Optimization of the Reaction between 5-O-Caffeoylquinic Acid (5-CQA) and Tryptophan—Isolation of the Product and Its Evaluation as a Food Dye
Abstract
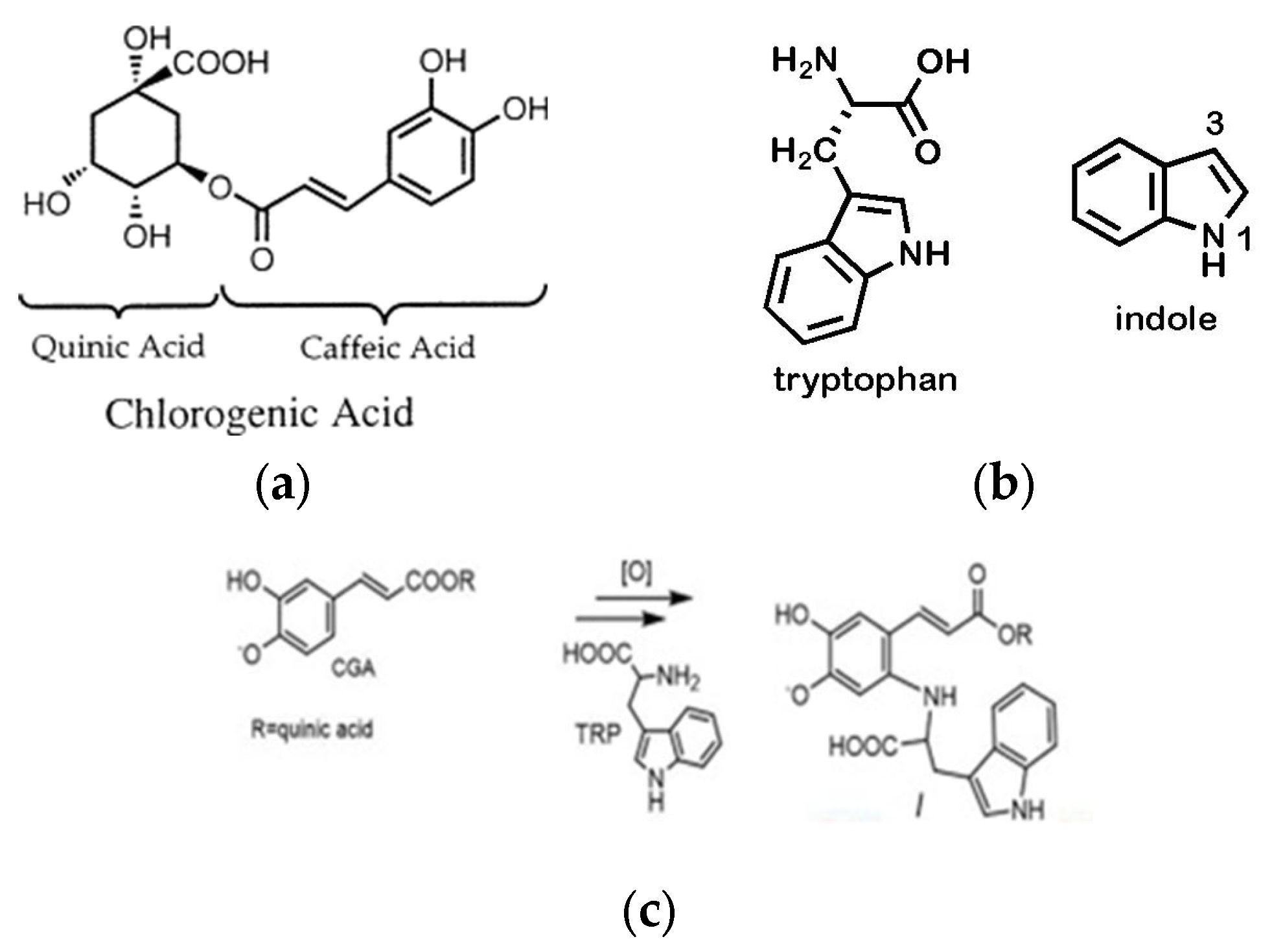
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. 5-CQA and Red Pigment Determination
2.3. Reaction of 5-CQA with TRP; Effect of Reactant Concentrations, pH, and Temperature
2.4. Order of the Reaction and Investigation of Reaction Mechanism
2.5. Recovery of the Red Pigment
2.6. Thermal Stability of the Red Pigment
2.7. Antimicrobial Activity of the Red Pigment
2.8. The Red Pigment as a Food Dye in Sausages: Antioxidant Ability and Color Stability
2.9. Statistical Analysis
3. Results and Discussion
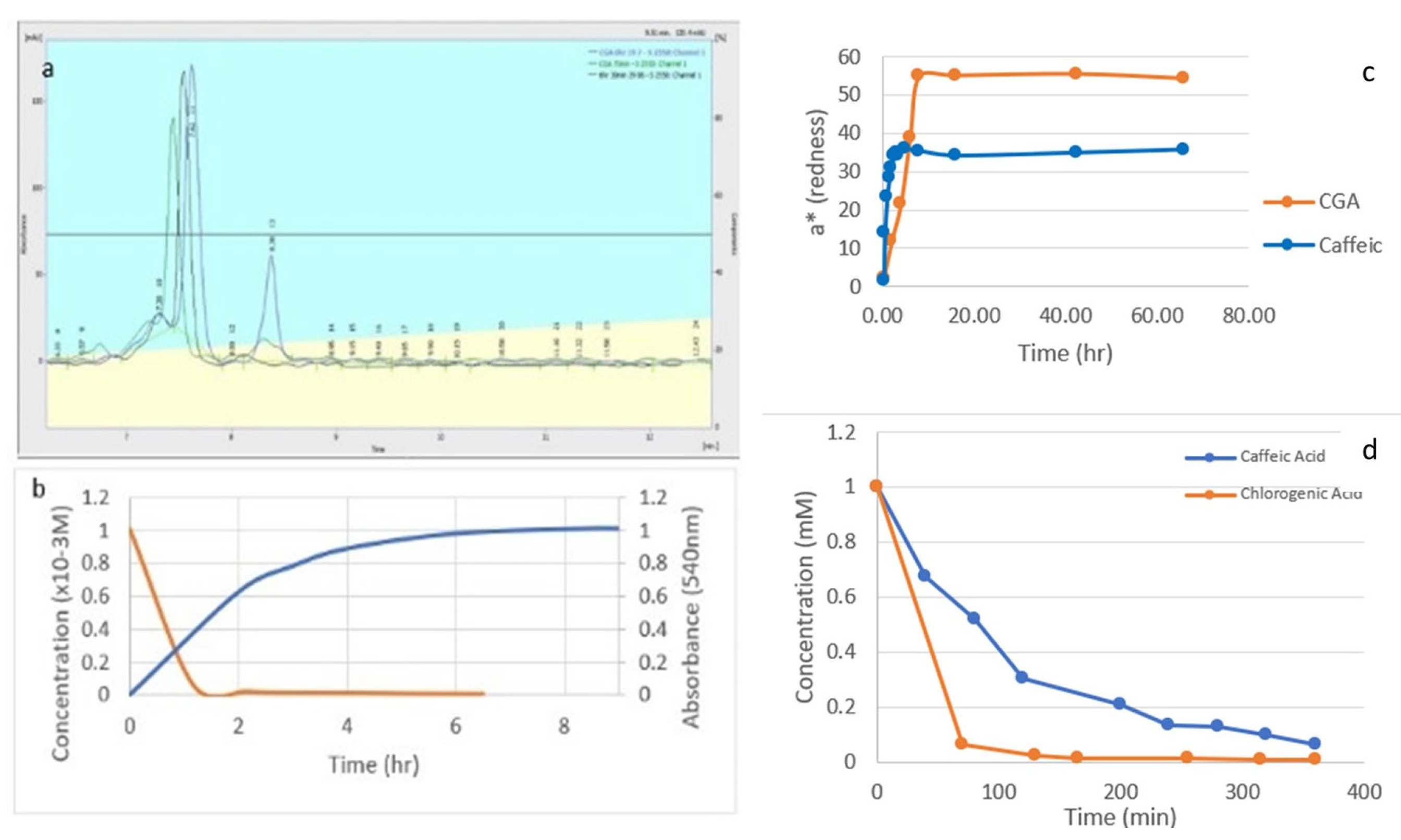
3.1. Reaction Kinetics
3.2. Reaction Order
3.3. Effect of Molar Ratios
3.4. Temperature Effect on the Reaction
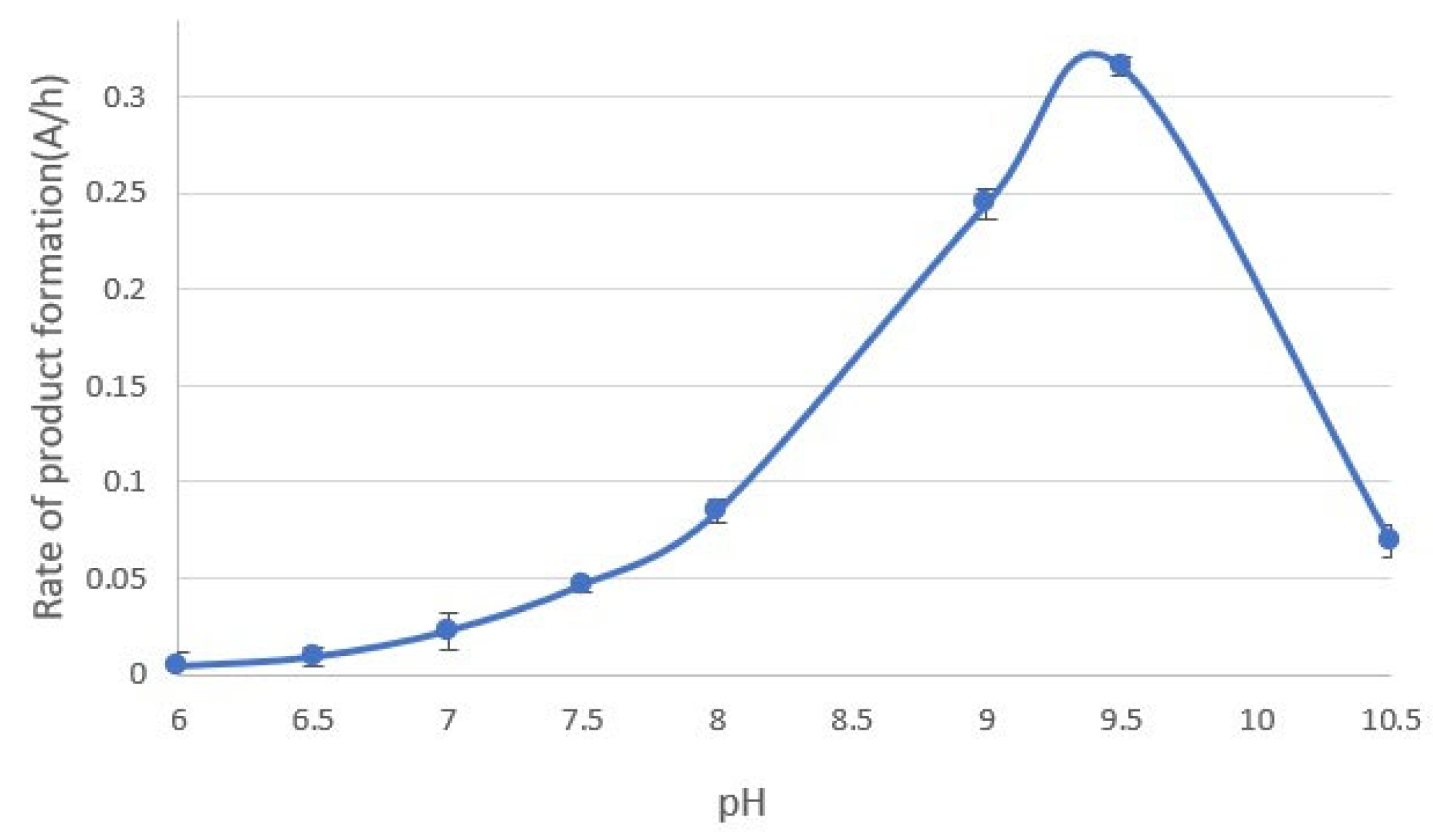
3.5. Effect of pH on the Reaction Rate
3.6. Recovery and Isolation of the Reaction Product
3.7. Physicochemical and Antimicrobial Properties of the Spray-Dried Pigment
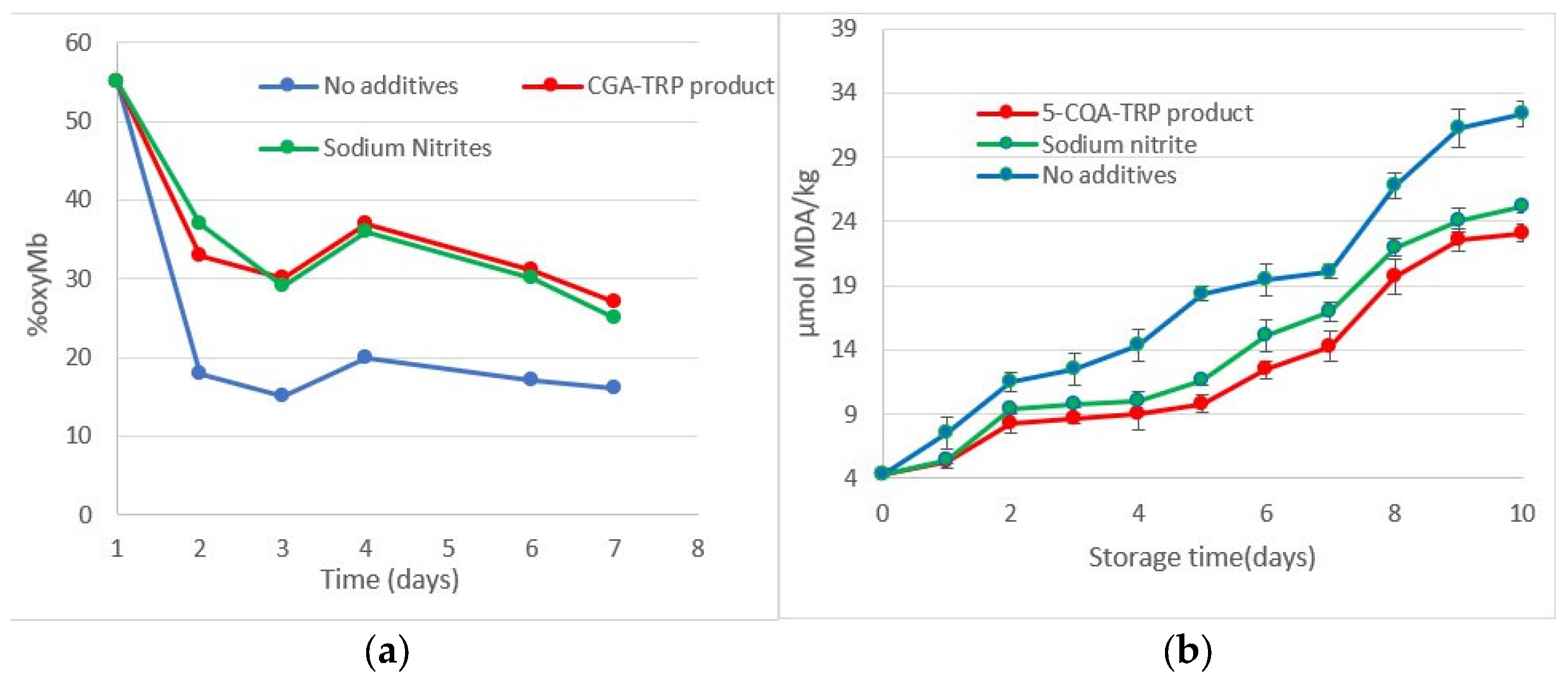
3.8. The Red Pigment as a Food Dye in Frankfurt-Type Sausages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food Colour Additives: A Synoptical Overview on Their Chemical Properties, Applications in Food Products and Health Side Effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef]
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Stewart, B.W.; et al. Carcinogenicity of Consumption of Red and Processed Meat. Lancet Oncol. 2015, 26, 1599–1600. [Google Scholar] [CrossRef]
- Kumar, G.; Upadhyay, S.; Yadav, D.K.; Malakar, S.; Dhurve, P.; Suri, S. Application of Ultrasound Technology for Extraction of Color Pigments from Plant Sources and Their Potential Bio-Functional Properties: A Review. J. Food Process. Eng. 2023, 46, e14238. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2021, 62, 1999–2049. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, S.; Li, Z.; Chou, S.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J.; et al. An Updated Review on the Stability of Anthocyanins Regarding the Interaction with Food Proteins and Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4378–4401. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and Their Applications in Food: The Current State of Processing, Stability and Future Opportunities in the Industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef] [PubMed]
- Neelwarne, B.; Halagur, S.B. Red Beet: An Overview. Red Beet Biotechnology: Food and Pharmaceutical Applications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–43. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds of Beetroot and Utilization in Food Processing Industry: A Critical Review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, Stability, Encapsulation and Application of Natural Pigments in Foods. Food Rev. Int. 2020, 38, 1735–1790. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary Protein-Phenolic Interactions: Characterization, Biochemical-Physiological Consequences, and Potential Food Applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 3589–3615. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein-Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Perez-Gregorio, M.R.; Simal-Gandara, J. A Critical Review of the Characterization of Polyphenol–Protein Interactions and of Their Potential Use for Improving Food Quality. Curr. Pharm. Des. 2017, 23, 2742–2753. [Google Scholar] [CrossRef]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic Acid Oxidation and Its Reaction with Sunflower Proteins to Form Green-Colored Complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef]
- Prigent, S.V.E.; Voragen, A.G.J.; Visser, A.J.W.G.; Van Koningsveld, G.A.; Gruppen, H. Covalent Interactions between Proteins and Oxidation Products of Caffeoylquinic Acid (Chlorogenic Acid). J. Sci. Food Agric. 2007, 87, 2502–2510. [Google Scholar] [CrossRef]
- Vercruysse, K.P. Preliminary Observations on the Interactions between Chlorogenic Acid and Select Amino Acids. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Bongartz, V.; Brandt, L.; Gehrmann, M.L.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Evidence for the Formation of Benzacridine Derivatives in Alkaline-Treated Sunflower Meal and Model Solutions. Molecules 2016, 21, 91. [Google Scholar] [CrossRef]
- Gil, M.; Wianowska, D. Chlorogenic Acids—Their Properties, Occurrence and Analysis. Ann. Univ. Mariae Curie-Sklodowska Sect. AA Chem. 2017, 72, 61. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and Its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216. [Google Scholar] [CrossRef]
- Šilarová, P.; Boulekbache-Makhlouf, L.; Pellati, F.; Česlová, L. Monitoring of Chlorogenic Acid and Antioxidant Capacity of Solanum melongena L. (Eggplant) under Different Heat and Storage Treatments. Antioxidants 2019, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Weber, F.; Gleichenhagen, M.; Pistorio, V.; Panzella, L.; Pizzo, E.; Schieber, A.; D’Ischia, M.; Napolitano, A. Stable Benzacridine Pigments by Oxidative Coupling of Chlorogenic Acid with Amino Acids and Proteins: Toward Natural Product-Based Green Food Coloring. J. Agric. Food Chem. 2017, 65, 6519–6528. [Google Scholar] [CrossRef] [PubMed]
- Santarcangelo, A.; Weber, F.; Kehraus, S.; Dickschat, J.S.; Schieber, A. Generation and Structure Elucidation of a Red Colorant Formed by Oxidative Coupling of Chlorogenic Acid and Tryptophan. Food Chem. 2023, 425, 136473. [Google Scholar] [CrossRef]
- Moccia, F.; Martín, M.Á.; Ramos, S.; Goya, L.; Marzorati, S.; DellaGreca, M.; Panzella, L.; Napolitano, A. A New Cyanine from Oxidative Coupling of Chlorogenic Acid with Tryptophan: Assessment of the Potential as Red Dye for Food Coloring. Food Chem. 2021, 348, 129152. [Google Scholar] [CrossRef]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-Hepatitis B Virus Activity of Chlorogenic Acid, Quinic Acid and Caffeic Acid in Vivo and in Vitro. Antiviral. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Meisel, J.; Patel, M.; Negin, S.; Gokel, M.; Cantwell, R.; Gokel, G. Tryptophan in Membrane-Active Synthetic Antimicrobials. J. Explor. Res. Pharmacol. 2017, 2, 7–15. [Google Scholar] [CrossRef]
- Xue, F.; Li, C.; Adhikari, B. Physicochemical Properties of Soy Protein Isolates-Cyanidin-3-Galactoside Conjugates Produced Using Free Radicals Induced by Ultrasound. Ultrason. Sonochem. 2020, 64, 104990. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and Food Technology: A Review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Gogate, P.R.; Tayal, R.K.; Pandit, A.B. Cavitation: A Technology on the Horizon. Curr. Sci. 2006, 91, 35–46. [Google Scholar]
- Ancheyta, J. Chemical Reaction Kinetics: Concepts, Methods and Case Studies; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–287. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of Pomegranate Peel Polyphenols Encapsulated in Orange Juice Industry By-Product and Their Incorporation in Cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Karavasili, C.; Adamoudi, E.; Tsitsos, A.; Economou, V.; Beltes, C.; Bouropoulos, N.; Katsamenis, O.L.; Doherty, R.; Bakopoulou, A.; et al. Electrospun Nanofiber Films Suppress Inflammation in Vitro and Eradicate Endodontic Bacterial Infection in an E. Faecalis-Infected Ex Vivo Human Tooth Culture Model. ACS Biomater. Sci. Eng. 2022, 8, 2096–2110. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Clinical and Laboratory Standards Institute: Pittsburgh, PA, USA, 2023. [Google Scholar]
- Herrmann, S.S.; Granby, K.; Duedahl-Olesen, L. Formation and Mitigation of N-Nitrosamines in Nitrite Preserved Cooked Sausages. Food Chem. 2015, 174, 516–526. [Google Scholar] [CrossRef]
- Floros, S.; Toskas, A.; Pasidi, E.; Vareltzis, P. Bioaccessibility and Oxidative Stability of Omega-3 Fatty Acids in Supplements, Sardines and Enriched Eggs Studied Using a Static In Vitro Gastrointestinal Model. Molecules 2022, 27, 415. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Silva, L.K.R.; da Silva, M.V. Natural Antioxidants Used in Meat Products. In Plant Antioxidants Health; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Yu, Q.P.; Feng, D.Y.; Xiao, J.; Wu, F.; He, X.J.; Xia, M.H.; Dong, T.; Liu, Y.H.; Tan, H.Z.; Zou, S.G.; et al. Studies on Meat Color, Myoglobin Content, Enzyme Activities, and Genes Associated with Oxidative Potential of Pigs Slaughtered at Different Growth Stages. Asian-Australas. J. Anim. Sci. 2017, 30, 1739. [Google Scholar] [CrossRef]
- Tang, S.; Kerry, J.P.; Shehan, D.; Bucklry, J.D.; Morrissey, P.A. Antioxidant Effect of Added Tea Catechin on Susceptibility of Cooked Red Meat, Poultry and Fish Parties to Lipid Oxidation. Food Res. Int. 2001, 34, 651–657. [Google Scholar] [CrossRef]
- Paniwnyk, L. Applications of Ultrasound in Processing of Liquid Foods: A Review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Engelke, G.H.; Schieber, A. Structure Elucidation and Tentative Formation Pathway of a Red Colored Enzymatic Oxidation Product of Caffeic Acid. Food Chem. 2019, 297, 124932. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant Activity of Polyphenolics in Diets: Rate Constants of Reactions of Chlorogenic Acid and Caffeic Acid with Reactive Species of Oxygen and Nitrogen. Biochim. Biophys. Acta (BBA) Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Cuq, J.C.; Cheftel, J.C. Tryptophan Degradation during Heat Treatments: Part 1—The Degradation of Free Tryptophan. Food Chem. 1983, 12, 1–14. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. The Influence of PH on the Thermal Stability of 5-O-Caffeoylquinic Acids in Aqueous Solutions. Eur. Food Res. Technol. 2011, 233, 223–232. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of Different Phenolic Acids and Flavonoids with Soy Proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Li, M.; Ritzoulis, C.; Du, Q.; Liu, Y.; Ding, Y.; Liu, W.; Liu, J. Recent Progress on Protein-Polyphenol Complexes: Effect on Stability and Nutrients Delivery of Oil-in-Water Emulsion System. Front. Nutr. 2021, 8, 765589. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–Polyphenol Conjugates: Antioxidant Property, Functionalities and Their Applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of Sodium Nitrite Reduction on Lipid Oxidation and Antioxidant Properties of Cooked Meat Products. Antioxidants 2020, 9, 9. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Beta, T.; Cai, Z.; Zhang, X.; Li, Y.; Huang, W. Effect of in Vitro Gastro-Intestinal Digestion on the Phenolic Composition and Antioxidant Capacity of Burdock Roots at Different Harvest Time. Food Chem. 2021, 358, 129897. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The Antidiabetic and Antioxidant Properties of Some Phenolic Phytochemicals: A Review Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T.; Ikemoto, N.; Terashima, M. Designing Antioxidant Peptides Based on the Antioxidant Properties of the Amino Acid Side-Chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.N.; Buttar, H.S. Evaluation of the Antioxidant Properties of Tryptophan and Its Metabolites in in Vitro Assay. J. Complement. Integr. Med. 2016, 13, 129–136. [Google Scholar] [CrossRef]


| 5-CGA (mM) | TRP (mM) | L* | a* | b* | ΔΕ |
|---|---|---|---|---|---|
| 1 | 7 | 22.63 | 46.09 a | 30.44 | - |
| 7 | 3.5 | 3.00 | 0.51 b | 2.80 | 56.87 a |
| 3.5 | 7 | 10.93 | 27.09 c | 16.03 | 26.80 b |
| 1 | 5 | 36.68 | 54.78 d | 50.81 | 26.10 b |
| 1 | 3.5 | 45.49 | 35.24 e | 29.07 | 25,21 b |
| 7 | 7 | 3.18 | 0.31 b | 0.05 | 58.43 a |
| 1 | 14 | 54.17 | 57.63 d | 30.81 | 31.29 b |
| Isolation Method | Yield% |
|---|---|
| Acidification + 10 min centrifugation | 9.1 ± 0.1 |
| Acidification + 20 min centrifugation | 11.1 ± 0.2 |
| Acidification + 45 min centrifugation | 15.2 ± 0.1 |
| Spray drying | 62.1 ± 1.1 |
| pH | 3 | 4 | 7 | 9 |
|---|---|---|---|---|
| Thermal process | Colour difference (ΔΕ) | |||
| Ultrasounds | 1.50 | 2.68 | 3.20 | 3.06 |
| Microwave | 5.54 | 5.88 | 6.54 | 7.37 |
| Water bath | 3.37 | 5.03 | 2.27 | 3.95 |
| Freezing | 3.63 | 4.42 | 4.93 | 5.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vareltzis, P.; Karatsioli, P.; Kazakos, I.; Menelaou, A.-M.; Parmaxi, K.; Economou, V. Optimization of the Reaction between 5-O-Caffeoylquinic Acid (5-CQA) and Tryptophan—Isolation of the Product and Its Evaluation as a Food Dye. Separations 2024, 11, 60. https://doi.org/10.3390/separations11020060
Vareltzis P, Karatsioli P, Kazakos I, Menelaou A-M, Parmaxi K, Economou V. Optimization of the Reaction between 5-O-Caffeoylquinic Acid (5-CQA) and Tryptophan—Isolation of the Product and Its Evaluation as a Food Dye. Separations. 2024; 11(2):60. https://doi.org/10.3390/separations11020060
Chicago/Turabian StyleVareltzis, Patroklos, Panagiota Karatsioli, Ioannis Kazakos, Anna-Maria Menelaou, Konstantina Parmaxi, and Vangelis Economou. 2024. "Optimization of the Reaction between 5-O-Caffeoylquinic Acid (5-CQA) and Tryptophan—Isolation of the Product and Its Evaluation as a Food Dye" Separations 11, no. 2: 60. https://doi.org/10.3390/separations11020060








