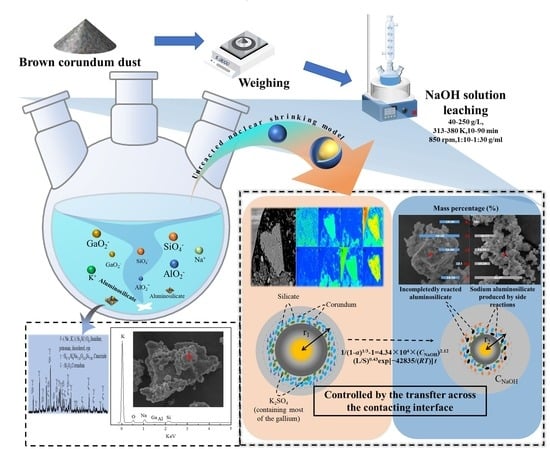
Extraction of Gallium from the Brown Corundum Dust with a One-Step Alkaline Leaching Process
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Procedure and Method
3. Results and Discussion
3.1. Thermodynamics of the Leaching Reactions
3.2. Relationships between Gallium Recovery and Leaching Factors
3.2.1. The Effect of NaOH Solution Concentration
3.2.2. The Effect of Leaching Duration and Temperature
3.2.3. The Effect of Stirring Speed
3.2.4. The Effect of Solid–Liquid Ratio
3.3. Kinetics Equation for the Leaching Process
- (1)
- If the leaching process is regulated by an internal diffusion (Ginstling–Brounshtein), the kinetics should be as follows:
- (2)
- If the leaching process is regulated by chemical reactions (interface), the leaching kinetics should be as follows:
- (3)
- If the leaching process is regulated by a transfer across the sphere’s contacting interface [42], the kinetics of the leaching process should be as follows:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moskalyk, R.R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Naumov, A.V. Status and prospects of world gallium production and the gallium market. Metallurgist 2013, 57, 367–371. [Google Scholar] [CrossRef]
- Font, O.; Querol, X.; Juan, R.; Casado, R.; Ruiz, C.R.; López-Soler, Á.; Coca, P.; Peña, F.G. Recovery of gallium and vanadium from gasification fly ash. J. Hazard. Mater. 2007, 139, 413–423. [Google Scholar] [CrossRef]
- Négrel, P.; Ladenberger, A.; Reimann, C.; Birke, M.; Sadeghi, M. Distribution of Rb, Ga and Cs in agricultural land soils at European continental scale (GEMAS): Implications for weathering conditions and provenance. Chem. Geol. 2018, 479, 188–203. [Google Scholar] [CrossRef]
- Strunz, H. Söhngeit, Ga(OH)3, Ein Neues Mineral. Die Naturwissenschaft. 1965, 52, 493. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Xiao, Y.; Fan, Y. Recovery of gallium from Bayer liquor: A review. Hydrometallurgy 2012, 125–126, 115–124. [Google Scholar] [CrossRef]
- Okudan, M.D.; Akcil, A.; Tuncuk, A.; Deveci, H. Recovery of gallium and aluminum from electrofilter dust of alumina calcination plant in Bayer process. Sep. Sci. Technol. 2015, 50, 2596–2605. [Google Scholar] [CrossRef]
- Gladyshev, S.V.; Akcil, A.; Abdulvaliyev, R.A.; Tastanov, E.A.; Beisembekova, K.O.; Temirova, S.S.; Deveci, H. Recovery of vanadium and gallium from solid waste by-products of Bayer process. Miner. Eng. 2015, 74, 91–98. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Zhou, G.; Gu, Y. Exploring a promising technology for the extraction of gallium from coal fly ash. Int. J. Coal Prep. Util. 2022, 42, 1712–1723. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, L.; Guo, Y.; Gao, J.; Li, H.; Cheng, F. A stepwise separation process for selective recovery of gallium from hydrochloric acid leach liquor of coal fly ash. Sep. Purif. Technol. 2021, 265, 118455. [Google Scholar] [CrossRef]
- Xu, K.; Deng, T.; Liu, J.; Peng, W. Study on the recovery of gallium from phosphorus flue dust by leaching with spent sulfuric acid solution and precipitation. Hydrometallurgy 2007, 86, 172–177. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, N.; Liu, H.; Wu, P.; Zhang, X.; Zhong, Z. Recovery of gallium from waste light emitting diodes by oxalic acidic leaching. Resour. Conserv. Recycl. 2019, 146, 366–372. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Flerus, B.; Friedrich, B. Recovery of Gallium from Smartphones—Part II: Oxidative Alkaline Pressure Leaching of Gallium from Pyrolysis Residue. Metals 2020, 10, 1565. [Google Scholar] [CrossRef]
- Macías-Macías, K.Y.; Ceniceros-Gómez, A.E.; Gutiérrez-Ruiz, M.E.; González-Chávez, J.L.; Martínez-Jardines, L.G. Extraction and recovery of the strategic element gallium from an iron mine tailing. J. Environ. Chem. Eng. 2019, 7, 102964. [Google Scholar] [CrossRef]
- Wu, X.L.; Qin, W.Q.; Wu, S.K.; Ma, X.H.; Niu, Y.J.; Yang, C.R. Recovery of gallium from zinc concentrate by pressure oxygen leaching. Rare Met. 2013, 32, 622–626. [Google Scholar] [CrossRef]
- Rao, S.; Liu, Z.; Wang, D.; Cao, H.; Zhu, W.; Zhang, K.; Tao, J. Hydrometallurgical process for recovery of Zn, Pb, Ga and Ge from Zn refinery residues. Trans. Nonferrous Met. Soc. China 2021, 31, 555–564. [Google Scholar] [CrossRef]
- Liu, S.; Sweatman, K.; McDonald, S.; Nogita, K. Ga-Based Alloys in Microelectronic Interconnects: A Review. Materials 2018, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- De Paula, W.J.; Tavares, P.L.; Pereira, D.D.C.; Tavares, G.M.; Silva, F.L.; Almeida, P.S.; Braga, H.A.C. A review on gallium nitride switching power devices and applications. In Proceedings of the 2017 Brazilian Power Electronics Conference (COBEP), Juiz de Fora, Brazil, 19–22 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–7. [Google Scholar]
- Hasan, M.N.; Swinnich, E.; Seo, J.-H. Recent Progress in Gallium Oxide and Diamond Based High Power and High-Frequency Electronics. Int. J. High Speed Electron. Syst. 2019, 28, 1940004. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-Bandgap Semiconductors: Research Opportunities and Challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Li, W.; Nomoto, K.; Hu, Z.; Jena, D.; Xing, H.G. Guiding principles for trench schottky barrier diodes based on ultrawide bandgap semiconductors: A case study in Ga2O3. IEEE Trans. Electron Devices 2020, 67, 3938–3947. [Google Scholar] [CrossRef]
- Dalla Vecchia, M.; Ravyts, S.; Van den Broeck, G.; Driesen, J. Gallium-nitride semiconductor technology and its practical design challenges in power electronics applications: An overview. Energies 2019, 12, 2663. [Google Scholar] [CrossRef]
- Breeman, W.A.P.; de Blois, E.; Sze Chan, H.; Konijnenberg, M.; Kwekkeboom, D.J.; Krenning, E.P. 68Ga-labeled dota-peptides and 68ga-labeled radiopharmaceuticals for positron emission tomography: Current status of research, clinical applications, and future perspectives. Semin. Nucl. Med. 2011, 41, 314–321. [Google Scholar] [CrossRef] [PubMed]
- De Assis, A.S.J.; Pegoraro, G.M.; Duarte, I.C.S. Evolution of gallium applications in medicine and microbiology: A timeline. BioMetals 2022, 35, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef]
- Cook, T.; Franconi, N.; Shea, B.; Wilson, C.; Grainger, B.; George, A.; Barchowsky, A. Radiation-tolerant, gan-based point of load converters for small spacecraft missions. In Proceedings of the Small Satellite Conference 2018, Logan, UT, UAS, 4–9 August 2018. [Google Scholar]
- Hall, C.; Pastra, C.L.; Burell, A.; Gladin, J.; Mavris, D. Projecting Power Converter Specific Power Through 2050 for Aerospace Applications. In Proceedings of the 2022 IEEE/AIAA Transportation Electrification Conference and Electric Aircraft Technologies Symposium, Anaheim, CA, USA, 15–17 June 2022. [Google Scholar]
- Zhao, P.; Zhang, H.; Gao, H.; Zhu, Y.; Yu, J.; Chen, Q.; Zhao, H. Separation and characterisation of fused alumina obtained from aluminium-chromium slag. Ceram. Int. 2018, 44, 3590–3595. [Google Scholar] [CrossRef]
- Wen, K.; Jiang, F.; Zhou, X.; Sun, Z. Leaching of gallium from corundum flue dust using mixed acid solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1862–1868. [Google Scholar] [CrossRef]
- Wen, K.; Jiang, F.; Zhou, X.; Sun, Z. Recovery of gallium from corundum flue dust by two-stage alkali leaching, carbonation, acid leaching and solvent extraction process. Metals 2018, 8, 545. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xiao, J. Mechanism and kinetics study on ultrasound assisted leaching of gallium and zinc from corundum flue dust. Miner. Eng. 2022, 183, 107624. [Google Scholar] [CrossRef]
- Østergaard, J. UV/Vis Spectrophotometry and UV Imaging. In Analytical Techniques in the Pharmaceutical Sciences. Advances in Delivery Science and Technology; Müllertz, A., Perrie, Y., Rades, T., Eds.; Springer: New York, NY, USA, 2016; pp. 3–27. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=565066AD4FE96E560C4CA0A3DC5EB220 (accessed on 1 October 2007).
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer: Berlin/Heidelberg, Germany, 1988; ISBN 978-3-642-73095-5. [Google Scholar]
- Hui, X.; Zhang, J.; Liang, Y.; Chang, Y.; Zhang, W.; Zhang, G. Comparison and evaluation of vanadium extraction from the calcification roasted vanadium slag with carbonation leaching and sulfuric acid leaching. Sep. Purif. Technol. 2022, 297, 121466. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xue, Z. Oxidation kinetics of vanadium slag roasting in the presence of calcium oxide. Miner. Process. Extr. Metall. Rev. 2017, 38, 265–273. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Xue, L.; Zou, Y. Extraction of rare earth elements from fluoride molten salt electrolytic slag by mineral phase reconstruction. J. Clean. Prod. 2018, 177, 567–572. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Process optimization and kinetics for leaching of rare earth metals from the spent Ni–metal hydride batteries. Waste Manag. 2016, 51, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Schilm, J.; Herrmann, M.; Michael, G. Kinetic study of the corrosion of silicon nitride materials in acids. J. Eur. Ceram. Soc. 2003, 23, 577–584. [Google Scholar] [CrossRef]
- Dickinson, C.F.; Heal, G.R. Solid–liquid diffusion controlled rate equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
- Dehghan, R.; Noaparast, M.; Kolahdoozan, M. Leaching and kinetic modelling of low-grade calcareous sphalerite in acidic ferric chloride solution. Hydrometallurgy 2009, 96, 275–282. [Google Scholar] [CrossRef]
- Rath, P.C.; Paramguru, R.K.; Jena, P.K. Kinetics of dissolution of zinc sulphide in aqueous ferric chloride solution. Hydrometallurgy 1981, 6, 219–225. [Google Scholar] [CrossRef]
- Li, M.T.; Wei, C.; Zhou, X.J.; Qiu, S.; Deng, Z.G.; Li, X.B. Kinetics of vanadium leaching from black shale in non-oxidative conditions. Miner. Process. Extr. Metall. 2012, 121, 40–47. [Google Scholar] [CrossRef]
- Nikiforova, A.; Kozhura, O.; Pasenko, O. Leaching of vanadium by sulfur dioxide from spent catalysts for sulfuric acid production. Hydrometallurgy 2016, 164, 31–37. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.; Zou, D.; Liu, T.; Li, D. Kinetics of nitric acid leaching of cerium from oxidation roasted Baotou mixed rare earth concentrate. J. Rare Earths 2019, 37, 198–204. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Cheng, G.; Xue, X.; Yang, H. Kinetics and mechanism of hydrochloric acid leaching of rare earths from Bayan Obo slag and recovery of rare earth oxalate and high purity oxides. Hydrometallurgy 2022, 208, 105782. [Google Scholar] [CrossRef]



















| SiO2 | K2O | Al2O3 | SO3 | Fe2O3 | P2O5 | MnO | MgO | Na2O | PbO | F |
| 42.90 | 20.50 | 19.71 | 5.01 | 4.29 | 1.26 | 1.08 | 0.947 | 0.881 | 0.681 | 0.624 |
| ZnO | CuO | Ga2O3 | CaO | Cl | SnO2 | Cr2O3 | ZrO2 | Rb2O | As2O3 | SrO |
| 0.254 | 0.227 | 0.133 | 0.211 | 0.135 | 0.0507 | 0.0497 | 0.0446 | 0.0397 | 0.0298 | 0.0178 |
| CeO2 | Bi2O3 | NiO | V2O5 | Nb2O5 | La2O3 | GeO2 | Co3O4 | MFe | ||
| 0.0177 | 0.0152 | 0.0089 | 0.0085 | 0.0072 | 0.0056 | 0.0034 | 0.0031 | 0.2 |
| Point | O | Na | Al | Si | K | Ga |
|---|---|---|---|---|---|---|
| 1 | 40.62 | 12.49 | 13.70 | 24.07 | 9.12 | - |
| 2 | 34.56 | 3.41 | 8.35 | 41.63 | 12.06 | - |
| 3 | 18.83 | - | 20.72 | 40.38 | 20.07 | - |
| 4 | 45.30 | 7.87 | 25.60 | 14.91 | 6.33 | - |
| 5 | 14.75 | 13.11 | 22.42 | 23.00 | 26.72 | - |
| 6 | 41.74 | 17.89 | 14.43 | 21.82 | 4.12 | - |
| 7 | 39.44 | 34.99 | 6.37 | 11.12 | - | 8.08 |
| 8 | 46.92 | 12.38 | 15.48 | 20.39 | 4.83 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gao, C.; Hui, X.; Chang, Y. Extraction of Gallium from the Brown Corundum Dust with a One-Step Alkaline Leaching Process. Separations 2023, 10, 510. https://doi.org/10.3390/separations10090510
Zhang J, Gao C, Hui X, Chang Y. Extraction of Gallium from the Brown Corundum Dust with a One-Step Alkaline Leaching Process. Separations. 2023; 10(9):510. https://doi.org/10.3390/separations10090510
Chicago/Turabian StyleZhang, Juhua, Cong Gao, Xujie Hui, and Yuwei Chang. 2023. "Extraction of Gallium from the Brown Corundum Dust with a One-Step Alkaline Leaching Process" Separations 10, no. 9: 510. https://doi.org/10.3390/separations10090510