Recovery of Omega-3-Rich Lipids: Toward the Sustainable Valorization of Sea-Bass Industry Side Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Solvents
2.1.1. Raw Materials
2.1.2. Solvents and Reagents
2.2. Methods
2.2.1. Total Oil Content—Soxhlet
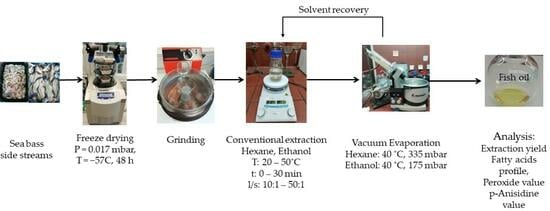
2.2.2. Conventional Extraction
2.2.3. Extraction Yield—Oil Recovery Determination
2.2.4. Oil Quality Determination and Fatty Acid Analysis
2.3. Mathematical Modeling
2.4. Statistical Analysis
3. Results and Discussion
3.1. Oil Content and Fatty Acid Composition of Raw Materials
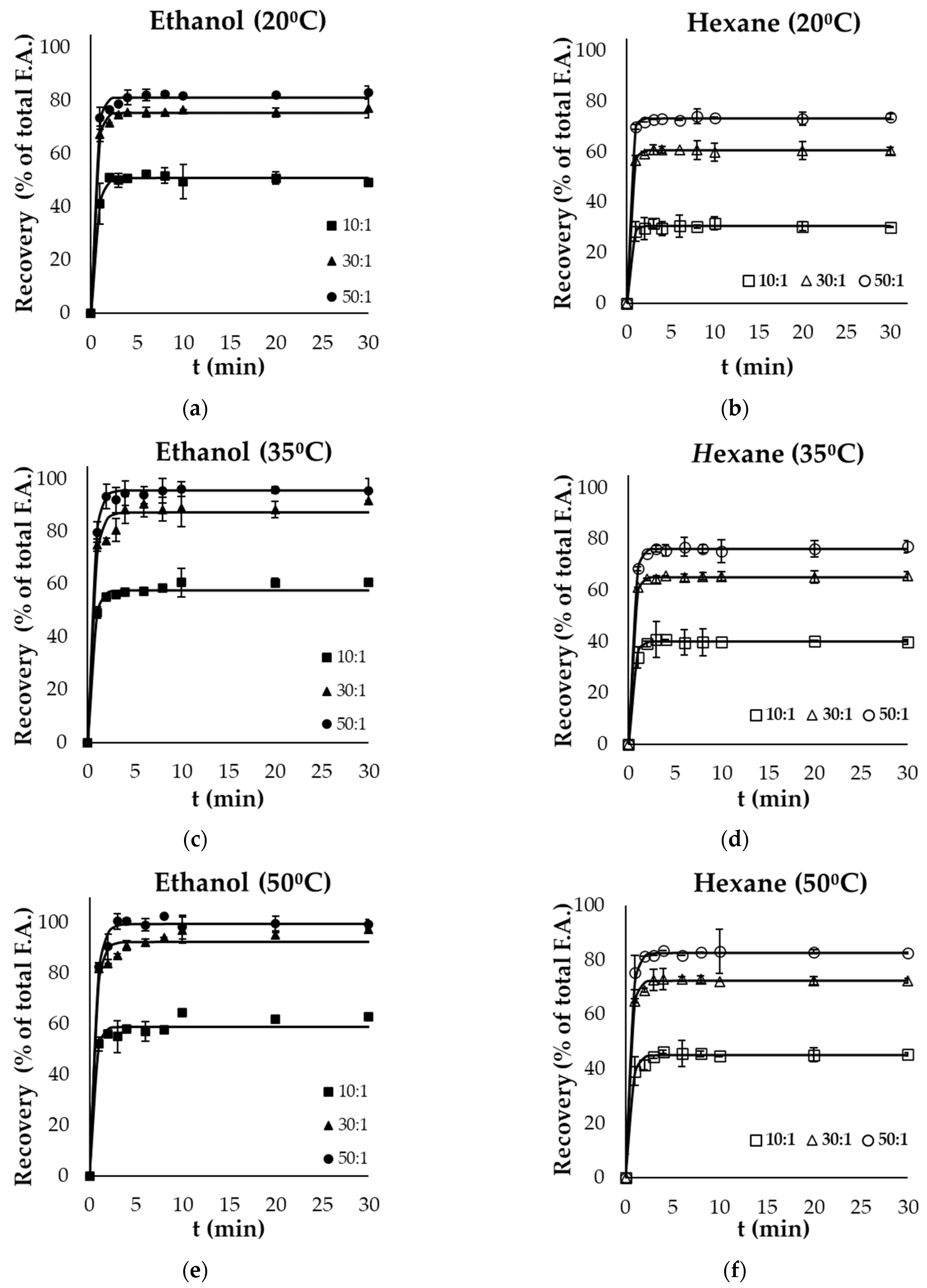
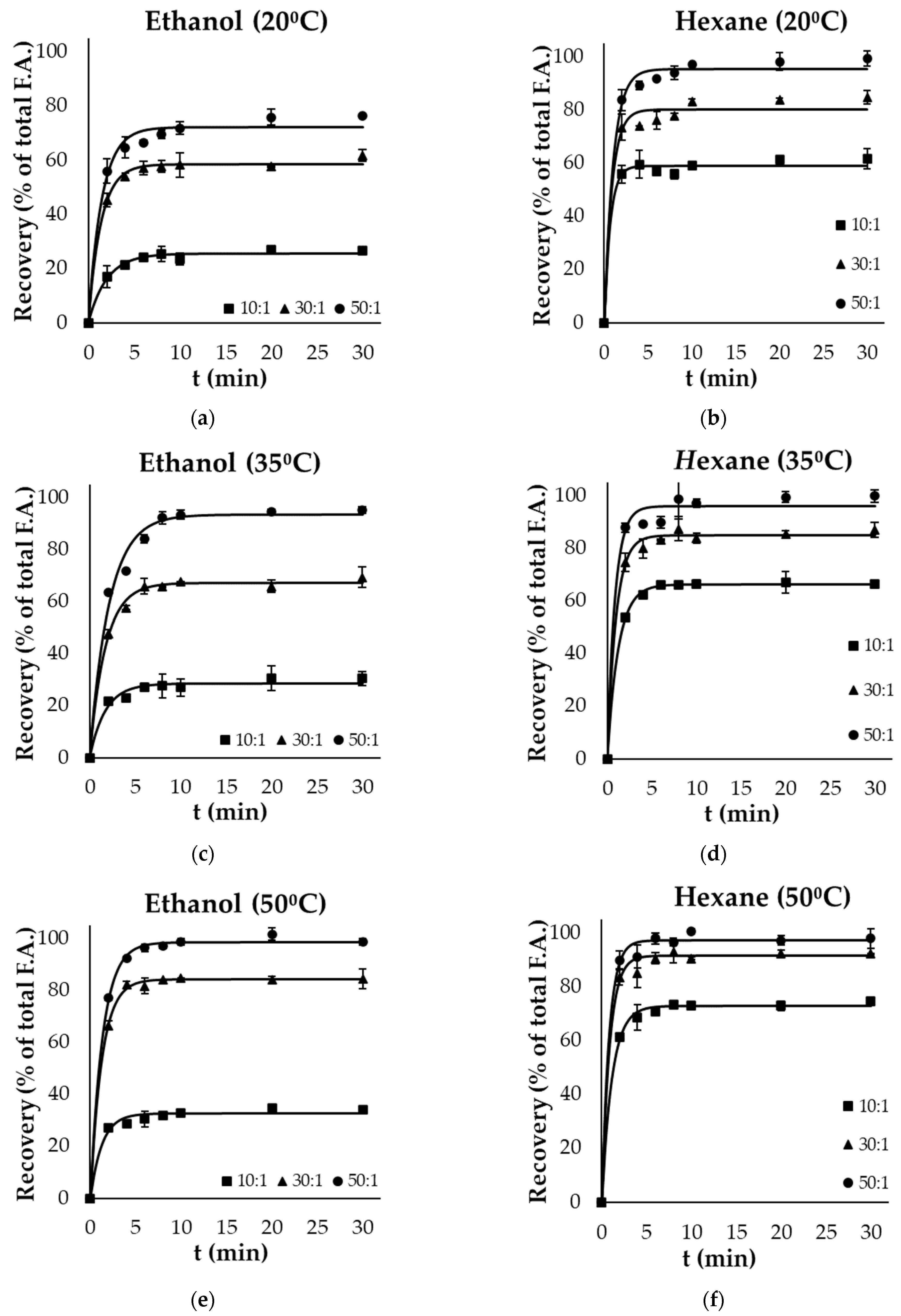
3.2. Lipid Extraction Kinetics
3.3. Fatty Acid Composition of the Extracts
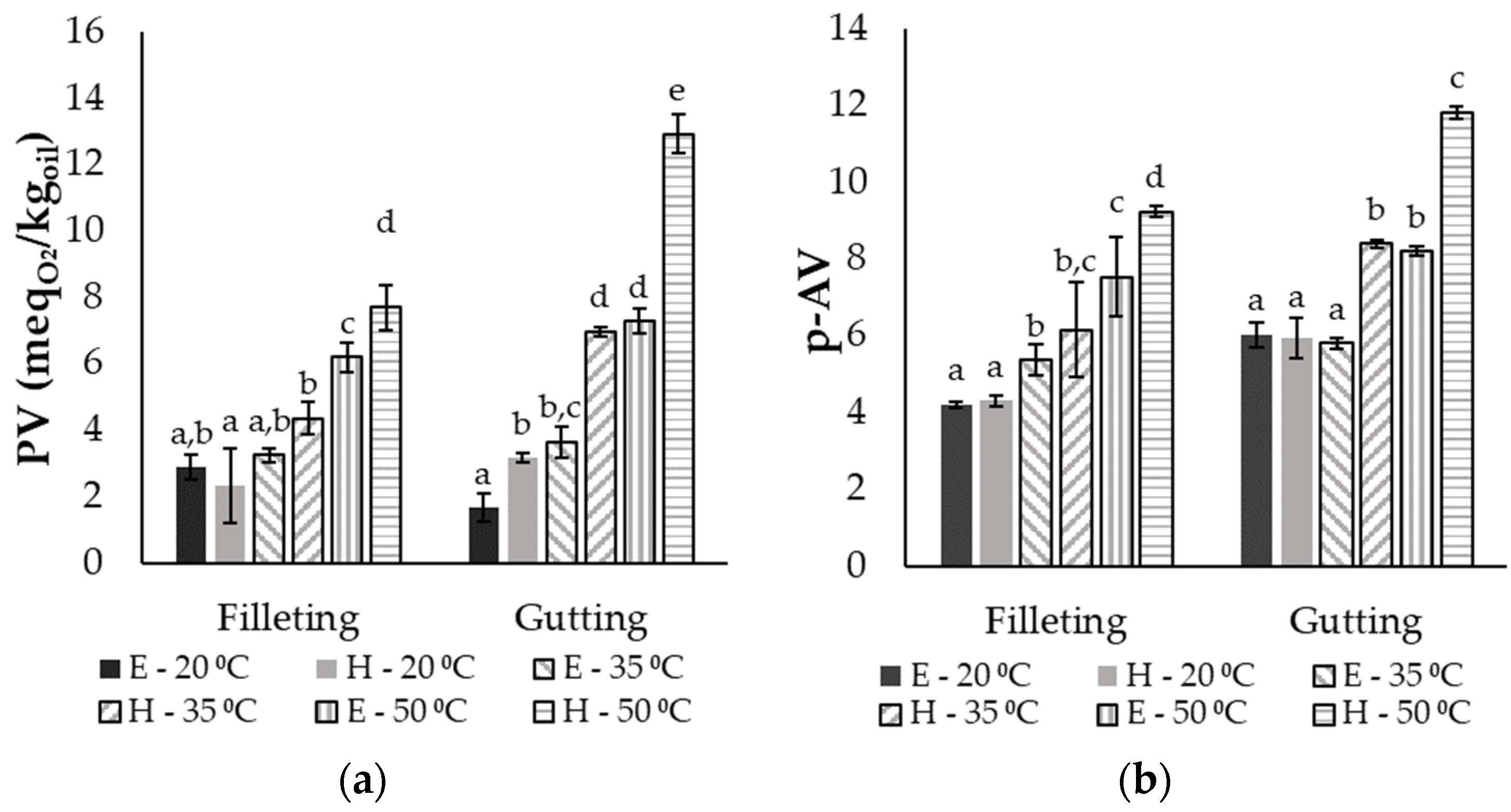
3.4. Oxidation Level of the Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022, towards Blue Transformation; FAO: Rome, Italy, 2022; ISBN 9789251363645. [Google Scholar]
- Tsironi, T.; Semenoglou, I.; Taoukis, P. New Product Development from Marine Sources and Side Streams Valorization Using Nonthermal Processing Technologies. In Nonthermal Processing in Agri-Food-Bio Sciences; Režek Jambrak, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 597–621. ISBN 9783030924157. [Google Scholar]
- Semenoglou, I.; Dimopoulos, G.; Tsironi, T.; Taoukis, P. Mathematical Modelling of the Effect of Solution Concentration and the Combined Application of Pulsed Electric Fields on Mass Transfer during Osmotic Dehydration of Sea Bass Fillets. Food Bioprod. Process. 2020, 121, 186–192. [Google Scholar] [CrossRef]
- HAPO. Annual Report: Aquaculture in Greece. 2023. Available online: https://fishfromgreece.com/wp-content/uploads/2023/10/HAPO_AR23_WEB-NEW.pdf (accessed on 26 March 2024).
- Semenoglou, I.; Eliasson, L.; Uddstål, R.; Tsironi, T.; Taoukis, P.; Xanthakis, E. Supercritical CO2 Extraction of Oil from Arctic Charr Side Streams from Filleting Processing. Innov. Food Sci. Emerg. Technol. 2021, 71, 102712. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the Utilisation of Marine By-Products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9783540699330. [Google Scholar]
- Erkan, N.; Özden, Ö. Proximate Composition and Mineral Contents in Aqua Cultured Sea Bass (Dicentrarchus labrax), Sea Bream (Sparus aurata) Analyzed by ICP-MS. Food Chem. 2007, 102, 721–725. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Zhou, J.; Barba, F.J.; Lorenzo, J.M. Nutritional Characterization of Sea Bass Processing By-Products. Biomolecules 2020, 10, 232. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of Fish Oil Using Green Extraction Methods: A Short Review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020—Sustainability in Action; FAO: Rome, Italy, 2020; ISBN 9789251055687. [Google Scholar]
- Carvajal, A.K.; Mozuraityte, R.; Fisheries, S. Fish Oils: Production and Properties, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780123849472. [Google Scholar]
- Einarsson, M.I.; Jokumsen, A.; Bæk, A.M.; Jacobsen, C.; Samuelsen, T.A.; Pálsson, J.; Eliasen, O. Nordic Centre of Excellence Network in Fishmeal and Fish Oil; Matís: Reykjavík, Iceland, 2019. [Google Scholar] [CrossRef]
- Adeoti, I.A.; Hawboldt, K. A Review of Lipid Extraction from Fish Processing By-Product for Use as a Biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Hathwar, S.C.; Bijinu, B.; Rai, A.K.; Narayan, B. Simultaneous Recovery of Lipids and Proteins by Enzymatic Hydrolysis of Fish Industry Waste Using Different Commercial Proteases. Appl. Biochem. Biotechnol. 2011, 164, 115–124. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.S. Characterization of Phospholipids Extracted from Atlantic Salmon By-Product Using Supercritical CO2 with Ethanol as Co-Solvent. J. Clean. Prod. 2018, 178, 186–195. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in Fish: Extraction, Fractionation, Importance in Health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green. Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. 2009/32/EC: Decision of 23 April 2009 on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients; European Parliament and the Council of the European Union: Strasbourg, France, 2009. [Google Scholar]
- Xie, D.; Jin, J.; Sun, J.; Liang, L.; Wang, X.; Zhang, W.; Wang, X.; Jin, Q. Comparison of Solvents for Extraction of Krill Oil from Krill Meal: Lipid Yield, Phospholipids Content, Fatty Acids Composition and Minor Components. Food Chem. 2017, 233, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted from Cephalothorax of Pacific White Shrimp (Litopenaeus vannamei). Eur. J. Lipid Sci. Technol. 2018, 120, 1700495. [Google Scholar] [CrossRef]
- Aubourg, S.P.; Rodríguez, A.; Trigo, M.; Medina, I. Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction. Foods 2023, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- AOCS Official Method Cd 8-53; AOCS: Champaign, IL, USA, 1998.
- AOCS Official Method Cd 18-90; AOCS: Champaign, IL, USA, 2004.
- de la Fuente, B.; Pinela, J.; Calhelha, R.C.; Heleno, S.A.; Ferreira, I.C.F.R.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata) Head Oils Recovered by Microwave-Assisted Extraction: Nutritional Quality and Biological Properties. Food Bioprod. Process. 2022, 136, 97–105. [Google Scholar] [CrossRef]
- Messina, C.M.; Renda, G.; La Barbera, L.; Santulli, A. By-Products of Farmed European Sea Bass (Dicentrarchus labrax L.) as a Potential Source of n-3 PUFA. Biologia 2013, 68, 288–293. [Google Scholar] [CrossRef]
- Ntzimani, A.; Angelakopoulos, R.; Stavropoulou, N.; Semenoglou, I.; Dermesonlouoglou, E.; Tsironi, T.; Moutou, K.; Taoukis, P. Seasonal Pattern of the Effect of Slurry Ice during Catching and Transportation on Quality and Shelf Life of Gilthead Sea Bream. J. Mar. Sci. Eng. 2022, 10, 443. [Google Scholar] [CrossRef]
- Zotos, A.; Vouzanidou, M. Seasonal Changes in Composition, Fatty Acid, Cholesterol and Mineral Content of Six Highly Commercial Fish Species of Greece. Food Sci. Technol. Int. 2012, 18, 139–149. [Google Scholar] [CrossRef]
- Skalli, A.; Robin, J.H.; Le Bayon, N.; Le Delliou, H.; Person-Le Ruyet, J. Impact of Essential Fatty Acid Deficiency and Temperature on Tissues’ Fatty Acid Composition of European Sea Bass (Dicentrarchus labrax). Aquaculture 2006, 255, 223–232. [Google Scholar] [CrossRef]
- Santos, S.B.D.; Martins, M.A.; Caneschi, A.L.; Aguilar, P.R.M.; Coimbra, J.S.D.R. Kinetics and Thermodynamics of Oil Extraction from Jatropha curcas L. Using Ethanol as a Solvent. Int. J. Chem. Eng. 2015, 2015, 871236. [Google Scholar] [CrossRef]
- Amarante, R.C.A.; Oliveira, P.M.; Schwantes, F.K.; Morón-Villarreyes, J.A. Oil Extraction from Castor Cake Using Ethanol: Kinetics and Thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 6824–6829. [Google Scholar] [CrossRef]
- Sinanoglou, V.; Houhoula, D.; Kyrana, V.; Lougovois, V. Visceral Oil from Farmed Sparus aurata, Dicentrarchus labrax and Diplodus puntazzo as a Source of ω-3 PUFA. Czech J. Food Sci. 2017, 35, 414–423. [Google Scholar] [CrossRef]
- Delgado, A.; Estevez, A.; Hortelano, P.; Alejandre, M.J. Analyses of Fatty Acids from Different Lipids in Liver and Muscle of Sea Bass (Dicentrarchus zabrax L.). Influence of Temperature and Fasting. Comp. Biochem. Physiol. A Physiol. 1994, 108, 673–680. [Google Scholar] [CrossRef]
- Takama, K.; Suzuki, T.; Yoshida, K.; Arai, I.H.; Anma, H. Lipid Content and Fatty Acid Composition of Phospholipids in White-Flesh Fish Species. Fish. Sci. 1994, 60, 177–184. [Google Scholar] [CrossRef]
- Wang, J.L.; Yu, Z.L.; Yin, F.W.; Li, D.Y.; Liu, H.L.; Song, L.; Zhou, D.Y. Comparison of Different Solvents for Extraction of Oils from By-Products of Shrimps Penaeus vannamei and Procambarus clarkia. J. Food Process Preserv. 2021, 45, e15754. [Google Scholar] [CrossRef]
- Sun, W.; Shi, B.; Xue, C.; Jiang, X. The Comparison of Krill Oil Extracted through Ethanol–Hexane Method and Subcritical Method. Food Sci. Nutr. 2019, 7, 700–710. [Google Scholar] [CrossRef]
- Go, A.W.; Pham, T.Y.N.; Ju, Y.-H.; Agapay, R.C.; Angkawijaya, A.E.; Quijote, K.L. Extraction of Lipids from Post-Hydrolysis Spent Coffee Grounds for Biodiesel Production with Hexane as Solvent: Kinetic and Equilibrium Data. Biomass Bioenergy 2020, 140, 105704. [Google Scholar] [CrossRef]
- Mathimani, T.; Uma, L.; Prabaharan, D. Optimization of Direct Solvent Lipid Extraction Kinetics on Marine Trebouxiophycean Alga by Central Composite Design—Bioenergy Perspective. Energy Convers. Manag. 2017, 142, 334–346. [Google Scholar] [CrossRef]
- Mat Yasin, N.H.; Ahmad, N.A.N.; Mohd Hanapi, M.F. Extraction of FAME from Fish Waste by Using Modified Soxhlet Method. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012015. [Google Scholar] [CrossRef]
- Iberahim, N.I.; Tan, B.C. Hexane-Isopropanol Extraction and Quality Assessment of Omega-3 Fish Oil from Atlantic Salmon (Salmo salar). Proc. IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012038. [Google Scholar] [CrossRef]
- Toda, T.A.; Sawada, M.M.; Rodrigues, C.E.C. Kinetics of Soybean Oil Extraction Using Ethanol as Solvent: Experimental Data and Modeling. Food Bioprod. Process. 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Evaluation of Food Grade Solvents for Lipid Extraction and Impact of Storage Temperature on Fatty Acid Composition of Edible Seaweeds Laminaria digitata (Phaeophyceae) and Palmaria palmata (Rhodophyta). Food Chem. 2016, 208, 161–168. [Google Scholar] [CrossRef]
- Schaich, K.M. Challenges in Elucidating Lipid Oxidation Mechanisms: When, Where, and How Do Products Arise? In Lipid Oxidation: Challenges in Food Systems; Logan, A., Nienaber, U., Pan, X., Eds.; AOCS Press: Champaign, IL, USA, 2013; pp. 1–52. ISBN 9780988856516. [Google Scholar]
- FAO/WHO Codex Alimentarius Commision. Standard for Fish Oils (CXS 329-2017) Adopted in 2017; FAO/WHO Codex Alimentarius Commision: Rome, Italy, 2017. [Google Scholar]

| Fatty Acids | Filleting By-Products | Gutting By-Products |
|---|---|---|
| C14:0 | 3.72 ± 0.13 | 2.41 ± 0.01 |
| C16:0 | 18.7 ± 0.4 | 11.0 ± 0.5 |
| C16:1 | 5.36 ± 0.14 | 3.31 ± 0.27 |
| C18:0 | 3.00 ± 0.03 | 3.07 ± 0.16 |
| C18:1 n-9 | 36.3 ± 0.9 | 34.8 ± 0.4 |
| C18:2 n-6 | 16.1 ± 0.7 | 14.5 ± 0.2 |
| C20:1 n-9 | 2.05 ± 0.15 | 1.89 ± 0.15 |
| C20:2 | 0.25 ± 0.05 | 1.07 ± 0.01 |
| C20:4 n-6 | 0.28 ± 0.03 | 1.21 ± 0.12 |
| C20:5 n-3 | 3.54 ± 0.04 | 9.08 ± 0.31 |
| C22:1 n-9 | 1.93 ± 0.37 | 1.27 ± 0.11 |
| C22:6 n-3 | 7.04 ± 0.73 | 12.8 ± 0.3 |
| SFA | 26.9 ± 0.7 | 17.2 ± 0.7 |
| MUFA | 45.8 ± 1.6 | 42.0 ± 1.0 |
| PUFA | 27.3 ± 1.6 | 40.8 ± 1.2 |
| Yf (%) Filleting Side Streams | ||||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Ethanol 10:1 | Ethanol 30:1 | Ethanol 50:1 | Hexane 10:1 | Hexane 30:1 | Hexane 50:1 |
| 20 | 25.6 ± 0.6 aAx | 58.6 ± 0.7 aAy | 72.2 ± 1.6 aAz | 59.1 ± 0.9 aBx | 80.3 ± 1.7 aBy | 95.5 ± 1.4 aBz |
| 35 | 28.6 ± 0.9 bAx | 67.2 ± 0.9 bAy | 93.4 ± 2.3 bAz | 66.4 ± 0.3 bBx | 84.9 ± 0.9 bBy | 96.2 ± 1.8 bBz |
| 50 | 32.7 ± 0.8 cAx | 84.3 ± 0.5 cAy | 98.7 ± 0.7 cAz | 72.9 ± 0.6 cBx | 91.5 ± 1.1 cBy | 97.2 ± 1.1 cBz |
| Yf (%) Gutting Side Streams | ||||||
| 20 | 51.0 ± 0.5 aAx | 75.6 ± 0.5 aAy | 81.2 ± 0.8 aAz | 30.5 ± 0.3 aBx | 60.5 ± 0.2 aBy | 73.1 ± 0.3 aBz |
| 35 | 57.6 ± 0.7 bAx | 87.4 ± 1.6 bAy | 96.8 ± 0.5 bAz | 40.1 ± 0.4 bBx | 65.3 ± 0.2 bBy | 76.2 ± 0.3 bBz |
| 50 | 59.1 ± 1.2 cAx | 92.5 ± 1.8 cAy | 99.7 ± 1.1 cAz | 45.1 ± 0.5 cBx | 72.4 ± 0.4 cBy | 82.6 ± 0.3 cBz |
| k (min−1) Filleting Side Streams | ||||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Ethanol 10:1 | Ethanol 30:1 | Ethanol 50:1 | Hexane 10:1 | Hexane 30:1 | Hexane 50:1 |
| 20 | 0.50 ± 0.06 xa | 0.72 ± 0.06 xa | 0.67 ± 0.09 xa | 1.46 ± 0.36 xb | 1.15 ± 0.27 xb | 0.99 ± 0.13 xb |
| 35 | 0.58 ± 0.11 ya | 0.58 ± 0.04 ya | 0.47 ± 0.06 ya | 0.80 ± 0.03 yb | 1.01 ± 0.10 yb | 1.15 ± 0.24 yb |
| 50 | 0.78 ± 0.13 xa | 0.79 ± 0.03 xa | 0.74 ± 0.04 xa | 0.88 ± 0.06 xb | 1.16 ± 0.17 xb | 1.24 ± 0.17 xb |
| k (min−1) Gutting Side Streams | ||||||
| 20 | 1.71 ± 0.14 xa | 2.16 ± 0.16 xa | 2.25 ± 0.24 xa | 2.59 ± 0.18 xb | 2.71 ± 0.14 xb | 3.10 ± 0.23 xb |
| 35 | 1.91 ± 0.21 ya | 1.70 ± 0.18 ya | 1.85 ± 0.08 ya | 2.27 ± 0.17 yb | 2.48 ± 0.13 yb | 2.26 ± 0.10 yb |
| 50 | 2.06 ± 0.25 xa | 1.77 ± 0.30 xa | 1.66 ± 0.14 xa | 1.90 ± 0.19 xb | 2.22 ± 0.15 xb | 2.04 ± 0.11 xb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenoglou, I.; Katsouli, M.; Giannakourou, M.; Taoukis, P. Recovery of Omega-3-Rich Lipids: Toward the Sustainable Valorization of Sea-Bass Industry Side Streams. Separations 2024, 11, 101. https://doi.org/10.3390/separations11040101
Semenoglou I, Katsouli M, Giannakourou M, Taoukis P. Recovery of Omega-3-Rich Lipids: Toward the Sustainable Valorization of Sea-Bass Industry Side Streams. Separations. 2024; 11(4):101. https://doi.org/10.3390/separations11040101
Chicago/Turabian StyleSemenoglou, Ioanna, Maria Katsouli, Maria Giannakourou, and Petros Taoukis. 2024. "Recovery of Omega-3-Rich Lipids: Toward the Sustainable Valorization of Sea-Bass Industry Side Streams" Separations 11, no. 4: 101. https://doi.org/10.3390/separations11040101