Development of a Combined Heart-Cut and Comprehensive Two-Dimensional Gas Chromatography System to Extend the Carbon Range of Volatile Organic Compounds Analysis in a Single Instrument
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bachok Demonstration ‘International Opportunities Fund’ Campaign
2.2. Compound Identification and Quantification
2.3. Supporting Measurements
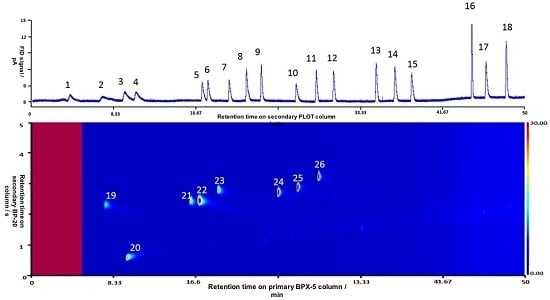
3. Results and Discussion
3.1. Development of the GC-GC×GC
3.2. Comparison of GC-GC×GC with an Established DC-GC
3.3. Bachok Demonstration ‘International Opportunities Fund’ campaign
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1D | one-dimensional |
| 2D | two-dimensional |
| BMRS | Bachok marine research station |
| ClearfLo | Clean air for London campaign |
| DC-GC | dual-channel gas chromatography |
| FID | flame ionisation detector |
| GC | gas chromatography |
| GC-GC | heart-cut gas chromatography |
| GC-GC×GC | combined heart-cut and comprehensive two-dimensional gas chromatography |
| GC×GC | comprehensive two-dimensional gas chromatography |
| IOES | Institute of Ocean and Earth Sciences |
| MS | mass spectrometer |
| NCAS | National Centre for Atmospheric Science |
| NERC | National Environment Research Council |
| O3 | ozone |
| OVOCs | oxygenated volatile organic compounds |
| PLOT | porous layer open tubular column |
| PTR-MS | proton transfer reaction - mass spectrometer |
| SOA | secondary organic aerosol |
| TD | thermal desorption |
| UM | University of Malaya |
| VOCs | volatile organic compounds |
Appendix

References
- Singh, H. Guidance for the Collection and Use of Ambient Hydrocarbon Species Data in Development of Ozone Control Strategies; U.S. Environment Protection Agency, EPA 450/4-80-008: 1980. Available online: http://nepis.epa.gov/Exe/ZyNET.exe/91010WPX.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1976+Thru+1980&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C76thru80%5CTxt%5C00000021%5C91010WPX.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=p%7Cf&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 1 June 2016).
- Goldstein, A.; Galbally, I. Known and unexplored organic constituents in the Earth’s atmosphere. Environ. Sci. Technol. 2007, 41, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Dunmore, R.; Hopkins, J.; Lidster, R.; Lee, J.; Evans, M.; Rickard, A.; Lewis, A.; Hamilton, J. Diesel-related hydrocarbons can dominate gas phase reactive carbon in megacities. Atmos. Chem. Phys. 2015, 15, 9983–9996. [Google Scholar] [CrossRef]
- Amador-Munoz, O.; Marriott, P. Quantification in comprehensive two-dimensional gas chromatography and a model of quantification based on selected summed modulated peaks. J. Chromatogr. A 2008, 1184, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.; Jussila, M.; Rissanen, T.; Anttila, P.; Hartonen, K.; Reissell, A.; Vreuls, R.; Adahchour, M.; Hyotylainen, T. Comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry in the identification of organic compounds in atmospheric aerosols from coniferous forest. J. Chromatogr. A 2006, 1125, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Bohnenstengel, S.; Belcher, S.; Aiken, A.; Allan, J.; Allen, G.; Bacak, A.; Bannan, T.; Barlow, J.; Beddows, D.; Bloss, W.; et al. Meteorology, air quality, and health in London: The ClearfLo project. Bull. Am. Meteorol. Soc. 2014. [Google Scholar] [CrossRef]
- Hopkins, J.; Jones, C.; Lewis, A. A dual channel gas chromatograph for atmospheric analysis of volatile organic compounds including oxygenated and monoterpene compounds. J. Environ. Monit. 2011, 13, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.; Lee, J.; MacKenzie, A.; Barkley, M.; Carslaw, N.; Carver, G.; Chappell, N.; Coe, H.; Collier, C.; Commane, R.; et al. Overview: oxidant and particle photochemical processes above a south-east Asian tropical rainforest (the OP3 project): introduction, rationale, location characteristics and tools. Atmos. Chem. Phys. 2010, 10, 169–199. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.; Eyres, G.; Marriott, P. System Design for Integrated Comprehensive and Multidimensional Gas Chromatography with Mass Spectrometry and Olfactometry. Anal. Chem. 2012, 84, 9154–9162. [Google Scholar] [CrossRef] [PubMed]
- Maikhunthod, B.; Morrison, P.; Small, D.; Marriott, P. Development of a switchable multidimensional/ comprehensive two-dimensional gas chromatographic analytical system. J. Chromatogr. A 2010, 1217, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Hartmann, C.; Marriott, P. Multidimensional gas chromatography methods for bioanalytical research. Bioanalysis 2014, 6, 2461–2479. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.; Lewis, A.; Read, K. A two-column method for long-term monitoring of non-methane hydrocarbons (NMHCs) and oxygenated volatile organic compounds (OVOCs). J. Environ. Monit. 2003, 5, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lidster, R.; Hamilton, J.; Lewis, A. The application of two total transfer valve modulators for comprehensive two-dimensional gas chromatography of volatile organic compounds. J. Sep. Sci. 2011, 34, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, M.; Szulejko, J. Simulation of the breakthrough behavior of volatile organic compounds against sorbent tube sampler as a function of concentration level and sampling volume. Anal. Chim. Acta 2014, 835, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Carslaw, D.; Ropkins, K. Openair—An R package for air quality data analysis. Environ. Model. Softw. 2012, 27–28, 52–61. [Google Scholar] [CrossRef]
- Carslaw, D.; Ropkins, K. Openair: Open-Source Tools for the Analysis of Air Pollution Data. R package version 0.6-2. 2012. Available online: http://journal.r-project.org/archive/2012-1/RJournal 2012-1 Ropkins+Carslaw.pdf (accessed on 1 June 2016).
- R Development Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2012. [Google Scholar]
- Murphy, J.; Oram, D.; Reeves, C. Measurements of volatile organic compounds over West Africa. Atmos. Chem. Phys. 2010, 10, 5281–5294. [Google Scholar] [CrossRef]








| Compounds | Gradient | Intercept/10 | R | |
|---|---|---|---|---|
| 1 | n-Propane | −1.5010 | 4.1427 | 0.15 |
| 2 | -Butane | −1.8479 | 4.5295 | 0.17 |
| 3 | n-Butane | −0.3147 | 4.5533 | 0.58 |
| 5 | -2-Butene | 0.0995 | −0.7799 | 0.81 |
| 6 | 1-Butene | 0.1419 | −1.0857 | 0.69 |
| 7 | -Butene | 0.0937 | −0.7115 | 0.83 |
| 8 | -Pentane | 0.0916 | −0.9669 | 0.87 |
| 9 | n-Pentane | 0.0368 | −0.0771 | 0.99 |
| 10 | 1,3-Butadiene | 0.0654 | 0.2000 | 0.43 |
| 11 | -2-Pentene | 0.0393 | −0.0736 | 0.99 |
| 13 | 2/3-methyl Pentane | 0.0322 | −0.1313 | 0.99 |
| 14 | n-Hexane | 0.0302 | −0.0658 | 0.99 |
| 15 | Isoprene | 0.0397 | −0.0623 | 0.99 |
| 16 | n-Heptane | 0.0264 | −0.0751 | 0.99 |
| 17 | Benzene | 0.0312 | −0.0910 | 0.99 |
| 18 | 2,2,4-trimethyl-Pentane | 0.0231 | −0.0602 | 0.99 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunmore, R.E.; Hopkins, J.R.; Lidster, R.T.; Mead, M.I.; Bandy, B.J.; Forster, G.; Oram, D.E.; Sturges, W.T.; Phang, S.-M.; Samah, A.A.; et al. Development of a Combined Heart-Cut and Comprehensive Two-Dimensional Gas Chromatography System to Extend the Carbon Range of Volatile Organic Compounds Analysis in a Single Instrument. Separations 2016, 3, 21. https://doi.org/10.3390/separations3030021
Dunmore RE, Hopkins JR, Lidster RT, Mead MI, Bandy BJ, Forster G, Oram DE, Sturges WT, Phang S-M, Samah AA, et al. Development of a Combined Heart-Cut and Comprehensive Two-Dimensional Gas Chromatography System to Extend the Carbon Range of Volatile Organic Compounds Analysis in a Single Instrument. Separations. 2016; 3(3):21. https://doi.org/10.3390/separations3030021
Chicago/Turabian StyleDunmore, Rachel E, James R Hopkins, Richard T Lidster, Mohammed Iqbal Mead, Brian J Bandy, Grant Forster, David E Oram, William T Sturges, Siew-Moi Phang, Azizan Abu Samah, and et al. 2016. "Development of a Combined Heart-Cut and Comprehensive Two-Dimensional Gas Chromatography System to Extend the Carbon Range of Volatile Organic Compounds Analysis in a Single Instrument" Separations 3, no. 3: 21. https://doi.org/10.3390/separations3030021