Carbon–Carbon Composite Membranes Derived from Small-Molecule-Compatibilized Immiscible PBI/6FDA-DAM-DABA Polymer Blends
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of 6FDA-DAM:6FDA-DABA [3:2] Polymer (6FDD)
2.3. Membrane Preparation
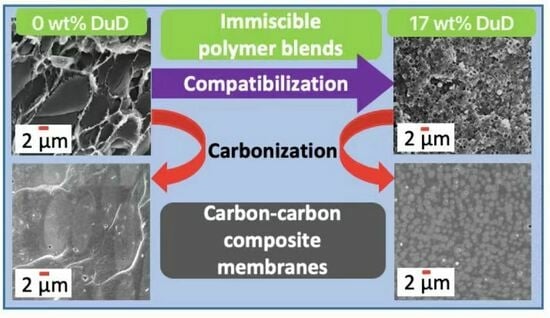
2.3.1. Preparation of Polymer Blend Membranes
2.3.2. Preparation of Compatibilized Polymer Blend Membranes
2.3.3. Preparation of Carbon Membranes
2.4. Characterization
2.4.1. Characterization of the Synthesized Polymer 6FDD
2.4.2. Characterization of Membranes (SEM, TGA, FTIR, Raman)
2.4.3. Gas Permeation Testing
3. Results and Discussion
3.1. Membrane Microstructure
3.1.1. SEM Images of Membrane Cross-Sections
3.1.2. 6FDD Domain Size Distributions
3.2. Spectroscopic Characterization of Membranes
3.2.1. FTIR Spectroscopy
3.2.2. Raman Spectroscopy
3.3. Gas Permeation Properties of Membranes
3.3.1. Gas Permeation of Polymer Blend Membranes
3.3.2. Gas Permeation of CMSMs from Polymer Blends
3.4. Understanding the Applicability of Gas Permeation Models for Precursors and CMSMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α | Ideal gas selectivity |
| ∆p | Differential gas pressure across the membrane |
| 1H-NMR | Proton nuclear magnetic resonance |
| 6FDA | 4,4-(hexafluoroisopropylidene)diphthalic anhydride |
| 6FDD | 6FDA-DAM:6FDA-DABA [3:2] polymer |
| A | Membrane exposed area |
| Å | Angstrom |
| ATR-FTIR | Attenuated total reflectance Fourier transform infrared |
| CMSM | Carbon molecular sieve membrane |
| D | Raman band from disordered domains |
| DABA | 3,5-diaminobenzoic acid |
| DAM | 2,4,6-trimethyl-1,3-phenylenediamine |
| DMAc | N,N-dimethylacetamide |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| DSC | Differential scanning calorimeter |
| DuD | 2,3,5,6-tetramethyl-1,4-phenylenediamine (durene diamine) |
| EBM | Equivalent box model of gas transport in composite materials |
| fn | The average number of PBI passages in a unit length at n% DuD loading |
| G | Raman band from graphitic domains |
| ID | Intensity from disordered domains |
| IG | Intensity from graphitic domains |
| J | Flow rate |
| kDa | Kilodalton |
| L | Membrane thickness |
| La | Crystallite size along the a axis |
| LiCl | Lithium chloride |
| Mw | Molecular weight |
| NMP | N-methyl pyrrolidone |
| PBI | Polybenzimidazole |
| PDI | Polydispersity index |
| P | Permeability |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| THF | Tetrahydrofuran |
| TMS | Tetramethylsilane |
| tn | Average thickness of PBI passages in membrane at n% DuD loading |
| UHP | Ultra-high purity |
References
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Tanco, M.L.; Tanaka, D.P. Recent advances on carbon molecular sieve membranes (CMSMs) and reactors. Processes 2016, 4, 29. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kwon, Y.; Kim, D.; Park, H.; Cho, Y.H.; Nam, S.-E.; Park, Y.-I. A review on polymer precursors of carbon molecular sieve membranes for olefin/paraffin separation. Membranes 2021, 11, 482. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F. Carbon membranes for gas separation processes: Recent progress and future perspective. J. Membr. Sci. Res. 2015, 1, 2–15. [Google Scholar]
- Williams, P.J.; Koros, W.J. Gas Separation by Carbon Membranes. In Advanced Membrane Technology and Applications; Li, N.N., Fane, A.G., Ho, W.S.W., Matsuura, T., Eds.; John Wiley & Sons, Incorporated: New York, NY, USA, 2008; pp. 599–631. [Google Scholar]
- Rahimalimamaghani, A.; Ramezani, R.; Tanaka, D.A.P.; Gallucci, F. Carbon molecular sieve membranes for selective CO2/CH4 and CO2/N2 separation: Experimental study, optimal process design, and economic analysis. Ind. Eng. Chem. Res. 2023, 62, 19116–19132. [Google Scholar] [CrossRef]
- Ngamou, P.H.T.; Ivanova, M.E.; Guillon, O.; Meulenberg, W.A. High-performance carbon molecular sieve membranes for hydrogen purification and pervaporation dehydration of organic solvents. J. Mater. Chem. A 2019, 7, 7082–7091. [Google Scholar] [CrossRef]
- Panapitiya, N.P.; Wijenayake, S.N.; Huang, Y.; Bushdiecker, D.; Nguyen, D.; Ratanawanate, C.; Kalaw, G.J.; Gilpin, C.J.; Musselman, I.H.; Balkus, K.J.; et al. Stabilization of immiscible polymer blends using structure directing metal-organic frameworks (MOFs). Polymer 2014, 55, 2028–2034. [Google Scholar] [CrossRef]
- Panapitiya, N.P.; Wijenayake, S.N.; Nguyen, D.D.; Huang, Y.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Gas separation membranes derived from high-performance immiscible polymer blends compatibilized with small molecules. ACS Appl. Mater. Interfaces 2015, 7, 18618–18627. [Google Scholar] [CrossRef]
- Bhuwania, N.; Labreche, Y.; Achoundong, C.S.K.; Baltazar, J.; Burgess, S.K.; Karwa, S.; Xu, L.; Henderson, C.L.; Williams, P.J.; Koros, W.J. Engineering substructure morphology of asymmetric carbon molecular sieve hollow fiber membranes. Carbon 2014, 76, 417–434. [Google Scholar] [CrossRef]
- Hatori, H.; Kobayashi, T.; Hanzawa, Y.; Yamada, Y.; Iimura, Y.; Kimura, T.; Shiraishi, M. Mesoporous carbon membranes from polyimide blended with poly(ethylene glycol). J. Appl. Polym. Sci. 2001, 79, 836–841. [Google Scholar] [CrossRef]
- Gilron, J.; Soffer, A. Knudsen diffusion in microporous carbon membranes with molecular sieving character. J. Membr. Sci. 2002, 209, 339–352. [Google Scholar] [CrossRef]
- Hu, L.; Bui, V.T.; Krishnamurthy, A.; Fan, S.; Guo, W.; Pal, S.; Chen, X.; Zhang, G.; Ding, Y.; Singh, R.P.; et al. Tailoring sub-3.3 Å ultramicropores in advanced carbon molecular sieve membranes for blue hydrogen production. Sci. Adv. 2022, 8, eabl8160. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Park, H.B.; Lee, Y.M. Gas separation properties of carbon molecular sieve membranes derived from polyimide/polyvinylpyrrolidone blends: Effect of the molecular weight of polyvinylpyrrolidone. J. Membr. Sci. 2005, 251, 159–167. [Google Scholar] [CrossRef]
- Tin, P.S.; Xiao, Y.; Chung, T.S. Polyimide-carbonized membranes for gas separation: Structural, composition, and morphological control of precursors. Sep. Purif. Rev. 2006, 35, 285–318. [Google Scholar] [CrossRef]
- Park, H.B.; Kim, Y.K.; Lee, J.M.; Lee, S.Y.; Lee, Y.M. Relationship between chemical structure of aromatic polyimides and gas permeation properties of their carbon molecular sieve membranes. J. Membr. Sci. 2004, 229, 117–127. [Google Scholar] [CrossRef]
- Briceño, K.; Montané, D.; Garcia-Valls, R.; Iulianelli, A.; Basile, A. Fabrication variables affecting the structure and properties of supported carbon molecular sieve membranes for hydrogen separation. J. Membr. Sci. 2012, 415–416, 288–297. [Google Scholar] [CrossRef]
- Fu, S.; Wenz, G.B.; Sanders, E.S.; Kulkarni, S.S.; Qiu, W.; Ma, C.; Koros, W.J. Effects of pyrolysis conditions on gas separation properties of 6FDA/DETDA: DABA(3:2) derived carbon molecular sieve membranes. J. Membr. Sci. 2016, 520, 699–711. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kouvelos, E.P.; Romanos, G.E.; Pilatos, G.I.; Mitropoulos, A.C.; Kanellopoulos, N.K. Characterization of highly selective microporous carbon hollow fiber membranes prepared from a commercial co-polyimide precursor. J. Porous Mater. 2008, 15, 625–633. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Omidkhah, M.R.; Moghaddam, A.Z.; Pirouzfar, V.; Krantz, W.B.; Tan, N.R. Enhancing the properties and gas separation performance of PBI–polyimides blend carbon molecular sieve membranes via optimization of the pyrolysis process. Sep. Purif. Technol. 2014, 122, 278–289. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. An investigation of the effects of pyrolysis parameters on gas separation properties of carbon materials. Carbon 2005, 43, 1843–1856. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Quan, W.; Koros, W.J. Advanced carbon molecular sieve membranes derived from molecularly engineered cross-linkable copolyimide for gas separations. Nat. Mater. 2023, 22, 109–116. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Centeno, T.A. Carbon molecular sieve membranes from polyetherimide. Micropor. Mesopor. Mat. 1998, 26, 23–26. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Shih, K.; Shiu, P.-T.; Wey, M.-Y. Influence of support structure on the permeation behavior of polyetherimide-derived carbon molecular sieve composite membrane. J. Membr. Sci. 2012, 405–406, 250–260. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F. Effect of stabilization temperature on gas permeation properties of carbon hollow fiber membrane. J. Appl. Polym. Sci. 2013, 127, 2840–2846. [Google Scholar] [CrossRef]
- Teixeira, M.; Campo, M.C.; Tanaka, D.A.P.; Tanco, M.A.L.; Magen, C.; Mendes, A. Composite phenolic resin-based carbon molecular sieve membranes for gas separation. Carbon 2011, 49, 4348–4358. [Google Scholar] [CrossRef]
- Teixeira, M.; Rodrigues, S.C.; Campo, M.; Tanaka, D.A.P.; Tanco, M.A.L.; Madeira, L.M.; Sousa, J.M.; Mendes, A. Boehmite-phenolic resin carbon molecular sieve membranes—Permeation and adsorption studies. Chem. Eng. Res. Des. 2014, 92, 2668–2680. [Google Scholar] [CrossRef]
- Wei, W.; Qin, G.; Hu, H.; You, L.; Chen, G. Preparation of supported carbon molecular sieve membrane from novolac phenol–formaldehyde resin. J. Membr. Sci. 2007, 303, 80–85. [Google Scholar] [CrossRef]
- Nie, J.; Okada, F.; Kita, H.; Tanaka, K.; Mihara, T.; Kondo, D.; Yamashita, Y.; Yahagi, N. Fabrication of carbon molecular sievemembranes supported on a novel porous carbon fiber. Energy Fuels 2022, 36, 7147–7157. [Google Scholar] [CrossRef]
- Perez, E.V.; Ferraris, J.P.; Balkus, K.J.; Musselman, I.H. Effect of the annealing temperature of polybenzimidazole membranes in high pressure and high temperature H2/CO2 gas separations. J. Membr. Sci. 2023, 677, 121619. [Google Scholar] [CrossRef]
- Asano, A.; Takegoshi, K. Polymer Blends and Miscibility. In Solid State NMR of Polymers; Ando, I., Asakura, T., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1998; pp. 351–414. [Google Scholar]
- Hosseini, S.S.; Chung, T.S. Carbon membranes from blends of PBI and polyimides for N2/CH4 and CO2/CH4 separation and hydrogen purification. J. Membr. Sci. 2009, 328, 174–185. [Google Scholar] [CrossRef]
- Dickinson, L.C.; Yang, H.; Chu, C.-W.; Stein, R.S.; Chien, J.C.W. Limits to compatibility in poly(x-methylstyrene)/Poly (2,6-dimethylphenylene oxide) blends by NMR. Macromolecules 1987, 20, 1757–1760. [Google Scholar] [CrossRef]
- Kaplan, D.S. Structure–property relationships in copolymers to composites: Molecular interpretation of the glass transition phenomenon. J. Appl. Polym. Sci. 1976, 20, 2615–2629. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Hu, C.-C.; Lin, D.-W.; Tsai, H.-A.; Huang, S.-H.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Adjustable microstructure carbon molecular sieve membranes derived from thermally stable polyetherimide/polyimide blends for gas separation. Carbon 2017, 113, 10–17. [Google Scholar] [CrossRef]
- Kyotani, T. Control of pore structure in carbon. Carbon 2000, 38, 269–286. [Google Scholar] [CrossRef]
- Lee, H.J.; Suda, H.; Haraya, K. Preparation of carbon membranes derived from polymer blends in the presence of a thermally labile polymer. Sep. Sci. Technol. 2007, 42, 59–71. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, H.B.; Lee, Y.M. Carbon molecular sieve membranes derived from thermally labile polymer containing blend polymers and their gas separation properties. J. Membr. Sci. 2004, 243, 9–17. [Google Scholar] [CrossRef]
- Saufi, S.M.; Ismail, A.F. Fabrication of carbon membranes for gas separation––A review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Centeno, T.A.; Fuertes, A.B. Carbon molecular sieve membranes derived from a phenolic resin supported on porous ceramic tubes. Sep. Purif. Technol. 2001, 25, 379–384. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.-S.; Hill, A.J. Advanced fabrication of carbon molecular sieve membranes by nonsolvent pretreatment of precursor polymers. Ind. Eng. Chem. Res. 2004, 43, 6476–6483. [Google Scholar] [CrossRef]
- Perez, E.; Karunaweera, C.; Musselman, I.; Balkus, K.; Ferraris, J. Origins and evolution of inorganic-based and MOF-based mixed-matrix membranes for gas separations. Processes 2016, 4, 32. [Google Scholar] [CrossRef]
- Lua, A.C.; Shen, Y. Preparation and characterization of polyimide–silica composite membranes and their derived carbon–silica composite membranes for gas separation. Chem. Eng. J. 2013, 220, 441–451. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, T.; Wu, Y.; Liu, Q.; Liu, S.; Zhang, S.; Qiu, J. Preparation and gas permeation of composite carbon membranes from poly(phthalazinone ether sulfone ketone). Sep. Purif. Technol. 2008, 60, 259–263. [Google Scholar] [CrossRef]
- Rao, P.S.; Wey, M.-Y.; Tseng, H.-H.; Kumar, I.A.; Weng, T.-H. A comparison of carbon/nanotube molecular sieve membranes with polymer blend carbon molecular sieve membranes for the gas permeation application. Micropor. Mesopor. Mat. 2008, 113, 499–510. [Google Scholar] [CrossRef]
- Richter, H.; Voss, H.; Kaltenborn, N.; Kämnitz, S.; Wollbrink, A.; Feldhoff, A.; Caro, J.; Roitsch, S.; Voigt, I. High-flux carbon molecular sieve membranes for gas separation. Angew. Chem. Int. Ed. 2017, 56, 7760–7763. [Google Scholar] [CrossRef]
- Hayashi, J.-I.; Mizuta, H.; Yamamoto, M.; Kusakabe, K.; Morooka, S. Pore size control of carbonized BPDA-pp′ ODA polyimide membrane by chemical vapor deposition of carbon. J. Membr. Sci. 1997, 124, 243–251. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer Blends: A Comprehensive Review; Hanser: Cincinnati, OH, USA, 2007. [Google Scholar]
- Panapitiya, N.; Wijenayake, S.; Nguyen, D.; Karunaweera, C.; Huang, Y.; Balkus, K.; Musselman, I.; Ferraris, J. Compatibilized immiscible polymer blends for gas separations. Materials 2016, 9, 643. [Google Scholar] [CrossRef]
- Omole, I.C.; Miller, S.J.; Koros, W.J. Increased molecular weight of a cross-linkable polyimide for spinning plasticization resistant hollow fiber membranes. Macromolecules 2008, 41, 6367–6375. [Google Scholar] [CrossRef]
- Grubb, T.L.; Ulery, V.L.; Smith, T.J.; Tullos, G.L.; Yagci, H.; Mathias, L.J.; Langsam, M. Highly soluble polyimides from sterically hindered diamines. Polymer 1999, 40, 4279–4288. [Google Scholar] [CrossRef]
- Fu, S.; Sanders, E.S.; Kulkarni, S.S.; Koros, W.J. Carbon molecular sieve membrane structure–property relationships for four novel 6FDA based polyimide precursors. J. Membr. Sci. 2015, 487, 60–73. [Google Scholar] [CrossRef]
- Pye, D.; Hoehn, H.; Panar, M. Measurement of gas permeability of polymers. I. Permeabilities in constant volume/variable pressure apparatus. J. Appl. Polym. Sci. 1976, 20, 1921–1931. [Google Scholar] [CrossRef]
- Yasuda, H. Units of gas permeability constants. J. Appl. Polym. Sci. 1975, 19, 2529–2536. [Google Scholar] [CrossRef]
- Robeson, L.M. Polymer Blends in Membrane Transport Processes. Ind. Eng. Chem. Res. 2010, 49, 11859–11865. [Google Scholar] [CrossRef]
- Vallerot, J.-M.; Bourrat, X.; Mouchon, A.; Chollon, G. Quantitative structural and textural assessment of laminar pyrocarbons through Raman spectroscopy, electron diffraction and few other techniques. Carbon 2006, 44, 1833–1844. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Bacsa, W.S.; Lannin, J.S.; Pappas, D.L.; Cuomo, J.J. Raman scattering of laser-deposited amorphous carbon. Phys. Rev. B 1993, 47, 10931–10934. [Google Scholar] [CrossRef]
- McEvoy, N.; Peltekis, N.; Kumar, S.; Rezvani, E.; Nolan, H.; Keeley, G.P.; Blau, W.J.; Duesberg, G.S. Synthesis and analysis of thin conducting pyrolytic carbon films. Carbon 2012, 50, 1216–1226. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 2011, 4, 385–393. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Materials Science and Engineering of Carbon: Characterization; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cosey, W.K.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Reduced aging in carbon molecular sieve membranes derived from PIM-1 and MOP-18. Ind. Eng. Chem. Res. 2021, 60, 9962–9970. [Google Scholar] [CrossRef]
- Aili, D.; Cleemann, L.N.; Li, Q.; Jensen, J.O.; Christensen, E.; Bjerrum, N.J. Thermal curing of PBI membranes for high temperature PEM fuel cells. J. Mater. Chem. 2012, 22, 5444–5453. [Google Scholar] [CrossRef]
- Kumar, A.; Tateyama, S.; Yasaki, K.; Ali, M.A.; Takaya, N.; Singh, R.; Kaneko, T. 1H NMR and FT-IR dataset based structural investigation of poly(amic acid)s and polyimides from 4,4′-diaminostilbene. Data Brief 2016, 7, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Glass, T.E.; Lyle, G.D.; McGrath, J.E. Kinetic and mechanistic investigations of the formation of polyimides under homogeneous conditions. Macromolecules 1993, 26, 1344–1358. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, C.-C.; Xu, L.; Cui, L.; Paul, D.R.; Koros, W.J. Sub-Tg cross-linking of a polyimide membrane for enhanced CO2 plasticization resistance for natural gas separation. Macromolecules 2011, 44, 6046–6056. [Google Scholar] [CrossRef]









| Membrane | Average 6FDD Domain Size (µm) | Relative Standard Deviation (%) |
|---|---|---|
| 0DuD-6FDD:PBI | 5.6 ± 5.6 | 100 |
| 5DuD-6FDD:PBI | 1.6 ± 0.6 | 38 |
| 9DuD-6FDD:PBI | 0.9 ± 0.3 | 33 |
| 17DuD-6FDD:PBI | 0.8 ± 0.2 | 19 |
| Membrane | ID/IG (Height) | La (nm) |
|---|---|---|
| 6FDD CMSM | 0.85 | 5.2 |
| PBI CMSM | 0.40 | 11.0 |
| 6FDD:PBI CMSM | 0.70 | 6.3 |
| 17DuD-6FDD:PBI CMSM | 0.44 | 10.0 |
| Membrane | P-H2 | P-CO2 | α (H2/CO2) |
|---|---|---|---|
| PBI | 1.30 | 0.05 | 22.6 |
| 6FDD | 100 | 51 | 1.9 |
| 0DuD-6FDD:PBI | 3.6 | 0.5 | 7.2 |
| 5DuD-6FDD:PBI | 10 ± 1 | 1.3 ± 0.1 | 8 ± 1 |
| 9DuD-6FDD:PBI | 17 ± 2 | 1.0 ± 0.1 | 18 ± 1 |
| 17DuD-6FDD:PBI | 27 ± 1 | 1.3 ± 0.1 | 20 ± 1 |
| Membrane | P-H2 | P-CO2 | α (H2/CO2) |
|---|---|---|---|
| PBI | 10.3 | 0.6 | 16.6 |
| 6FDD | 19,400 | 16,740 | 1.2 |
| 0DuD-6FDD:PBI | 97 | 29 | 3.4 |
| 5DuD-6FDD:PBI | 129 ± 9 | 21 ± 1 | 6.1 ± 0.2 |
| 9DuD-6FDD:PBI | 290 ± 10 | 27 ± 1 | 9.8 ± 0.4 |
| 17DuD-6FDD:PBI | 231 ± 5 | 15 ± 1 | 15.0 ± 0.1 |
| Membrane | P-H2 | P-CO2 | α (H2/CO2) |
|---|---|---|---|
| 9DuD-6FDD:PBI 550 CMSM | 290 ± 10 | 27 ± 1 | 9.8 ± 0.4 |
| 9DuD-6FDD:PBI 675 CMSM | 196 | 14 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunaweera, C.; Panapitiya, N.P.; Panangala, S.; Perez, E.V.; Musselman, I.H.; Balkus, K.J., Jr.; Ferraris, J.P. Carbon–Carbon Composite Membranes Derived from Small-Molecule-Compatibilized Immiscible PBI/6FDA-DAM-DABA Polymer Blends. Separations 2024, 11, 108. https://doi.org/10.3390/separations11040108
Karunaweera C, Panapitiya NP, Panangala S, Perez EV, Musselman IH, Balkus KJ Jr., Ferraris JP. Carbon–Carbon Composite Membranes Derived from Small-Molecule-Compatibilized Immiscible PBI/6FDA-DAM-DABA Polymer Blends. Separations. 2024; 11(4):108. https://doi.org/10.3390/separations11040108
Chicago/Turabian StyleKarunaweera, Chamaal, Nimanka P. Panapitiya, Samitha Panangala, Edson V. Perez, Inga H. Musselman, Kenneth J. Balkus, Jr., and John P. Ferraris. 2024. "Carbon–Carbon Composite Membranes Derived from Small-Molecule-Compatibilized Immiscible PBI/6FDA-DAM-DABA Polymer Blends" Separations 11, no. 4: 108. https://doi.org/10.3390/separations11040108