CO2 Adsorption by Bamboo Biochars Obtained via a Salt-Assisted Pyrolysis Route
Abstract
:1. Introduction
2. Preparation and Characterization of Bamboo Biochars
3. Results and Discussion
3.1. Physical Properties of Bamboo Biochars
3.2. Chemical Properties of Bamboo Biochars
3.3. Carbon Dioxide Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Satterthwaite, D. Cities’ contribution to global warming: Notes on the allocation of greenhouse gas emissions. Environ. Urban. 2008, 20, 539–549. [Google Scholar] [CrossRef]
- Bhave, A.; Taylor, R.H.; Fennell, P.; Livingston, W.R.; Shah, N.; Mac Dowell, N.; Dennis, J.; Kraft, M.; Pourkashanian, M.; Insa, M. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energy 2017, 190, 481–489. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Algozeeb, W.A.; Savas, P.E.; Yuan, Z.; Wang, Z.; Kittrell, C.; Hall, J.N.; Chen, W.; Bollini, P.; Tour, J.M. Plastic waste product captures carbon dioxide in nanometer pores. ACS Nano 2022, 16, 7284–7290. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, S.J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Kumar, S.; Srivastava, R.; Koh, J. Utilization of zeolites as CO2 capturing agents: Advances and future perspectives. J. CO2 Ultil. 2020, 41, 101251. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Y.; Gu, X. MOF based CO2 capture: Adsorption and membrane separation. Inorg. Chem. Commun. 2023, 152, 110722. [Google Scholar] [CrossRef]
- Liu, X.; Lim, G.J.H.; Wang, Y.; Zhang, L.; Mullangi, D.; Wu, Y.; Zhao, D.; Ding, J.; Cheetham, A.K.; Wang, J. Binder-free 3D printing of covalent organic framework (COF) monoliths for CO2 adsorption. Chem. Eng. J. 2021, 403, 126333. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wang, J.; Wu, C.; Gao, N.B. Biomass-based carbon materials for CO2 capture: A review. J. CO2 Util. 2023, 68, 102373. [Google Scholar] [CrossRef]
- Shen, Y.F. Preparation of renewable porous carbons for CO2 capture-A review. Fuel Process. Technol. 2022, 236, 107437. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Mattern, D.L.; Chen, W.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Egiebor, N.O.; Hammer, N. Ultrasound cavitation intensified amine functionalization: A feasible strategy for enhancing CO2 capture capacity of biochar. Fuel 2018, 225, 287–298. [Google Scholar] [CrossRef]
- Wen, C.; Liu, T.; Wang, D.; Wang, Y.; Chen, H.P.; Luo, G.Q.; Zou, Z.J.; Li, C.; Xu, M.H. Biochar as the effective adsorbent to combustion gaseous pollutants: Preparation, activation, functionalization and the adsorption mechanisms. Prog. Energy Combust. 2023, 99, 101098. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Mohammed, M.M.; Mohammed, M.F.; Shadhar, M.H. Reviewing of using nanomaterials for wastewater treatment. Pollution 2022, 8, 995–1013. [Google Scholar]
- Yang, L.; Qiu, J.; Wang, Y.; Guo, S.; Feng, Y.; Dong, D.; Yao, J. Molten salt synthesis of hierarchical porous carbon from wood sawdust for supercapacitors. J. Electroanal. Chem. 2020, 856, 113673. [Google Scholar] [CrossRef]
- Siddiqui, N.; Don, J.; Mondal, K.; Mahajan, A. Development of bamboo-derived sorbents for mercury removal in gas phase. Environ. Technol. 2011, 32, 383–394. [Google Scholar] [CrossRef]
- Cheng, L.; Adhikari, S.; Wang, Z.; Ding, Y. Characterization of bamboo species at different ages and bio-oil production. J. Anal. Appl. Pyrol. 2015, 116, 215–222. [Google Scholar] [CrossRef]
- Tan, Z.; Xiang, J.; Su, S.; Zeng, H.; Zhou, C.; Sun, L.S.; Hu, S.; Qiu, J. Enhanced capture of elemental mercury by bamboo-based sorbents. J. Hazard. Mater. 2012, 239, 160–166. [Google Scholar] [CrossRef]
- Goel, C.; Mohan, S.; Dinesha, P. CO2 capture by adsorption on biomass-derived activated char: A review. Sci. Total Environ. 2021, 798, 149296. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, M.; Zhu, X.; Guo, W.; Luo, Z.; Cai, W.; Zhu, X. Molten salt shielded pyrolysis of biomass waste: Development of hierarchical biochar, salt recovery, CO2 adsorption. Fuel 2023, 334, 126565. [Google Scholar] [CrossRef]
- Díez, N.; Fuertes, A.B.; Sevilla, M. Molten salt strategies towards carbon materials for energy storage and conversion. Energy Storage Mater. 2021, 38, 50–69. [Google Scholar] [CrossRef]
- Li, B.; Tang, J.Z.; Xie, X.; Wei, J.T.; Xu, D.; Shi, L.; Ding, K.; Zhang, S.; Hu, X.; Zhang, S.; et al. Char structure evolution during molten salt pyrolysis of biomass: Effect of temperature. Fuel 2023, 331, 125747. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Jin, H.; Wu, J.; Liu, K.; Xu, Z.; Wan, J.; Zhou, H.; Duan, J.; Hu, B.; et al. Salt-assisted synthesis of 2D materials. Adv. Funct. Mater. 2020, 30, 1908486. [Google Scholar] [CrossRef]
- Liu, D.J.; Yang, L.T.; Wu, J.; Li, B. Molten salt shielded preparation of rice straw biochars doped by copper sulfide for elemental mercury capture. J. Energy Inst. 2022, 102, 176–183. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Xie, X.; Li, C.E.; Liu, D.J. Pyrolysis of rice husk in molten lithium chloride: Biochar structure evolution and CO2 adsorption. J. Energy Inst. 2024, 113, 101526. [Google Scholar] [CrossRef]
- Oh, Y.; Morris, C.D.; Kanatzidis, M.G. Polysulfide chalcogels with ion-exchange properties and highly efficient mercury vapor sorption. J. Am. Chem. Soc. 2012, 134, 14604–14608. [Google Scholar] [CrossRef] [PubMed]
- López, R.H.; Vidales, A.M.; Zgrablich, G. Percolation effects on adsorption-desorption hysteresis. Langmuir 2000, 16, 6999–7005. [Google Scholar] [CrossRef]
- McKee, D.W. Mechanisms of the alkali metal catalysed gasification of carbon. Fuel 1983, 62, 170–175. [Google Scholar] [CrossRef]
- Lyon, L.A.; Keating, C.D.; Fox, A.P.; Baker, B.E.; He, L.; Nicewarner, S.R.; Mulvaney, S.P.; Natan, M.J. Raman spectroscopy. Anal. Chem. 1998, 70, 341–362. [Google Scholar] [CrossRef]
- Yan, S.; Lin, J.; Liu, P.; Zhao, Z.; Lian, J.; Chang, W.; Yao, L.; Liu, Y.; Lin, H.; Han, S. Preparation of nitrogen-doped porous carbons for high-performance supercapacitor using biomass of waste lotus stems. RSC Adv. 2018, 8, 6806–6813. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. Nitrogen-doped two-dimensional porous carbon sheets derived from clover biomass for high performance supercapacitors. J. Power Sources 2017, 363, 375–383. [Google Scholar] [CrossRef]
- Liu, X.; Antonietti, M. Molten salt activation for synthesis of porous carbon nanostructures and carbon sheets. Carbon 2014, 69, 460–466. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.J. Tuning ratios of KOH and NaOH on acetic acid-mediated chitosan-based porous carbons for improving their textural features and CO2 uptakes. J. CO2 Util. 2020, 40, 101212. [Google Scholar] [CrossRef]
- Shen, J.; Hu, H.Y.; Xu, M.; Liu, H.; Xu, K.; Zhang, X.; Yao, H.; Naruse, I. Interactions between molten salts and ash components during Zhundong coal gasification in eutectic carbonates. Fuel 2017, 207, 365–372. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Akhavan, O. The effect of heat treatment on formation of graphene thin films from graphene oxide nanosheets. Carbon 2010, 48, 509–519. [Google Scholar] [CrossRef]
- Jampaiah, D.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Selvakannan, P.R.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Ceria–zirconia modified MnOx catalysts for gaseous elemental mercury oxidation and adsorption. Catal. Sci. Technol. 2016, 6, 1792–1803. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Y.; Wu, Q.; Liu, B.; Li, D.; Chen, R.; Wang, C.; Li, H.; Zeng, Z.; Li, L. Underlying mechanism of CO2 uptake onto biomass-based porous carbons: Do adsorbents capture CO2 chiefly through narrow micropores? Fuel 2020, 282, 118727. [Google Scholar] [CrossRef]
- Liu, D.J.; Yang, L.T.; Wu, J.; Li, B. Tuning sulfur vacancies in CoS2 via a molten salt approach for promoted mercury vapor adsorption. Chem. Eng. J. 2022, 450, 137956. [Google Scholar] [CrossRef]
- Pu, Q.; Zou, J.; Wang, J.; Lu, S.; Ning, P.; Huang, L.; Wang, Q. Systematic study of dynamic CO2 adsorption on activated carbons derived from different biomass. J. Alloy Compd. 2021, 887, 161406. [Google Scholar] [CrossRef]
- García, S.; Gil, M.V.; Martín, C.F.; Pis, J.J.; Rubiera, F.; Pevida, C. Breakthrough adsorption study of a commercial activated carbon for pre-combustion CO2 capture. Chem. Eng. J. 2011, 171, 549–556. [Google Scholar] [CrossRef]
- Shahkarami, S.; Azargohar, R.; Dalai, A.K.; Soltan, J. Breakthrough CO2 adsorption in bio-based activated carbons. J. Environ. Sci. 2015, 34, 68–76. [Google Scholar] [CrossRef] [PubMed]


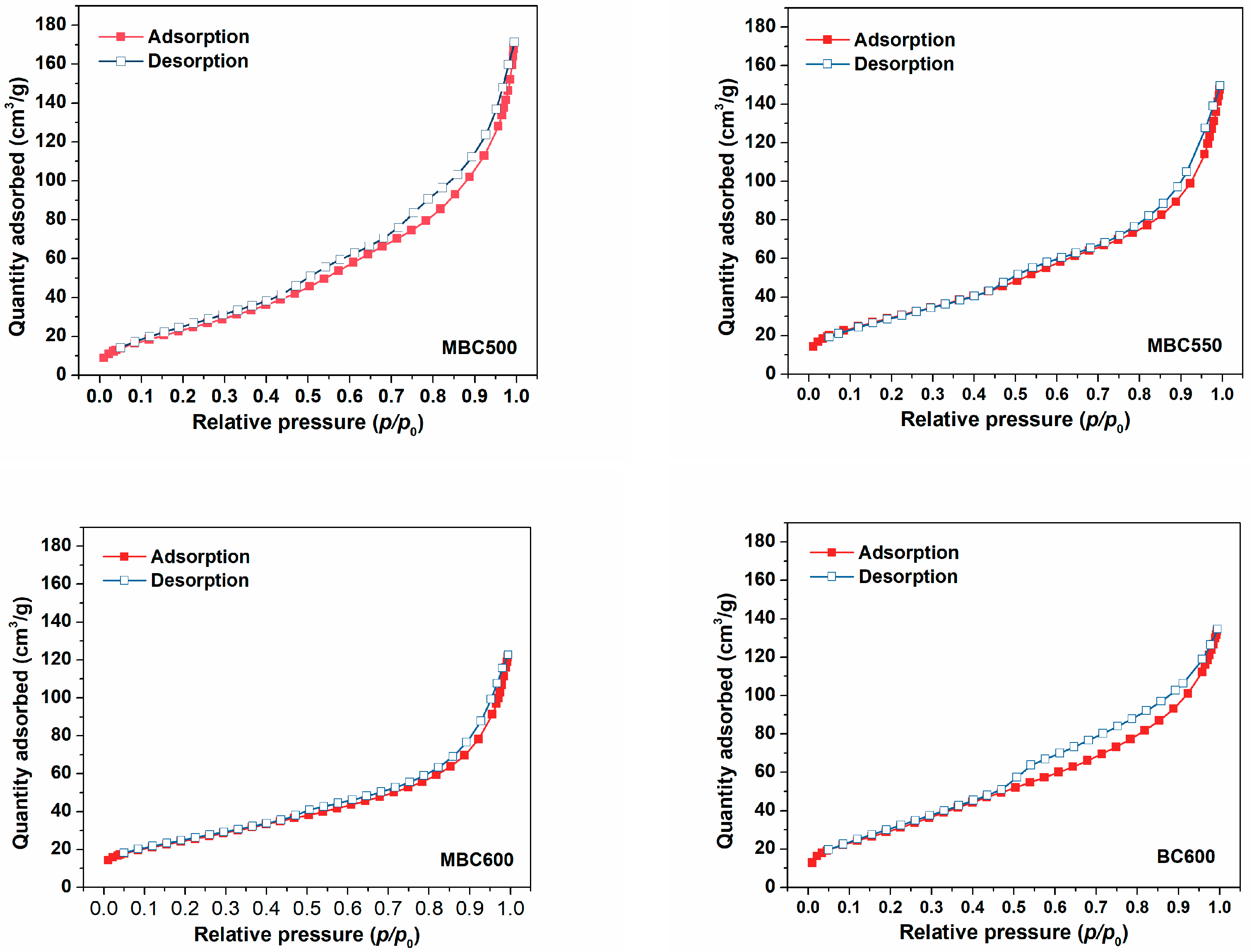

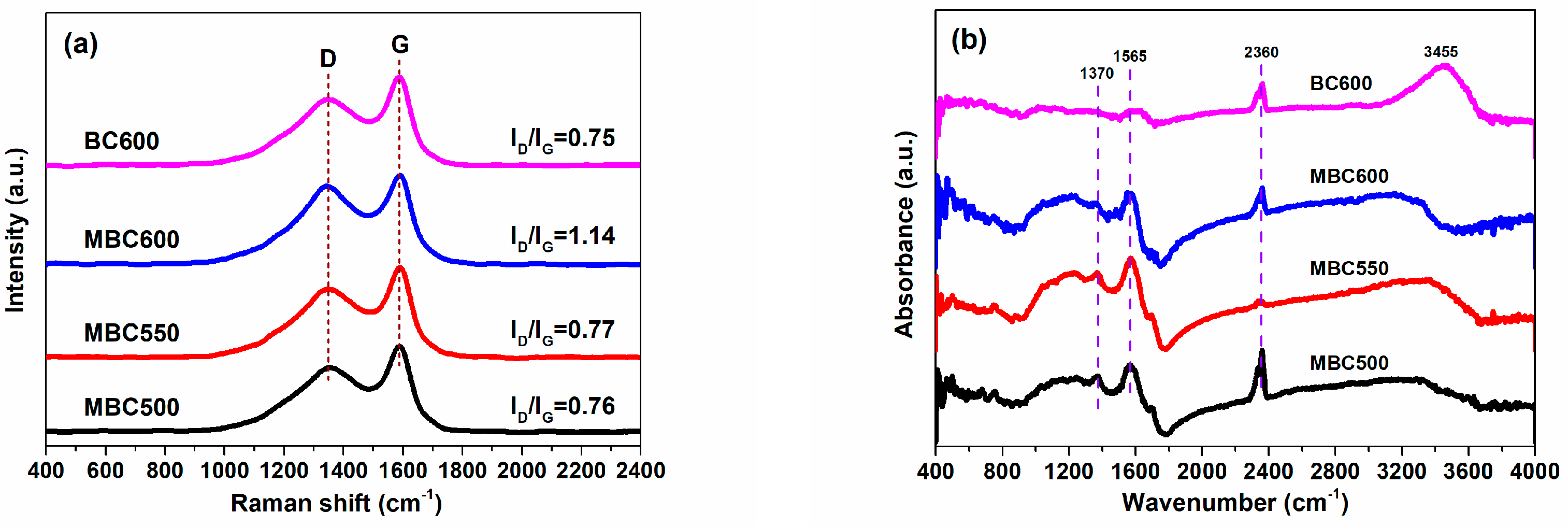
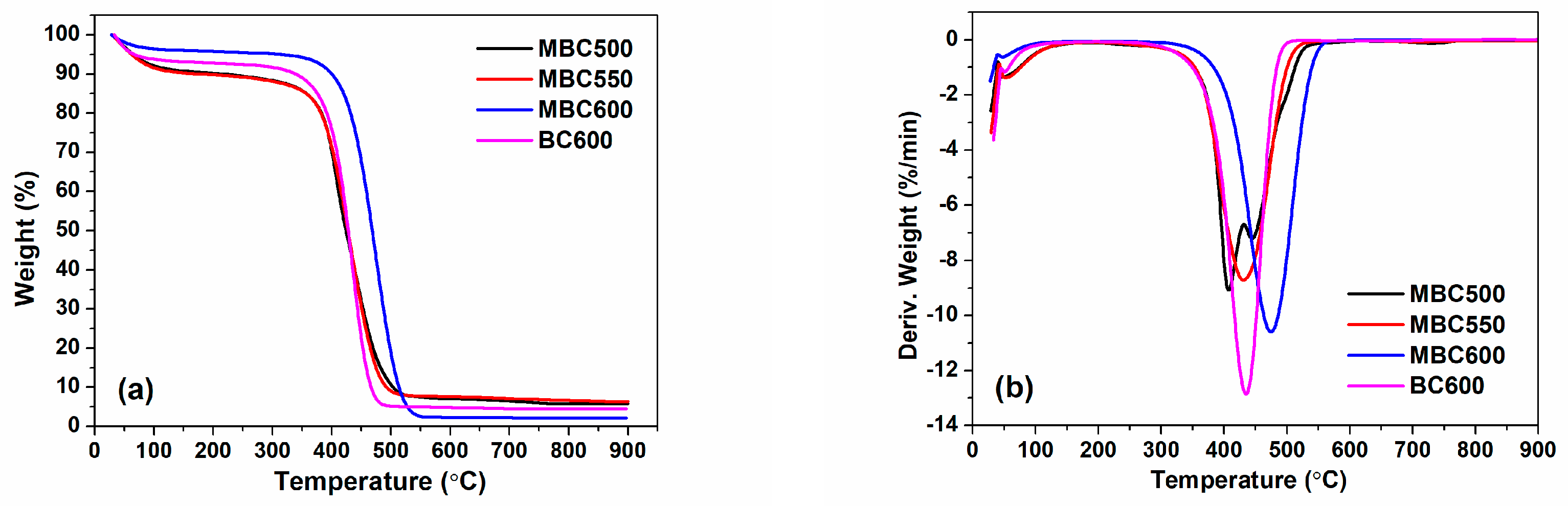
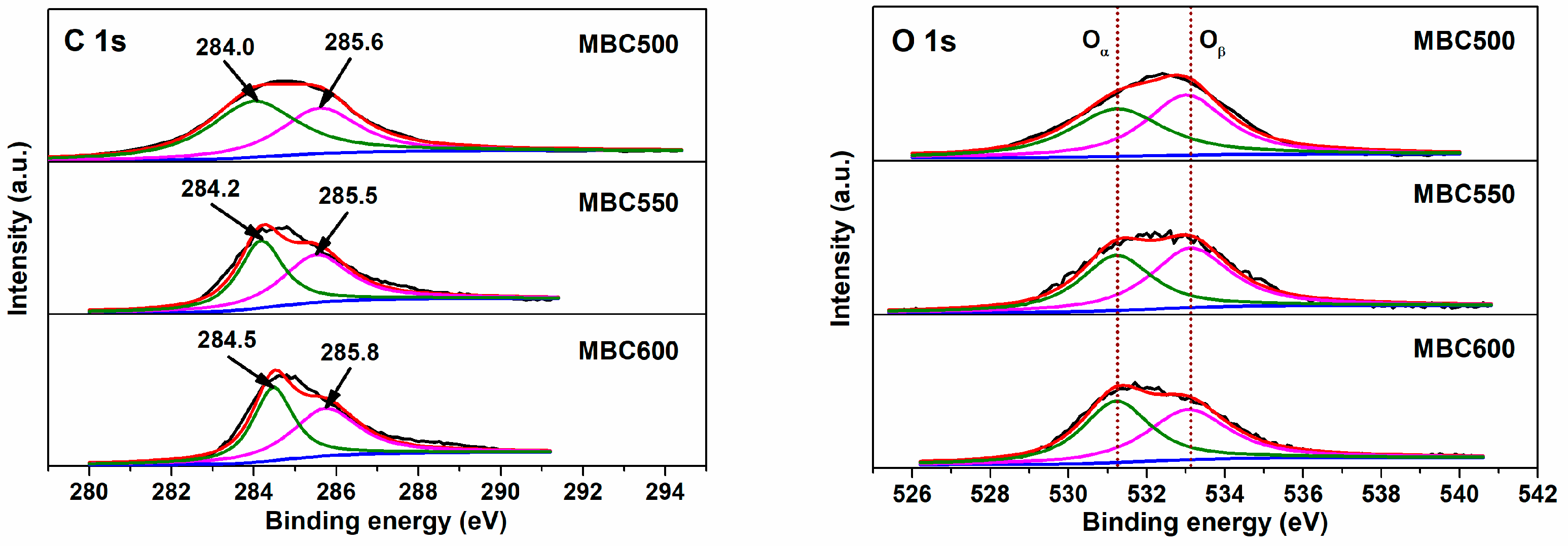
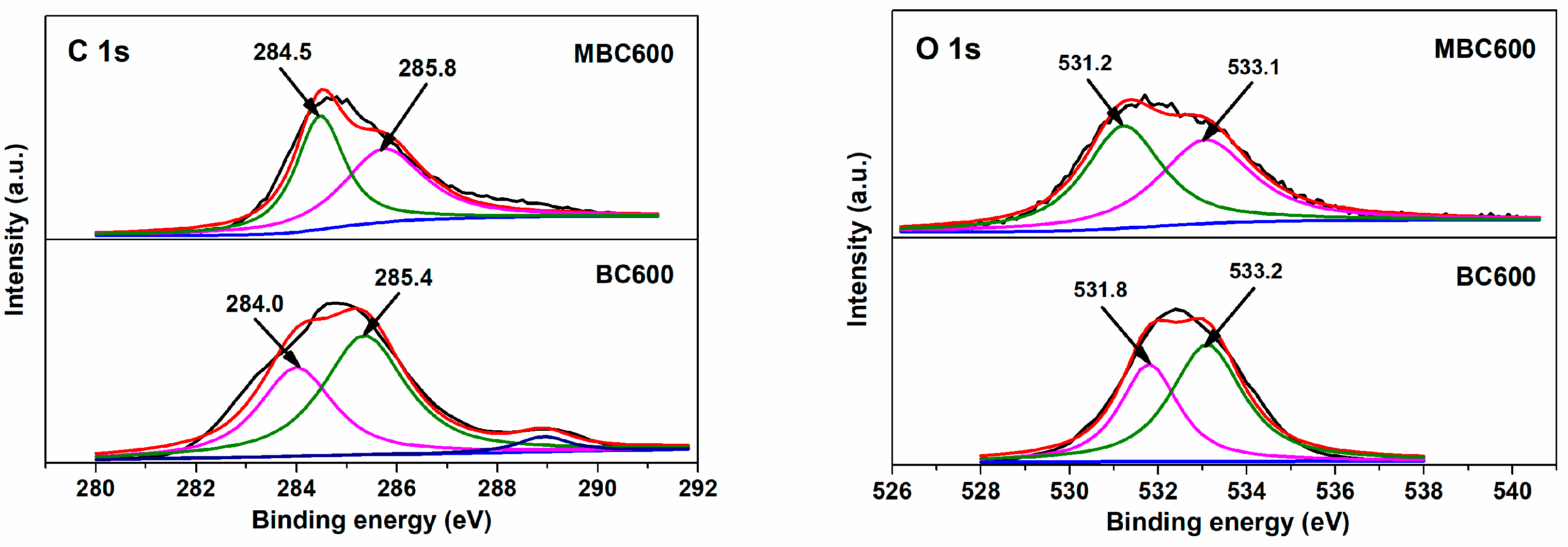
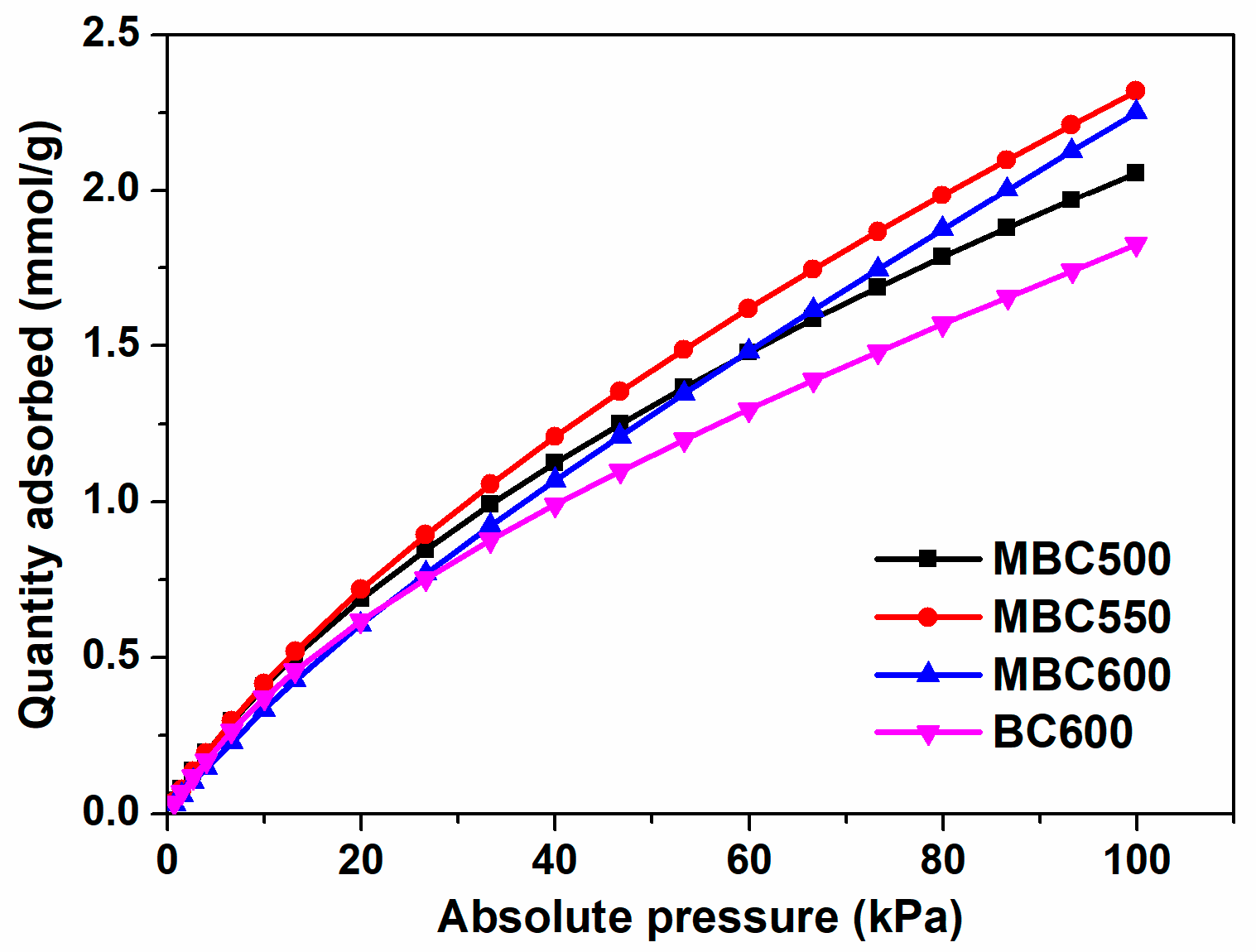
| Samples | SSA (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) | Yield | ID/IG | Oα/Oβ | CO2 Capacity (mmol/g) |
|---|---|---|---|---|---|---|---|
| MBC500 | 92 | 0.247 | 10.8 | 12.7% | 0.76 | 0.83 | 2.06 |
| MBC550 | 109 | 0.218 | 8.0 | 10.9% | 0.77 | 0.85 | 2.32 |
| MBC600 | 89 | 0.180 | 8.1 | 4.5% | 1.14 | 0.98 | 2.25 |
| BC600 | 113 | 0.201 | 7.1 | 23.8% | 0.75 | 0.68 | 1.83 |
| Samples | C * | H * | O * | N * | Ash * | H/C # | O/C # |
|---|---|---|---|---|---|---|---|
| MBC500 | 66.19 | 2.56 | 24.82 | 0.64 | 5.79 | 0.46 | 0.28 |
| MBC550 | 67.96 | 2.46 | 22.58 | 0.68 | 6.32 | 0.43 | 0.25 |
| MBC600 | 65.97 | 2.05 | 29.21 | 0.67 | 2.10 | 0.37 | 0.33 |
| BC600 | 78.13 | 2.28 | 14.52 | 0.55 | 4.52 | 0.35 | 0.14 |
| Sorbents | Precursors | SSA (m2/g) | Adsorption Conditions | CO2 Uptakes (mmol/g) | Ref. |
|---|---|---|---|---|---|
| MBC550 | bamboo | 109 | 25 °C (1 bar) | 2.32 | this work |
| CSAC | chestnut shell | 1254 | 25 °C (1 bar) | 1.70 | [41] |
| WSAC | walnut shell | 1489 | 25 °C (1 bar) | 2.10 | [41] |
| MSAC | macadamia shell | 1151 | 25 °C (1 bar) | 1.97 | [41] |
| AC | peat | 942 | 25 °C (1 bar) | 2.46 | [42] |
| AC | white wood | 1400 | 25 °C (1 bar) | 1.80 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Li, M.; Lin, D.; Li, B.; Li, C.; Liu, D. CO2 Adsorption by Bamboo Biochars Obtained via a Salt-Assisted Pyrolysis Route. Separations 2024, 11, 48. https://doi.org/10.3390/separations11020048
Xie X, Li M, Lin D, Li B, Li C, Liu D. CO2 Adsorption by Bamboo Biochars Obtained via a Salt-Assisted Pyrolysis Route. Separations. 2024; 11(2):48. https://doi.org/10.3390/separations11020048
Chicago/Turabian StyleXie, Xing, Mangmang Li, Dan Lin, Bin Li, Chaoen Li, and Dongjing Liu. 2024. "CO2 Adsorption by Bamboo Biochars Obtained via a Salt-Assisted Pyrolysis Route" Separations 11, no. 2: 48. https://doi.org/10.3390/separations11020048





