. Aluminium fumarate metal-organic framework: A super adsorbent for fluoride from water. J. Hazard. Mater. 2016, 303, 10–20. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.R.; Arjmand, M. UiO-66 metal–organic frameworks in water treatment: A critical review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- He, J.; Cai, X.; Chen, K.; Li, Y.; Zhang, K.; Jin, Z.; Meng, F.; Liu, N.; Wang, X.; Kong, L.; et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal. J. Colloid Interface Sci. 2016, 484, 162–172. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Fedin, V.P. Main Approaches to the Synthesis of Heterometallic Metal-Organic Frameworks. Russ. J. Coord. Chem. Khimiya 2020, 46, 443–457. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-Metal MOFs: Unique Opportunities in Metal–Organic Framework (MOF) Functionality and Design. Angew. Chemie-Int. Ed. 2019, 58, 15188–15205. [Google Scholar] [CrossRef]
- Castells-Gil, J.; Padial, N.M.; Almora-Barrios, N.; Gil-San-Millán, R.; Romero-Ángel, M.; Torres, V.; da Silva, I.; Vieira, B.C.J.; Waerenborgh, J.C.; Jagiello, J.; et al. Heterometallic Titanium-Organic Frameworks as Dual-Metal Catalysts for Synergistic Non-buffered Hydrolysis of Nerve Agent Simulants. Chem 2020, 6, 3118–3131. [Google Scholar] [CrossRef]
- Trousselet, F.; Archereau, A.; Boutin, A.; Coudert, F.X. Heterometallic metal-organic frameworks of MOF-5 and UiO-66 families: Insight from computational chemistry. J. Phys. Chem. C 2016, 120, 24885–24894. [Google Scholar] [CrossRef]
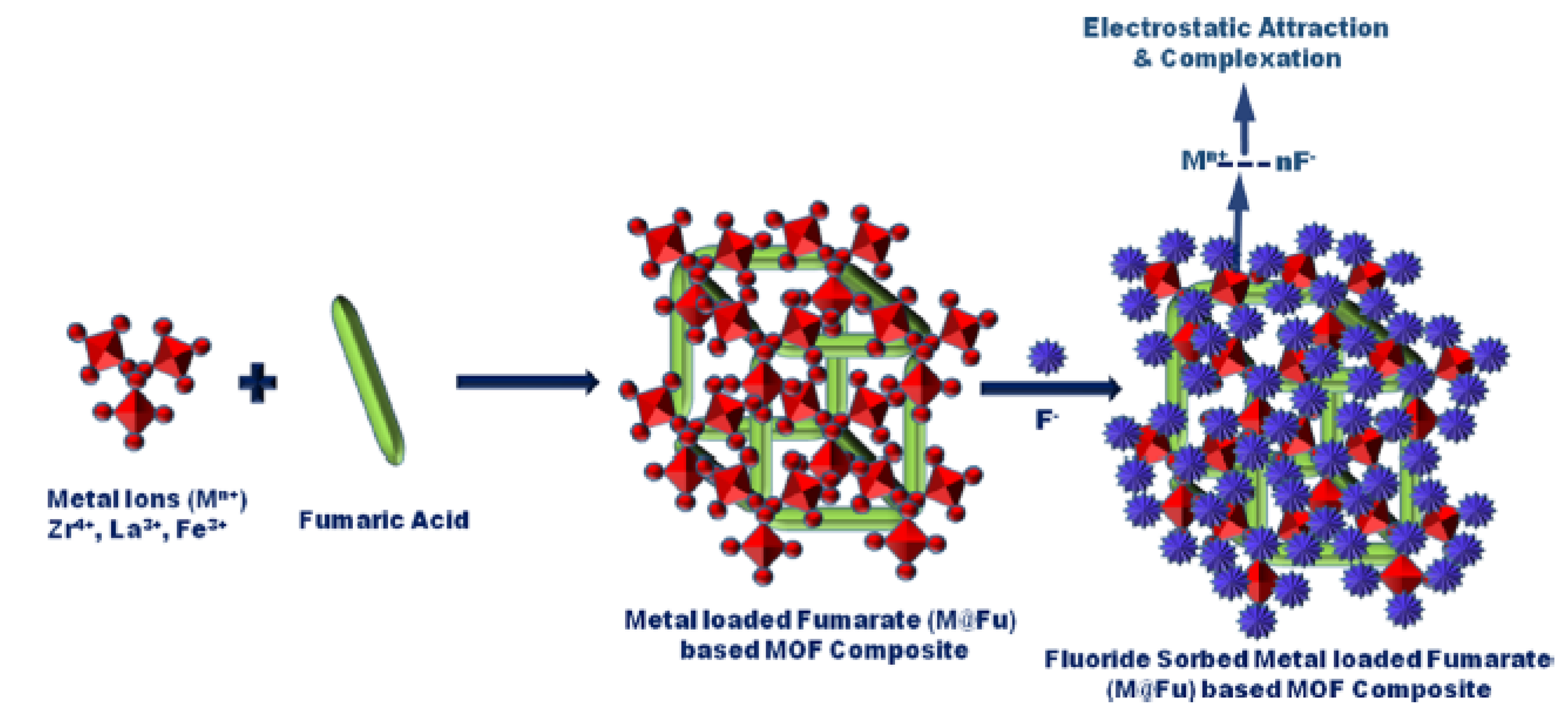
- Jeyaseelan, A.; Naushad, M.; Viswanathan, N. Development of Multivalent Metal-Ion-Fabricated Fumaric Acid-Based Metal-Organic Frameworks for Defluoridation of Water. J. Chem. Eng. Data 2020, 65, 2990–3001. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Vithanage, M.; Sarmah, A.; An, T.; Kim, K.H.; Ok, Y.S. Performance of metal-organic frameworks for the adsorptive removal of potentially toxic elements in a water system: A critical review. RSC Adv. 2019, 9, 34359–34376. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline metal-Organic frameworks (MOFs): Synthesis, structure and function. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Vemuri, R.S.; Kutnyakov, I.; McGrail, B.P.; Motkuri, R.K. An Efficient Synthesis Strategy for Metal-Organic Frameworks: Dry-Gel Synthesis of MOF-74 Framework with High Yield and Improved Performance. Sci. Rep. 2016, 6, 28050. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, M. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. In Electrochemical Applications of Metal-Organic Frameworks; Elsevier: Amsterdam, The Netherlands, 2022; pp. 17–35. [Google Scholar] [CrossRef]
- Bedia, J.; Muelas-Ramos, V.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodríguez, J.J.; Belver, C. A review on the synthesis and characterization of metal organic frameworks for photocatalytic water purification. Catalysts 2019, 9, 52. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Babu Arulmani, S.R.; Yan, J.; Li, Q.; Tang, J.; Wan, K.; Zhang, H.; Xiao, T.; Shao, M. Research progress of metal organic frameworks and their derivatives for adsorption of anions in water: A review. Environ. Res. 2022, 204, 112381. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Thünemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical synthesis of metal-organic frameworks: A fast and facile approach toward quantitative yields and high specific surface areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, F.; Qu, G.; Ren, N.; Ning, P.; Chen, X.; He, M.; Yang, Y.; Wang, Z.; Hu, Y. Extraction and preparation of metal organic frameworks from secondary aluminum ash for removal mechanism study of fluoride in wastewater. J. Mater. Res. Technol. 2023, 23, 3023–3034. [Google Scholar] [CrossRef]
- Wang, X.; Liu, K.; Zhu, H.; Sun, T.; Han, T.; Li, J.; Dai, H. An effective and selective stable metal-organic framework adsorben(Al-mof-5) for the removal of fluoride from water. Desalin. Water Treat. 2021, 216, 220–231. [Google Scholar] [CrossRef]
- Aliakbari, M.; Gholami, R.M.; Borghei, S.M. A zwitterion metal-organic framework for the removal of fluoride from an aqueous solution. J. Chem. Sci. 2022, 134, 74. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Wang, L.; Zhang, J. The simple synthesis of metal organic frameworks with high fluoride adsorption performance from water. J. Solid State Chem. 2022, 307, 122866. [Google Scholar] [CrossRef]
- Ghosh, A.; Das, G. Green synthesis of a novel water-stable Sn(ii)-TMA metal-organic framework (MOF): An efficient adsorbent for fluoride in aqueous medium in a wide pH range. New J. Chem. 2020, 44, 1354–1361. [Google Scholar] [CrossRef]
- Jeyaseelan, A.; Naushad, M.; Ahamad, T.; Viswanathan, N. Design and development of amine functionalized iron based metal organic frameworks for selective fluoride removal from water environment. J. Environ. Chem. Eng. 2021, 9, 104563. [Google Scholar] [CrossRef]
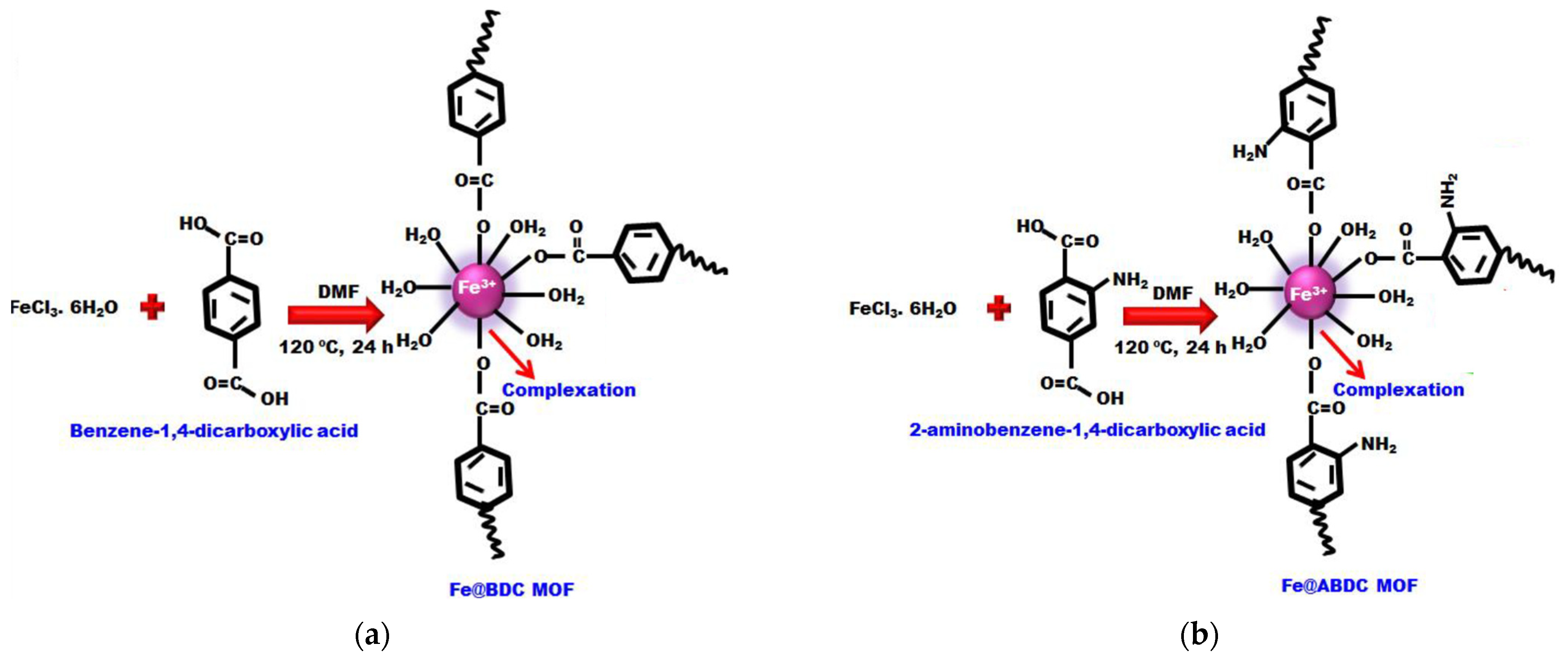
- Jeyaseelan, A.; Naushad, M.; Ahamad, T.; Viswanathan, N. Fabrication of amino functionalized benzene-1,4-dicarboxylic acid facilitated cerium based metal organic frameworks for efficient removal of fluoride from water environment. Environ. Sci. Water Res. Technol. 2021, 7, 384–395. [Google Scholar] [CrossRef]
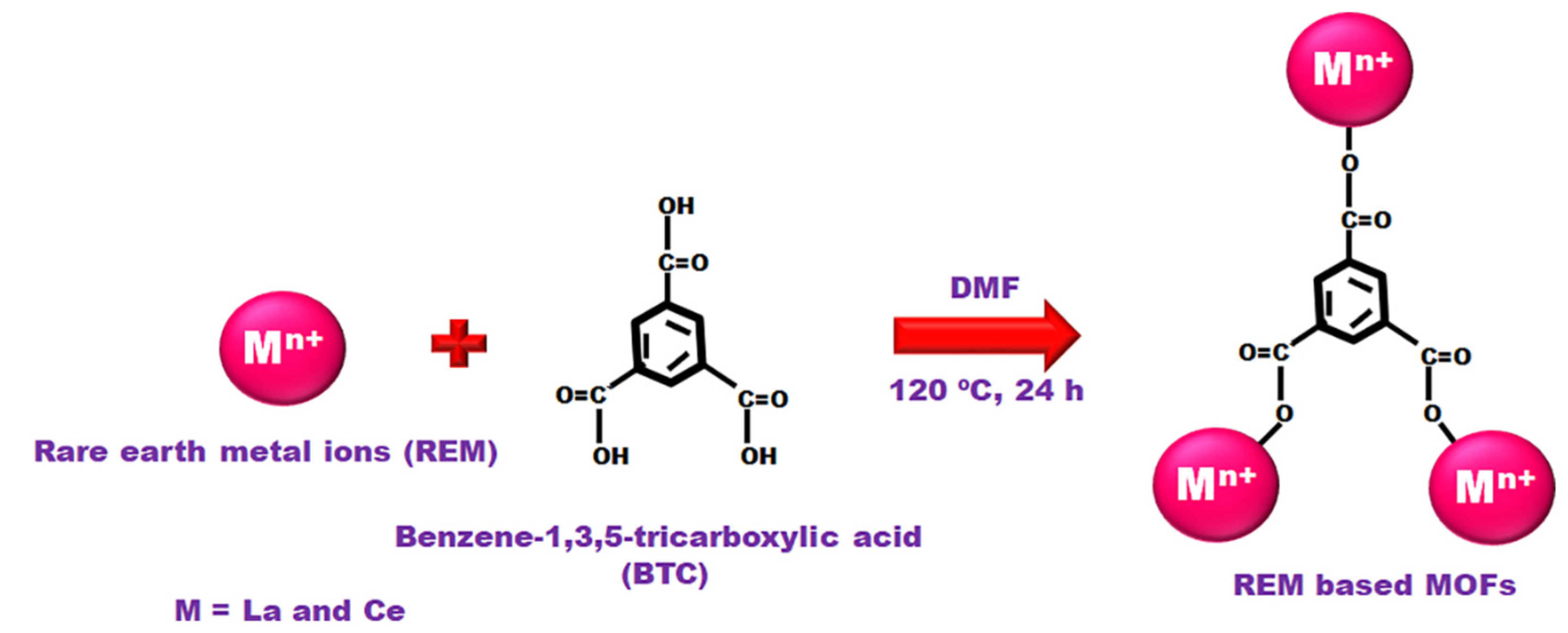
- Jeyaseelan, A.; Viswanathan, N. Facile synthesis of tunable rare earth based metal organic frameworks for enhanced fluoride retention. J. Mol. Liq. 2021, 326, 115163. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Q.; Zhu, G.; Liu, L.; Li, S.; Yang, X.; Wang, S. High-performance lanthanum-based metal–organic framework with ligand tuning of the microstructures for removal of fluoride from water. J. Colloid Interface Sci. 2022, 607, 1762–1775. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dhak, P.; Dhak, D. The solvothermal synthesis of a 3D rod-like Fe–Al bimetallic metal–organic-framework for efficient fluoride adsorption and photodegradation of water-soluble carcinogenic dyes. Environ. Sci. Adv. 2022, 1, 121–137. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, L.; Zhu, G.; Chen, D.; Ma, X.; Yang, X.; Wang, S. Outstanding performance of thiophene-based metal-organic frameworks for fluoride capture from wastewater. Sep. Purif. Technol. 2022, 298, 121567. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, A.; Sarkar, S.; Ghosh, T.K.; Basu, S. Zirconium fumarate metal-organic framework: A selective adsorbent for fluoride from industrial wastewater. Water Pract. Technol. 2023, 18, 1074–1085. [Google Scholar] [CrossRef]
- Zhu, X.H.; Yang, C.X.; Yan, X.P. Metal-organic framework-801 for efficient removal of fluoride from water. Microporous Mesoporous Mater. 2018, 259, 163–170. [Google Scholar] [CrossRef]
- Tan, T.L.; Krusnamurthy, P.A.; Nakajima, H.; Rashid, S.A. Adsorptive, kinetics and regeneration studies of fluoride removal from water using zirconium-based metal organic frameworks. RSC Adv. 2020, 10, 18740–18752. [Google Scholar] [CrossRef] [PubMed]
- Massoudinejad, M.; Shahsavani, A.; Kamarehie, B.; Jafari, A.; Ghaderpoori, M.; Amini, M.M.; Ghaderpoury, A. Highly efficient adsorption of fluoride from aqueous solutions by metal organic frameworks: Modeling, isotherms, and kinetics. Fluoride 2018, 51, 355–365. [Google Scholar]
- Lacalamita, D.; Hoyez, G.; Mongioví, C.; Ponchel, A.; Morin-Crini, N.; Rousseau, C.; Loup, C.; Rousseau, J.; Raschetti, M.; Monflier, E.; et al. Efficient removal of fluoride ions present in industrial effluents using metal-organic frameworks of UiO-66-NH2. J. Water Process Eng. 2023, 53, 103791. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Dai, Z.; Lei, Y.; Liu, X.; Liu, G. Simple preparation and efficient fluoride removal of la anchored Zr-based metal-organic framework adsorbent. J. Environ. Chem. Eng. 2022, 10, 108807. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Sun, T.; Dai, H. Synthesis of a Matériaux Institut Lavoisier metal-organic framework 96 (MIL-96(RM)) using red mud and its application to defluorination of water. Mater. Today Commun. 2020, 25, 101401. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Niazi, Z.; Heidari, K.; Afarinandeh, A.; Samadi Kazemi, M.; Haghighat, G.A.; Vasseghian, Y.; Rezania, S.; Barghi, A. Nickel and iron-based metal-organic frameworks for removal of organic and inorganic model contaminants. Environ. Res. 2022, 212, 113164. [Google Scholar] [CrossRef]
- Massoudinejad, M.; Ghaderpoori, M.; Shahsavani, A.; Amini, M.M. Adsorption of fluoride over a metal organic framework Uio-66 functionalized with amine groups and optimization with response surface methodology. J. Mol. Liq. 2016, 221, 279–286. [Google Scholar] [CrossRef]
- Hossien Saghi, M.; Chabot, B.; Rezania, S.; Sillanpää, M.; Akbar Mohammadi, A.; Shams, M.; Alahabadi, A. Water-stable zirconium and iron-based metal-organic frameworks (MOFs) as fluoride scavengers in aqueous medium. Sep. Purif. Technol. 2021, 270, 118645. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Kodali, J.; Mittal, S.; Samhith, G.D.; Rajesh, N. Probing the Interaction between Fluoride and the Polysaccharides in Al(III)- and Zr (IV)-Modified Tea Waste by Using Diverse Analytical Characterization Techniques. ChemistrySelect 2017, 2, 10123–10135. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. Aluminium hydroxide impregnated macroreticular aromatic polymeric resin as a sustainable option for defluoridation. J. Environ. Chem. Eng. 2015, 3, 630–641. [Google Scholar] [CrossRef]
- Barathi, M.; Krishna Kumar, A.S.; Kumar, C.U.; Rajesh, N. Graphene oxide-aluminium oxyhydroxide interaction and its application for the effective adsorption of fluoride. RSC Adv. 2014, 4, 53711–53721. [Google Scholar] [CrossRef]
- Wu, T.; Mao, L.; Wang, H. Adsorption of fluoride from aqueous solution by using hybrid adsorbent fabricated with Mg/Fe composite oxide and alginate via a facile method. J. Fluor. Chem. 2017, 200, 8–17. [Google Scholar] [CrossRef]



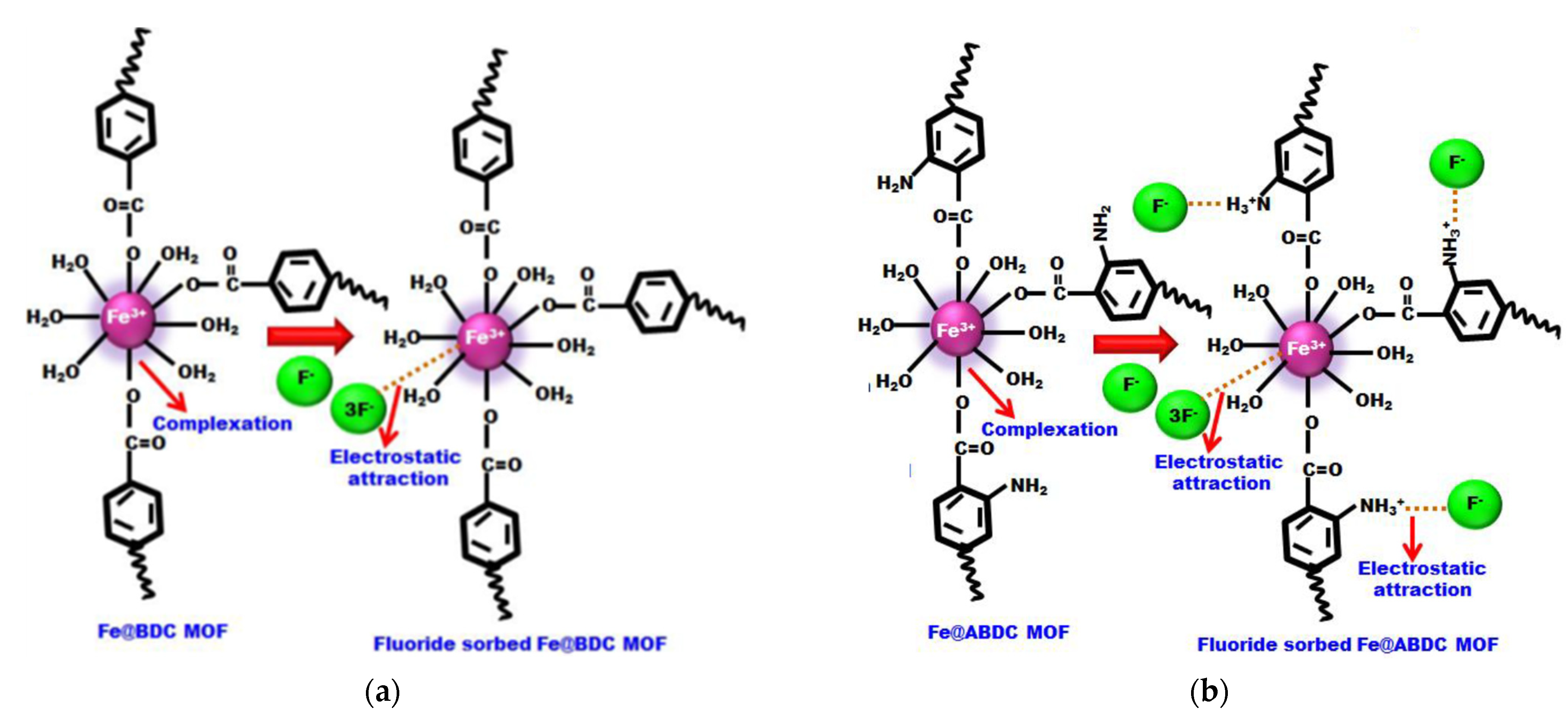
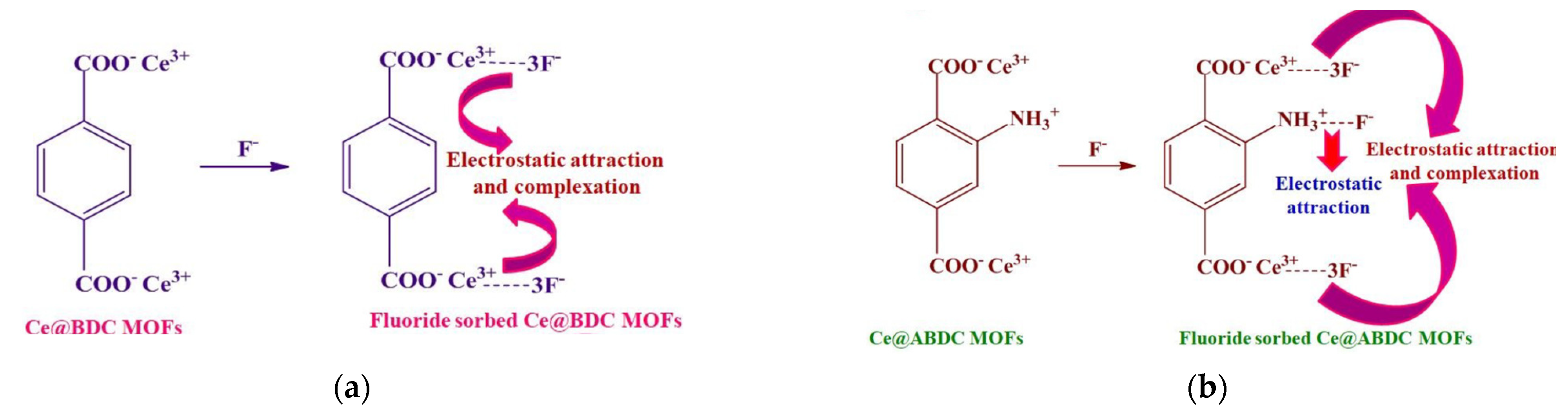
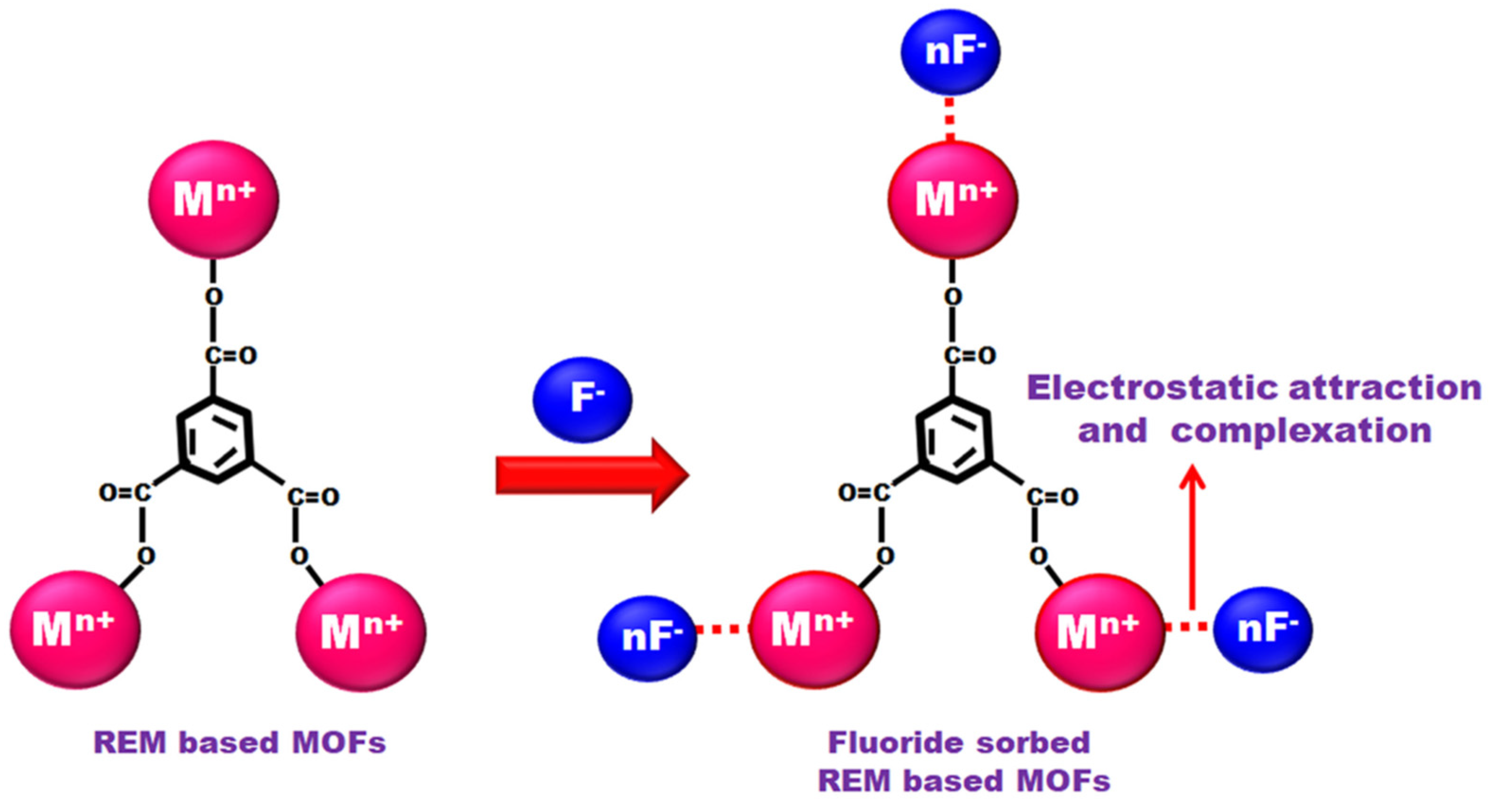

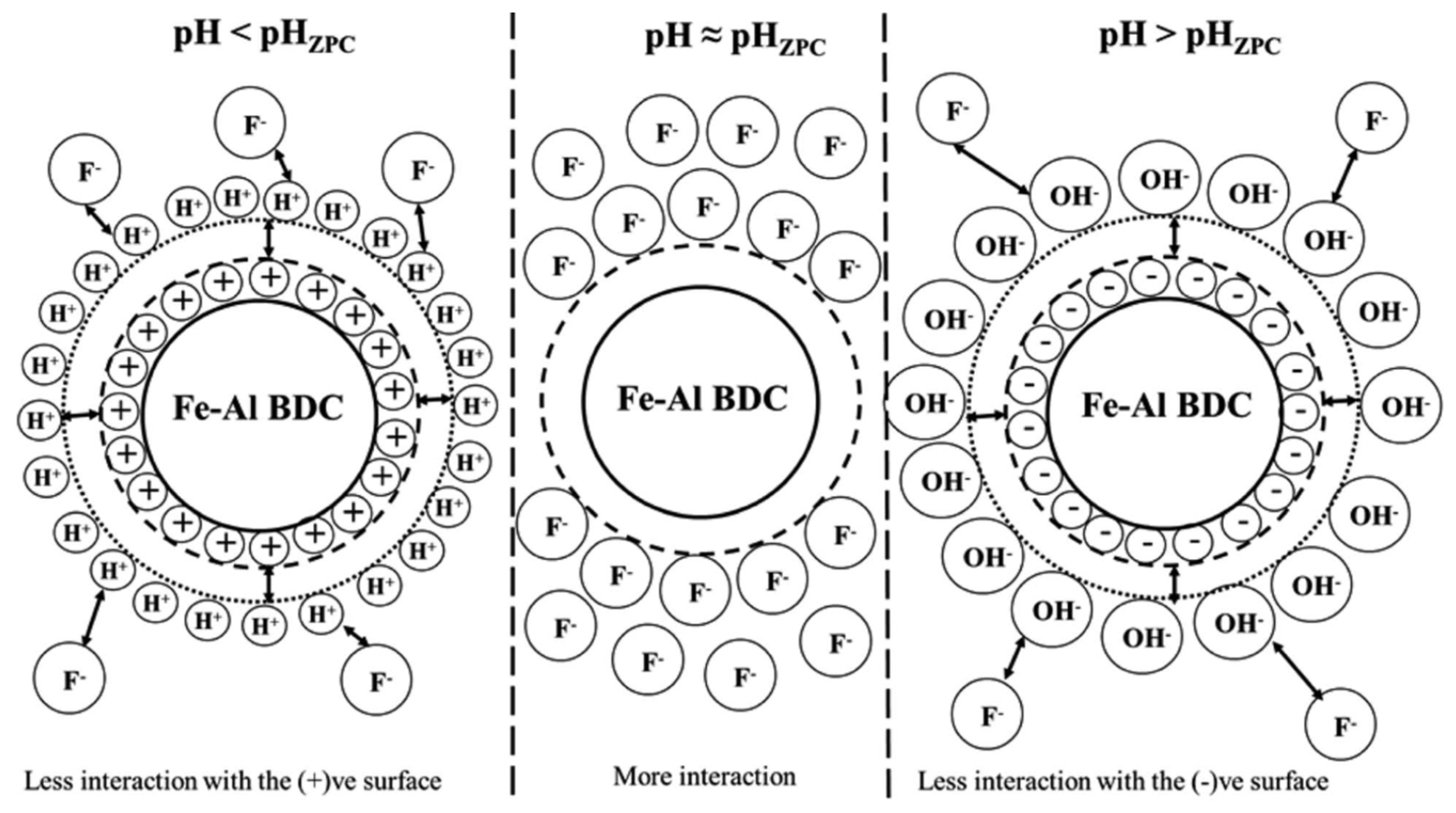

| Types of MOFs | Characteristics |
|---|---|
| The MILs Family (Materiaux de I’ Institut Lavoisier) | The MIL-53(Fe) and MIL-53(Cr) materials have shown lower adsorption capacity than the MIL-96 (Al). |
| The UiO-66 family (University of Oslo) | Have a basic framework of zirconium cluster that can attract the fluoride ions. They are best matched with the amine functional group for improving the adsorption of fluoride. |
| The MOF-801 family | They have a basic framework of an octahedral zirconium cluster that can attract fluoride ions together with the ion-exchange mechanism of the cluster’s hydroxyl groups. |
| The Metal-ion seeded MOFs | Used metals can be, e.g., cerium, zirconium, hafnium, etc.; as zirconium and hafnium present a similar electronic structure, UiO-66(Zr) and UiO-66(Hf) show related removal mechanisms. Aluminum can be also inserted inside the MOF, as this metal ion will attract the fluoride ions via electrostatic interactions due to the high electronegativity of fluoride anions. |
| The ZIFs Family | The zeolite imidazolate frameworks-7,8,9 show good fluoride adsorption, while not presenting higher efficiency, than the other adsorbents. |
| MOFs | Synthetic Method | BET Surface Area, SBET (m2/g) | Average Pore Size (nm) | Total Pore Volume, VT (cm3/g) | Ref. |
|---|---|---|---|---|---|
| AlFu | Hydrothermal | 1156.0 | 1.7 | NR 1 | [38] |
| b-CD@AlFuMoF | Hydrothermal | 779.2 | 5.6 | 0.36 | [53] |
| Al-MOF-5 | Hydrothermal | 1264.0 | 3.1 | NR 1 | [54] |
| Zr@Fu | Hydrothermal | NR 1 | NR 1 | NR 1 | [45] |
| La@Fu | Hydrothermal | NR 1 | NR 1 | NR 1 | |
| Fe@Fu | Hydrothermal | NR 1 | NR 1 | NR 1 | |
| [Ce(L1)0.5(NO3)(H2O)2]·2DMF | NR 1 | NR 1 | NR 1 | NR 1 | [29] |
| [Eu3(L2)2(OH)(DMF)0.22(H2O)5.78]·guest | NR 1 | NR 1 | NR 1 | NR 1 | |
| MOF1 ({[Zn3L3(BPE)1.5]∙4.5DMF}n) | Solvothermal | 270.3 | NR 1 | 0.15 | [55] |
| MOF1 | NR 1 | NR 1 | 10–20 | NR 1 | [56] |
| Sn(II)-TMA | Solvothermal | 360.8 | 4.0 | 0.46 | [57] |
| Fe@BDC | Hydrothermal | 53.7 | 9.0 | 0.14 | [58] |
| Fe@ABDC | Hydrothermal | 68.8 | 4.2 | 0.42 | |
| Ce@BDC | Hydrothermal | NR 1 | NR 1 | NR 1 | [59] |
| Ce@ABDC | Hydrothermal | NR 1 | NR 1 | NR 1 | |
| La@BTC | Hydrothermal | NR 1 | NR 1 | NR 1 | [60] |
| La-BTC | Solvothermal | 2.4 | 18.1 | 0.01 | [61] |
| Fe-Al BDC | Solvothermal | 120.3 | 1.4 | NR 1 | [62] |
| Al-TDC | Hydrothermal | 1251.7 | 1.3 | 0.87 | [63] |
| Ce-TDC | Hydrothermal | 859.7 | 1.7 | 0.36 | |
| Zr-TDC | Hydrothermal | 923.3 | 1.4 | 0.37 | |
| ZrFu MOF | NR 1 | 537.5 | 1.8 | 0.23 | [64] |
| Zn- MOF-801 | Hydrothermal | 725.0 | 0.1 | 0.40 | [65] |
| Zn- MOF-801 | Solvothermal | 522.0 | 0.3 | 1.51 | [66] |
| Uio-66 | NR 1 | NR 1 | NR 1 | NR 1 | [67] |
| UiO-66-NH2 | Hydrothermal | 945.0 | 2.0 | NR 1 | [68] |
| La-UiO-66-(COOH)2 | Solvothermal | 80.3 | 1.3–2.2 | 0.15 | [69] |
| MIL-96(RM) | Hydrothermal | NR 1 | NR 1 | NR 1 | [70] |
| MIL-53 (Fe) | NR 1 | 51.3 | NR 1 | NR 1 | [71] |
| MOFs | [F]o (mg/L) | Dosage (g/L) | pHinit | Contact Time (min) | Adsorption Capacity (mg/g) | 1 ΔH0 (kJ/mol) | Recycling Cycles | Ref. |
|---|---|---|---|---|---|---|---|---|
| AlFu | 30.0 | 0.75 | 7.0 | 60 | 600 | −32.06 | NR 2 | [38] |
| b-CD@AlFuMoF | 30.0 | 0.75 | 2.0 | 120 | 39.95 | −140.84 | 7 | [53] |
| Al-MOF-5 | 10.0 | 1.0 | 7.0 | 120 | 46.08 | 21.30 | 5 | [54] |
| Zr@Fu | 10.0 | 0.1 | 7.0 | 30 | 4.92 | 0.37 | 6 | [45] |
| La@Fu | 10.0 | 0.1 | 7.0 | 30 | 4.93 | 0.45 | 6 | |
| Fe@Fu | 10.0 | 0.1 | 7.0 | 30 | 4.85 | 0.31 | 6 | |
| [Ce(L1)0.5(NO3)(H2O)2]·2DMF | 12.5 | 2.0 | 3.0–7.0 | 120 | 103.95 | 18.52 | NR 2 | [29] |
| [Eu3(L2)2(OH)(DMF)0.22(H2O)5.78]·guest | 12.5 | 2.0 | 3.0–7.0 | 120 | 57.01 | 25.32 | NR 2 | |
| MOF1 ({[Zn3L3(BPE)1.5]∙4.5DMF}n) | NR 2 | NR 2 | 7.0 | 20 | NR 2 | NR 2 | 5 | [55] |
| MOF1 | 10.0 | 0.2 | 3.0–11.0 | 30 | 240 | NR 2 | 7 | [56] |
| Sn(II)-TMA | 12.0 | 1.0 | 3.0–10.0 | 150 | 30.86 | 10.1 | NR 2 | [57] |
| Fe@BDC | 10.0 | 0.1 | 7.0 | 30 | 4.90 | 0.56 | 6 | [58] |
| Fe@ABDC | 10.0 | 0.1 | 7.0 | 30 | 4.92 | 1.24 | 6 | |
| Ce@BDC | 10.0 | 0.1 | 7.0 | 30 | 4.88 | 0.38 | 6 | [59] |
| Ce@ABDC | 10.0 | 0.1 | 7.0 | 30 | 4.91 | 0.45 | 6 | |
| La@BTC | 10.0 | 0.1 | 7.85 | 30 | 4.98 | 0.58 | 6 | [60] |
| Al-TDC | 5.0 | 0.2 | 10.0–11.0 | 300 | 107.5 | −25.37 | 4 | [63] |
| Ce-TDC | 5.0 | 0.3 | 3.0–4.0 | 300 | 94.9 | −20.53 | 4 | |
| Zr-TDC | 5.0 | 0.3 | 3.0–4.0 | 300 | 97.0 | −21.18 | 4 | |
| Fe-Al BDC | 10.0 | 1.0 | 7.0 | 45 | NR 1 | NR 1 | NR 1 | [62] |
| La-BTC | 20.0 | 0.5 | 5.0 | 180 | 105.2 | 19.68 | 4 | [61] |
| La-BPDC | 20.0 | 0.25 | 5.0 | 180 | 125.9 | 35.94 | 4 | |
| La-BHTA | 20.0 | 0.15 | 5.0 | 180 | 145.5 | 25.66 | 4 | |
| La-PMA | 20.0 | 0.25 | 5.0 | 180 | 158.9 | 36.47 | 4 | |
| La-BDC | 20.0 | 0.15 | 5.0 | 180 | 171.7 | 30.22 | 4 | |
| ZrFu | 10.0 | 3.0 | 6.0 | 60 | 49.66 | NR 1 | 6 | [64] |
| Zn-MOF-801 | 10.0 | 0.7 | no pH adjusting | 40 | 40.0 | NR 2 | NR 2 | [65] |
| Zn-MOF-801 | 10.0 | 1.0 | NR 2 | 120 | 17.33 | NR 2 | 4 | [66] |
| Uio-66 | 14.6 | 0.4 | 7.0 | 41.5 | 31.09 | NR 2 | 5 | [67] |
| UiO-66-NH2 | 20.0 | 2.0 | 6.1 | 60 | 49.7 | NR 2 | NR 2 | [68] |
| La-UiO-66-(COOH)2 | 100.0 | 1.0 | 3.0 | 30 | 57.23 | 32.92 | 4 | [69] |
| MIL-96(RM) | 20.0 | 0.5 | 7.0 | 120 | 82.65 | 9.05 | 7 | [70] |
| MIL-53(Fe) | 10.0 | 0.25 | 4.0 | 60 | 4.34 | NR 2 | NR 2 | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolkou, A.K.; Zouboulis, A.I. Fluoride Removal from Water Sources by Adsorption on MOFs. Separations 2023, 10, 467. https://doi.org/10.3390/separations10090467
Tolkou AK, Zouboulis AI. Fluoride Removal from Water Sources by Adsorption on MOFs. Separations. 2023; 10(9):467. https://doi.org/10.3390/separations10090467
Chicago/Turabian StyleTolkou, Athanasia K., and Anastasios I. Zouboulis. 2023. "Fluoride Removal from Water Sources by Adsorption on MOFs" Separations 10, no. 9: 467. https://doi.org/10.3390/separations10090467








