GC-MS Analysis of Essential Oil and Volatiles from Aerial Parts of Peucedanum tauricum M.B. during the Phenological Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Chemicals
2.3. Hydrodistillation of the Essential Oil
2.4. HS-SPME Analysis of Plant Samples
2.5. Gas Chromatography-Mass Spectrometry
3. Results
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea. In Rosaceae to Umbelliferae; Cambridge University Press: Cambridge, UK, 1968; (reprint 1992); Volume 2, pp. 315–375. [Google Scholar]
- Kubeczka, K.-H.; Schmaus, G.; Schultze, W.; Ullmann, I. The essential root oil of Peucedanum lancifolium Lange and chemotaxonomic implications. Z. Naturfosch. C 1989, 44, 183–188. [Google Scholar] [CrossRef]
- Artur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Evolution of solid-phase microextraction technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, M.J.; Pawliszyn, J. Solid phase microextraction: A new solvent-free alternative for sample preparation. Anal. Chem. 1994, 66, 844A–853A. [Google Scholar] [CrossRef]
- Pawliszyn, J. New directions in sample preparation for analysis of organic compounds. Trends Anal. Chem. 1995, 14, 113–122. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Headspace solid phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Musci, M.; Mangia, A. Fish and food safety: Determination of formaldehyde in 12 fish species by SPME extraction and GC–MS analysis. Food Chem. 2007, 100, 1049–1053. [Google Scholar] [CrossRef]
- Richter, J.; Schellenberg, I. Comparison of different extraction methods for the determination of essential oils and related compounds from aromatic plants and optimization of solid-phase microextraction/gas chromatography. Anal. Bioanal. Chem. 2007, 387, 2207–2217. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Application of solid-phase micoextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef]
- Szyszkin, B.K. Flora USSR, Vol. XVII; USSR Academy of Sciences: Leningrad, Russia, 1951. [Google Scholar]
- Grossgiejm, A.A. Flora of the Caucasus, Vol. VII; Umbelliferae-Scrophulariaceae, Science Publishing House: Leningrad, Russia, 1967. [Google Scholar]
- Baranauskaite, D.I.; Nikonov, G.K.; Murav’eva, D.A. Peucedanin. Byul. Izobret. Tovarnyh. Znakov. 1963, 4, 158. [Google Scholar]
- Baranauskeite, D.I.; Nikonov, G.K. Chemical investigation of Peucedanum tauricum M.B. and Peucedanum calcareum Alb. Apotech. Delo 1965, 14, 25–28. [Google Scholar]
- Głowniak, K.; Bartnik, M.; Mroczek, T.; Zabża, A.; Wierzejska, A. Application of column chromatography and preparative TLC for isolation and purification of coumarins from Peucedanum tauricum Bieb. fruits. J. Planar Chromatogr. 2002, 15, 94–100. [Google Scholar] [CrossRef]
- Bartnik, M. Phytochemical Investigations of Aerial Parts of Peucedanum tauricum Bieb. Ph.D. Thesis, Department of Pharmacognosy, Medical University of Lublin, Lublin, Poland, 2004. [Google Scholar]
- Tesso, H.; König, W.A.; Kubeczka, K.-H.; Bartnik, M.; Głowniak, K. Secondary metabolites of Peucedanum tauricum fruits. Phytochemistry 2005, 66, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M.; Głowniak, K. Furanocoumarins from Peucedanum tauricum Bieb. and their variability in the aerial parts of the plant during development. Acta Chromatogr. 2007, 18, 5–14. [Google Scholar]
- Bartnik, M. Efficient Separation of the Methoxyfuranocoumarins Peucedanin, 8-Methoxypeucedanin, and Bergapten by Centrifugal Partition Chromatography (CPC). Molecules 2023, 28, 1923. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, M.; Głowniak, K.; Dul, R. Use of two-dimensional TLC to identify of phenolic acids in the foliage and fruit of Peucedanum tauricum Bieb. J. Planar Chromatogr. 2003, 16, 206–210. [Google Scholar] [CrossRef]
- Bartnik, M.; Głowniak, K.; Gromek, A. TLC and HPLC analysis of the flavonoid glycosides in the aerial parts of Peucedanum tauricum Bieb. J. Planar Chromatogr. 2007, 20, 127–130. [Google Scholar] [CrossRef]
- Bartnik, M.; Głowniak, K.; Mardarowicz, M. Essential oil from fruit of Peucedanum tauricum Bieb. Acta Pol. Pharm. Drug Res. 2002, 56, 457–459. [Google Scholar]
- Polish Ph, V.I. Polish Pharmacopoeia, 6th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 2002. [Google Scholar]
- Lancioni, C.; Castells, C.; Roberto Candal, R.; Tascon, M. Headspace solid-phase microextraction: Fundamentals and recent advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Worsfold, P.; Townshend, A.; Poole, C. (Eds.) Headspace Analysis/Static. In Encyclopedia of Analytical Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 229–236. ISBN 9780123693976. [Google Scholar] [CrossRef]
- Namiesnik, J.; Zygmunt, B.; Jastrzębska, A. Application of solid-phase microextraction for determination of organic vapours in gaseous matrices. J. Chrom. A 2000, 885, 405–518. [Google Scholar] [CrossRef]
- Banel, A.; Zygmunt, B. Application of combination of solid phase microextraction and gas chromatography for determination of volatile fatty acids in environmental and related samples. Ecol. Chem. Engin. S 2008, 15, 7–28. [Google Scholar]
- Joulein, D.; Koenig, W. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; EB-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing: Carol Stream, IL, USA, 2001. [Google Scholar]
- Boegelsack, N.; Sandau, C.; McMartin, D.W.; Withey, J.M.; O’Sullivan, G. Development of retention time indices for comprehensive multidimensional gas chromatography and application to ignitable liquid residue mapping in wildfire investigations. J. Chromatogr. A 2021, 1635, 461717. [Google Scholar] [CrossRef] [PubMed]
- Khruengsai, S.; Sripahco, T.; Rujanapun, N.; Charoensup, R.; Preepdeevech, P. Chemical composition and biological activity of Peucedanum dhana A. Ham essential oil. Sci. Rep. 2021, 11, 19079. [Google Scholar] [CrossRef] [PubMed]
- Silv, N.; Fortuna, A.; Cavaleiro, C. The essential oil from the fruits of P. oeroselinum (L.) Moench (Apiaceae) as a natural source of P-glycoprotein inhibitors. J. Herb. Med. 2021, 29, 100482. [Google Scholar] [CrossRef]
- Prats, E.; Llamas, M.J.; Jorrin, J.; Rubiales, D. Constitutive coumarin accumulation on Sunflower leaf surface prevents rust germ tube growth and appressorium differentiation. Crop. Sci. 2007, 47, 1119–1124. [Google Scholar] [CrossRef]
- Turkez, H.; Sozio, P.; Geyikoglu, F.; Tatar, A.; Hacimuftuoglu, A.; Di Stefano, A. Neuroprotective effects of farnesene against hydrogen peroxide-induced neurotoxicity in vitro. Cell. Mol. Neurobiol. 2014, 34, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Akpulat, H.A.; Sokmen, M. Evaluation of the chemical composition and antioxidant activity of the essential oils of Peucedanum longifolium (Waldst. & Kit.) and P. palimbioides (Boiss.). Rec. Nat. Prod. 2011, 5, 108–116. [Google Scholar]
- Masoudi, S.; Akhgar, M.R.; Rustaiyan, A. Essential oils of Peucedanum scoparium (Boiss.) Boiss and Serotinocarpum insignis Mozaffarian from Iran. J. Essent. Oil Res. 2004, 16, 117–119. [Google Scholar] [CrossRef]
- Figueredo, G.; Chalchat, J.-C.; Petrovic, S.; Maksimovic, Z.; Boza, P.; Radic, J. Composition of essential oils of flowers, leaves, stems and rhizome of Peucedanum officinale L. (Apiaceae). J. Essent. Oil Res. 2009, 21, 123–126. [Google Scholar] [CrossRef]
- Chizzola, R. Composition of the essential oils, from Peucedanum cervaria and P. alsaticum growing wild in the urban area of Vienna (Austria). Nat. Prod. Comm. 2012, 7, 1515–1518. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Łoś, R.; Głowniak, K.; Malm, A. Variation of the volatile content of the fruits of Peucedanum alsaticum L. Acta Chromatogr. 2008, 20, 119–133. [Google Scholar] [CrossRef]
- Motskute, D.; Nivinskene, O. Essential oil of Peucedanum oreoselinum fruits collected near Vilnius. Chem. Nat. Comp. 1999, 35, 635–637. [Google Scholar] [CrossRef]
- Domokos, J.; Palinkas, J.; Hethelyi, E.; Korany, K.; Peredi, J. Examination on volatile and fatty oils of the seed of broad-leaved spingel (Peucedanum cervaria L.). Acta Hortic. 2000, 532, 97–104. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Łoś, R.; Głowniak, K.; Malm, A. Volatile compounds in fruits of Peucedanum cervaria (Lap.) L. Chem. Biodiv. 2009, 6, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.M.; Deshmukh, V.K.; Saoji, A.N.; Parashar, V.V. Chemical examination of fruits and leaves of Peucedanum grande. J. Indian Chem. Soc. 1987, 64, 646–647. [Google Scholar]
- Fraternale, D.; Giamperi, L.; Ricci, D.; Manunta, A. Composition of the essential oil of Peucedanum verticillare. Biochem. Syst. Ecol. 2000, 28, 143–147. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Komeilizadeh, H.; Mojab, F.; Khazaie, A.; Masoudi, S.; Yari, M. Essential oil composition of Peucedanum petiolare (DC) Boiss. from Iran. J. Essent. Oil Res. 2001, 13, 49–50. [Google Scholar] [CrossRef]
- Menut, C.; Mve-Mba, C.E.; Lamaty, G.; Zollo, P.-H.A.; Tchoumboubnang, F.; Bessiere, J.-M. Aromatic plants of tropical Central Africa. XVIII. Essential oils of leaf and rhizome of Peucedanum zenkeri Engl. from Cameroon. J. Essent. Oil Res. 1995, 7, 77–79. [Google Scholar] [CrossRef]
- Schmaus, G.; Schultze, W.; Kubeczka, K.-H. Volatile constituents of Peucedanum palustre. Planta Med. 1989, 55, 482–486. [Google Scholar] [CrossRef]
- Alavi, S.H.R.; Yasa, N.; Fouladi, F.; Shafiee, A. Chemical composition of the essential oils of Peucedanum ruthenicum M. Bieb. leaves, flowers and fruits. Int. J. Prod. Res. 2006, 2, 143–147. [Google Scholar]
- Ulrich, S. Solid-phase microextraction in biomedical analysis. J. Chromatogr. A 2000, 902, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Steffen, A.; Pawliszyn, J. Analysis of flavor volatiles using headspace solid-phase microextraction. J. Agric. Food Chem. 1996, 44, 2187–2193. [Google Scholar] [CrossRef]
- Adio, A.M. (−)-trans-β-Elemene and related compounds: Occurrence, synthesis, and anticancer activity. Tetrahedron 2009, 65, 5145–5159. [Google Scholar] [CrossRef]
- Barreto, I.C.; de Almeida, A.S.; Sena Filho, J.G. Taxonomic Insights and Its Type Cyclization Correlation of Volatile Sesquiterpenes in Vitex Species and Potential Source Insecticidal Compounds: A Review. Molecules 2021, 26, 6405. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Paiss, M.S.S.; Scheffer, J.J.C. Seasonal variation in the composition of the essential oil of Crithmum maritimum L. Flavour Fragr. J. 1992, 7, 147–150. [Google Scholar] [CrossRef]
- Rasul, M.G. Conventional extraction methods use in medicinal plants, their advantages and disadvantages. Int. J. Basic Sci. Appl. Comput. 2018, 2, 10–14. [Google Scholar]
- Kowalczyk, A.; Kuś, P.; Marijanovic, Z.; Tuberoso, C.I.G.; Fecka, I.; Jerkovic, I. Headspace Solid-Phase Micro-Extraction Versus Hydrodistillation of Volatile Compounds from Leaves of Cultivated Mentha Taxa: Markers of Safe Chemotypes. Molecules 2022, 27, 6561. [Google Scholar] [CrossRef]
- Vellutini, M.; Baldovini, N.; De Rocca Serra, D.; Tomi, F.; Casanova, J. β-cyclolavandulyl and β-isocyclolavandulyl estersfrom Peucedanum paniculatum L. an endemic species to Corsica. Phytochemistry. 2005, 66, 1956–1962. [Google Scholar] [CrossRef]
- Bazgir, A.; Shaabani, A.; Sefidkon, F. Composition of the essential oil of Peucedanum ervariifolium C.A. Mey. from Iran. J. Essent. Oil Res. 2005, 17, 380–381. [Google Scholar] [CrossRef]
- Widelski, J.; Graikou, C.; Ganos, C.; Skalicka-Woźniak, K.; Chinou, I. Volatiles from selected Apiaceae species cultivated in Poland—Antimicrobial activities. Processes 2021, 9, 695. [Google Scholar] [CrossRef]
- Jovanovic, O.P.; Zlatkovic, B.K.; Jovanovic, S.C.; Petrovic, P.; Stojanovic, G.S. Composition of Peucedanum longifolium Waldst. & Kit. Essential oil and volatiles obtained by headspace. J. Essent. Oil Res. 2015, 27, 182–185. [Google Scholar]
- Sarkhail, P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar] [CrossRef] [PubMed]
- Saleh-E.-In, M.M.; Choi., Y.E. Anethum sowa Roxb. Ex. Fleming: A review on traditional uses, phytochemistry, pharmacological and toxicological activities. J. Ethnopharmacol. 2021, 280, 113967. [Google Scholar] [CrossRef] [PubMed]
| No | Compound Name | RI | L1 | FL | L2 | IF | L3 | MF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS SPME | HD | HS SPME | HD | HS SPME | HD | HS SPME | HD | HS SPME | HD | HS SPME | HD | |||
| 1 | cis-3-hexen-1-ol | 846 | 9.4 | - | - | - | - | - | - | - | - | - | - | - |
| 2 | 1-hexanol | 860 | 2.3 | - | - | - | - | - | - | - | - | - | - | - |
| 3 | α-pinene | 925 | - | 0.6 | tr | 0.2 | - | 0.2 | tr | 0.6 | - | - | tr | 0.7 |
| 4 | camphene | 942 | - | 0.8 | - | 0.2 | - | - | - | - | - | - | - | - |
| 5 | β-pinene | 978 | 0.3 | - | 0,1 | - | - | - | - | - | - | - | - | - |
| 6 | myrcene | 988 | 1.1 | 1.2 | 5.0 | 6.4 | 1.4 | 6.0 | 0.2 | tr | 0.9 | 0.2 | 0.2 | 0.5 |
| 7 | cis-hexenyl 3-acetate | 995 | 12.9 | - | - | - | - | - | - | - | - | - | - | - |
| 8 | Δ3-carene | 1004 | - | 0.8 | - | 0,3 | - | - | - | - | - | - | - | - |
| 9 | hexenyl acetate | 1007 | 2.3 | - | - | - | - | - | - | - | - | - | - | - |
| 10 | p-cymene | 1022 | - | - | - | - | - | 0.2 | tr | - | - | - | - | - |
| 11/12 | limonene + β-felandrene | 1026 | 1.8 | 0.4 | 1.3 | 1.4 | 0.4 | 2.0 | 0.1 | 0,1 | 0.3 | 0.4 | 0.1 | 0.2 |
| 13 | cis-β-ocimene | 1035 | 0.5 | 0.3 | 0.1 | 0.3 | 0.3 | 2.0 | tr | - | 0.3 | - | tr | - |
| 14 | trans-β-ocimene | 1046 | 6.3 | 3.9 | 0.9 | 3.6 | 1.2 | 22.7 | 0.1 | - | 1.7 | - | 0.1 | - |
| 15 | γ-terpinene | 1057 | 0.3 | - | 0.1 | - | - | - | - | - | - | - | - | - |
| 16 | terpinolene | 1083 | - | - | 0.3 | 0.2 | - | - | - | - | - | - | - | - |
| 17 | camphor | 1140 | 2.1 | - | 0.8 | - | - | - | - | - | - | - | - | - |
| 18 | bornyl acetate | 1288 | 0.6 | 4.4 | 0.2 | 1.3 | - | - | - | - | - | - | - | - |
| 19 | α-copaene | 1380 | 0.7 | 0.4 | 0.4 | 0.1 | 0.6 | 0.5 | 0.1 | tr | 1.4 | 0.5 | 0.2 | tr |
| 20 | NN | 1385 | 0.4 | - | 0.1 | - | 0.5 | 1.0 | 0.2 | - | 0.9 | 2.1 | 0.2 | 0.3 |
| 21 | β-bourbonene | 1386 | 0.7 | 2.0 | 0.3 | 0.5 | 1.6 | 0.7 | 0.1 | 0.2 | 0.8 | 1.0 | 0.4 | 0.3 |
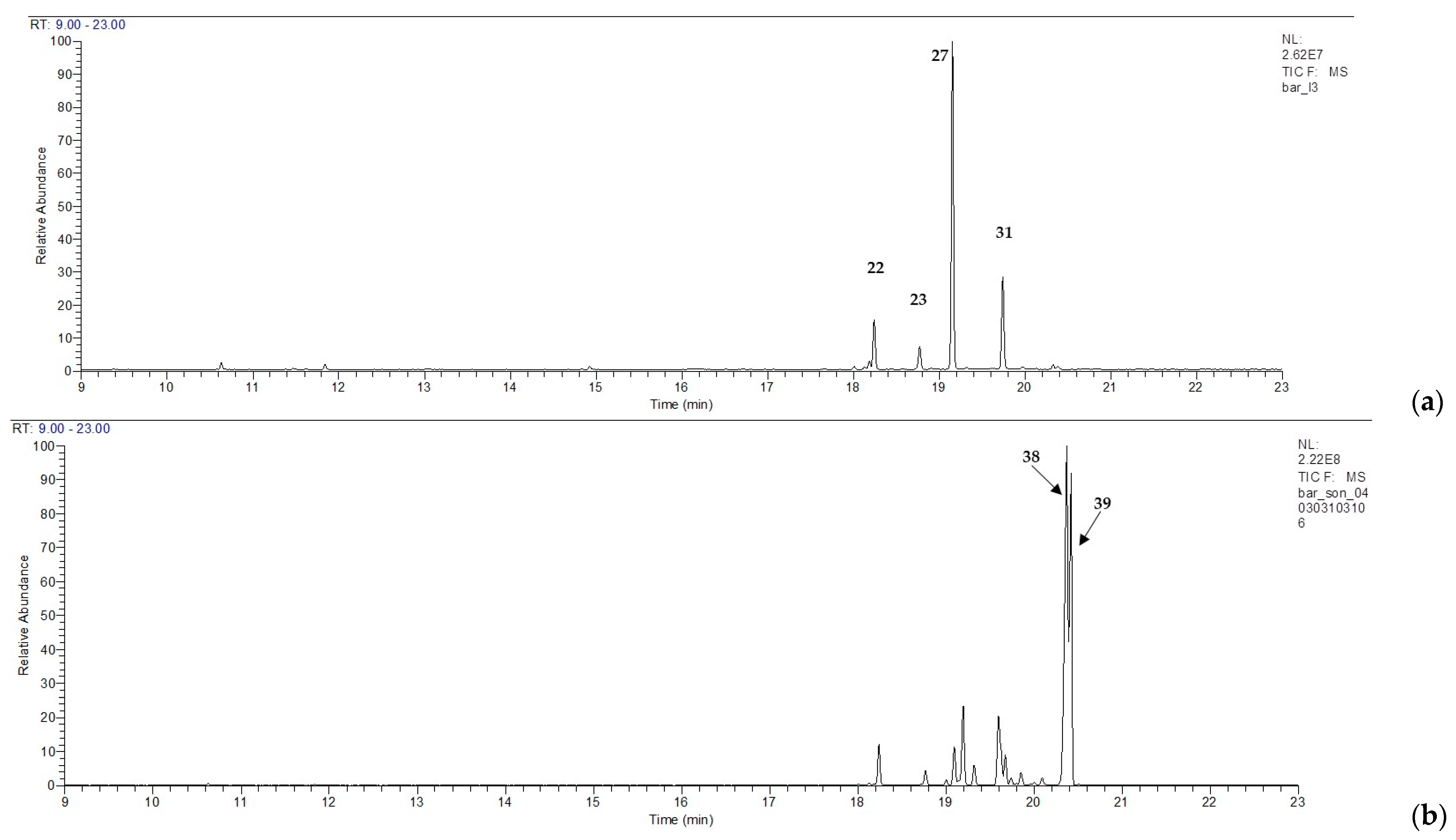
| 22 | β-elemene | 1392 | 6.6 | 19.5 | 2.2 | 5.3 | 9.6 | 11.5 | 3.4 | 5.6 | 17.3 | 31.1 | 3.1 | 3.7 |
| 23 | (E)-β-caryophyllene | 1423 | 2.9 | 6.6 | 2.7 | 1.6 | 4.9 | 2.7 | 1.3 | 1.7 | 8.2 | 9.2 | 1.7 | 1.1 |
| 24 | α-guaiene | 1439 | 0.2 | - | 0.6 | 1.8 | - | - | 0.5 | 0.2 | - | - | 0.5 | 0.2 |
| 25 | guaia-6,9-diene | 1445 | 1.9 | - | 4.0 | - | - | - | 3.5 | 2.4 | - | - | 3.8 | 2.0 |
| 26 | NN | 1454 | 8.6 | - | 8.1 | 3.6 | - | - | 6.8 | 4.4 | - | - | 6.9 | 3.5 |
| 27 | (Z)-β-farnesene + α-humulene | 1460 | - | 7.8 | - | 2.5 | 56.6 | 2.6 | - | - | 7.3 | 0.8 | - | - |
| 28 | α-humulene | 1460 | 0.8 | - | 0.6 | - | 0.5 | - | 1.8 | 2.7 | 0.8 | - | 2.1 | 2.1 |
| 29 | NN | 1478 | 1.8 | - | 9.7 | 5.0 | 0.4 | 0.1 | 8.7 | 6.3 | 0.7 | 0.3 | 10.2 | 6.0 |
| 30 | NN | 1482 | 0.6 | - | 2.8 | 1.8 | - | - | 2.6 | 2.2 | - | - | 2,4 | 2.0 |
| 31 | germacrene D | 1486 | 18.2 | 31.4 | 2.7 | 1.8 | 17.8 | 13.3 | 0.7 | 1.1 | 52.2 | 25.4 | 0.5 | 0.3 |
| 32 | β-selinene | 1493 | 0.3 | 0.6 | 1.3 | 0.9 | - | 0.2 | 1.3 | 1.2 | - | 1.0 | 1.4 | 1.3 |
| 33 | NN | 1495 | - | - | - | - | - | - | - | 0.4 | - | - | - | 1.1 |
| 34 | α-selinene | 1501 | 1.1 | 2.1 | 0.4 | 0.2 | - | 1.0 | 0.2 | 0.3 | 1.7 | 2.1 | 0.2 | 0.6 |
| 35 | α-muurolene | 1503 | - | - | - | - | - | 0.1 | - | - | - | 0.3 | - | - |
| 36 | α-bulnesene | 1506 | - | - | 0.6 | 0.5 | 0.3 | - | 0.8 | 0.7 | - | - | 0.7 | 0.7 |
| 37 | germacrene A | 1513 | 0.5 | 2.1 | 0.2 | 0.4 | - | 1.6 | - | 0.5 | - | 2.4 | - | 0.4 |
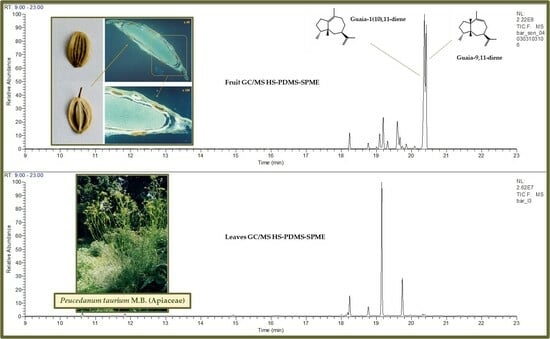
| 38 | guaia-1(10),11-diene | 1526 | 3.7 | - | 29.4 | 25.5 | 0.9 | - | 41.3 | 32.1 | 1.3 | - | 34.7 | 26.1 |
| 39 | guaia-9,11-diene | 1530 | 2.8 | - | 25.0 | 26.8 | 0.7 | 0.4 | 25.0 | 33.6 | 0.8 | - | 29.4 | 28.6 |
| 40 | NN | 1535 | - | - | - | 0.7 | - | - | - | tr | - | - | - | 1.3 |
| 41 | 4-β-hydroxygermacra-1(10),5-diene | 1585 | - | - | - | 0.6 | - | - | - | - | - | - | - | |
| 42 | caryolan-1-ol | 1583 | - | - | - | - | - | - | - | - | - | - | - | tr |
| 43 | spathulenol | 1584 | - | - | - | - | - | 1.6 | - | - | - | 0.8 | - | - |
| 44 | caryophylene oxide | 1589 | - | 0.4 | - | - | - | 3.8 | - | - | - | 2.5 | - | 0.6 |
| 45 | NN | 1601 | - | - | - | - | - | - | - | 0.1 | - | - | - | 1.4 |
| 46 | humulene epoxide | 1617 | - | - | - | - | - | 0.2 | - | tr | - | - | - | 0.6 |
| 47 | NN | 1620 | - | - | - | - | - | - | - | 0.2 | - | - | - | 3.1 |
| 48 | germacrene D-4-ol | 1630 | - | - | - | - | - | - | - | 0.2 | - | 0.5 | - | 0.5 |
| 49 | NN | 1630 | - | - | - | - | - | - | - | - | - | 0.3 | - | - |
| 50 | NN | 1634 | - | - | - | - | - | - | - | tr | - | - | - | 2.7 |
| 51 | cubenol | 1635 | - | - | - | - | - | tr | - | - | - | tr | - | - |
| 52 | NN | 1637 | - | - | - | - | - | - | - | 0.8 | - | - | - | 1.5 |
| 53 | eudesmol | 1640 | - | - | - | 2.3 | - | - | - | - | - | - | - | - |
| 54 | τ-cadinol | 1653 | - | - | - | - | - | 3.3 | - | 0.3 | - | 2.4 | - | 0.4 |
| 55 | eudesm-3-en-7-ol | 1655 | - | 0.6 | - | 0.6 | - | - | - | - | - | - | - | - |
| 66 | NN | 1656 | - | - | - | - | - | - | - | 0.1 | - | - | - | 1.9 |
| 57 | α-cadinol | 1665 | - | 0.3 | - | - | - | 1.5 | - | 0.1 | - | 1.7 | - | tr |
| 58 | NN | 1673 | - | 0.4 | - | - | - | - | - | 0.9 | - | - | - | 1.3 |
| 59 | NN | 1679 | - | - | - | - | - | 3.0 | - | - | - | 1.4 | - | - |
| 60 | NN | 1719 | - | - | - | - | - | 2.6 | - | - | - | 0.8 | - | - |
| 61 | NN | 1737 | - | - | - | - | - | 2.7 | - | - | - | 1.3 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartnik, M. GC-MS Analysis of Essential Oil and Volatiles from Aerial Parts of Peucedanum tauricum M.B. during the Phenological Period. Separations 2023, 10, 484. https://doi.org/10.3390/separations10090484
Bartnik M. GC-MS Analysis of Essential Oil and Volatiles from Aerial Parts of Peucedanum tauricum M.B. during the Phenological Period. Separations. 2023; 10(9):484. https://doi.org/10.3390/separations10090484
Chicago/Turabian StyleBartnik, Magdalena. 2023. "GC-MS Analysis of Essential Oil and Volatiles from Aerial Parts of Peucedanum tauricum M.B. during the Phenological Period" Separations 10, no. 9: 484. https://doi.org/10.3390/separations10090484