Screening and Evaluation of Variables for Determination of Sulfonylurea Herbicides in Water Samples by Capillary Zone Electrophoresis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Software
2.3. CE Analysis
2.4. Water Samples
3. Results and Discussion
3.1. Preliminary Studies
3.2. Method Optimization
3.3. Screening Process
3.4. Analytical Performance
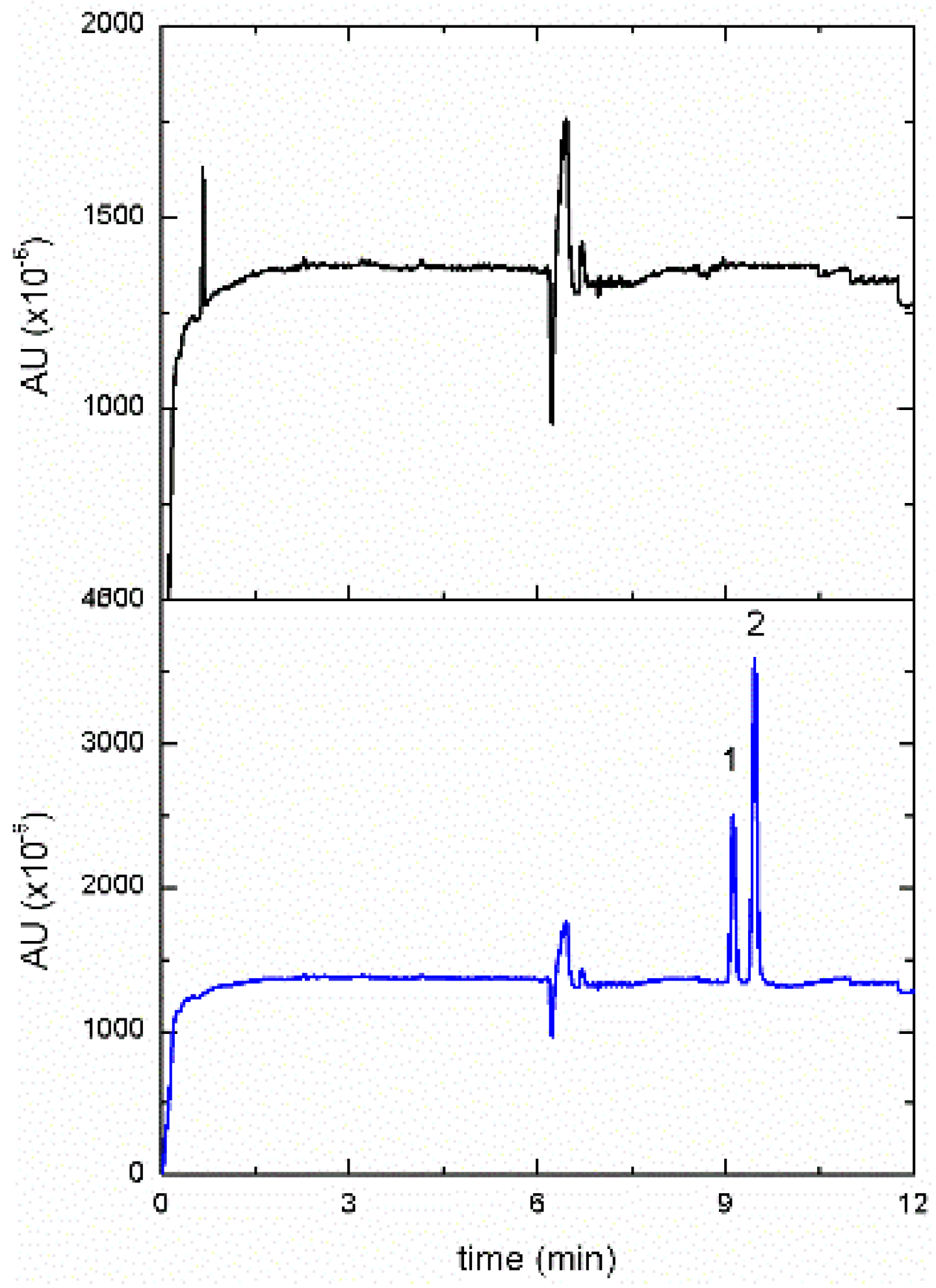
3.5. Analysis of Real Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brown, H.M. Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. Pestic. Sci. 1990, 29, 263–281. [Google Scholar] [CrossRef]
- Weber, J.B. Properties and behavior of pesticides in soil. In Mechanisms of Pesticide Movement into Groundwater; Honeycutt, R.C., Shabacker, D.J., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1994. [Google Scholar]
- Cessna, A.J.; Donald, D.B.; Bailey, J.; Waiser, M. Persistence of the Sulfonylurea Herbicides Sulfosulfuron, Rimsulfuron, and Nicosulfuron in Farm Dugouts (Ponds). J. Environ. Qual. 2015, 44, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Piola, J.C.; Prada, D.B.; Ezpeleta, D.C. Rabdomiolisis aguda por exposición percutánea a un herbicida en dos pacientes en Rosario Argentina. Acta Toxicol. Argent. 1997, 7, 11–15. [Google Scholar]
- INVESTEA-Asociación para la investigación y educación ambiental y Didáctica ambiental S.L. ISSN: 1698-5893. Revista de Didáctica Ambiental n° 10; Colombia, 2011. Available online: http://www.didacticaambiental.com/revista (accessed on 5 July 2016).
- Yin, X.H.; Li, S.N.; Zhang, L.; Zhu, G.N.; Zhuang, H.S. Evaluation of DNA damage in Chinese toad (Bufo bufo gargarizans) after in vivo exposure to sublethal concentrations of four herbicides using the comet assay. Ecotoxicology 2008, 17, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Cessna, A.J.; Donald, D.B.; Bailey, J.; Waiser, M.; Headley, J.V. Persistence of the Sulfonylurea Herbicides Thifensulfuron-Methyl, Ethametsulfuron-Methyl, and Metsulfuron-Methyl in Farm Dugouts (Ponds). J. Environ. Qual. 2006, 35, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Argentine Legislation 24051 Decreto 831, Régimen de desechos peligrosos. Anexo II, Tabla I, 1991. Available online: http://www2.medioambiente.gov.ar/mlegal/residuos/dec831/dec831_anxII_t1.htm (accessed on 5 July 2016).
- Official Journal of European Communities, EU legislation: Maximum pesticide limits for food products for human consumption and animal feeding stuffs. Council Regulation (EC) No 396/2005. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R0396 (accessed on 5 July 2016).
- Andreu, V.; Pico, Y. Determination of pesticides and their degradation products in soil: Critical review and comparison of methods. TRACs Trends Anal. Chem. 2004, 23, 772–789. [Google Scholar] [CrossRef]
- Bhadekar, R.; Pote, S.; Tale, V.; Nirichan, B. Developments in Analytical Methods for Detection of Pesticides in Environmental Samples. Am. J. Anal. Chem. 2011, 2, 1–15. [Google Scholar] [CrossRef]
- Guzman, N.A. Capillary Electrophoresis Technology; Marcel Dekker Inc. Press: New York, NY, USA, 1993. [Google Scholar]
- Orlandini, S.; Gotti, R.; Furlanetto, S. Multivariate optimization of capillary electrophoresis methods: A critical review. J. Pharm. Biomed. Anal. 2014, 87, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Volgas, G.C.; Downer, R.A.; Lopez, H.B. Pesticide Formulations and Application Systems, 23rd ed.; ASTM American Society for Testing and Materials International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Dinelli, G.; Vicari, A.; Brandolini, V. Detection and quantitation of sulfonylurea herbicides in soil at the ppb level by capillary electrophoresis. J. Chromatogr. A 1995, 700, 201–207. [Google Scholar] [CrossRef]
- El-Debs, R.; Nehmé, R.; Claude, B.; Motteau, S.; Togola, A.; Berho, C.; Morin, P. Coated capillaries with highly charged polyelectrolytes and carbon nanotubes co-aggregated with sodium dodecyl sulphate for the analysis of sulfonylureas by capillary electrophoresis. J. Chromatogr. A 2014, 1367, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.D. Chemometrics: Experimental Design; ACOL and John Wiley and Sons Press: Chichester, UK, 1991. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jons, S.; Lewi, P.J.; Smeyers-Verbeke, J. Hanbook of Chemometrics and Qualimetrics: Part A; Elsevier Science B.V.: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Menne, H.J.; Janowitz, K.; Berger, B.M. Comparison of capillary electrophoresis and liquid chromatography for determination of sulfonylurea herbicides in soil: Environmental Analysis Using Capillary Electrophoresis and Related Techniques, including Capillary Electrochromatography. J. AOAC Int. 1999, 82, 1534–1541. [Google Scholar]
- Berger, B.M.; Wolfe, N.L. Multiresidue determination of sulfonylurea herbicides by capillary electrophoresis for hydrolysis studies in water and sediments. J. Anal. Chem. 1996, 356, 508–511. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Henion, J. Fast capillary electrophoresis—Ion spray mass spectrometric determination of sulfonylureas. J. Chromatogr. A 1992, 606, 237–247. [Google Scholar] [CrossRef]
- Guibiao, Y.; Wei, Z.; Xin, C.; Canping, P.; Shuren, J. Determination and Quantitation of Ten Sulfonylurea Herbicides in Soil Samples Using Liquid Chromatography with Electrospray Ionization Mass Spectrometric Detection. Chin. J. Anal. Chem. 2006, 34, 1207–1212. [Google Scholar]
- Fenoll, J.; Hellín, P.; Sabater, P.; Flores, P.; Navarro, S. Trace analysis of sulfonylurea herbicides in water samples by solid-phase extraction and liquid chromatography-tandem mass spectrometry. Talanta 2012, 101, 273–282. [Google Scholar] [CrossRef] [PubMed]



| Factors | ||||
|---|---|---|---|---|
| Exp. | pH | Methanol %(v/v) | Voltage (kV) | Resolution (Rs) |
| 1 | 8.5 | 3 | 15 | 2.29 |
| 2 | 9.5 | 3 | 15 | 1.73 |
| 3 | 8.5 | 8 | 15 | 1.99 |
| 4 | 9.5 | 8 | 15 | 1.45 |
| 5 | 8.5 | 3 | 25 | 2.07 |
| 6 | 9.5 | 3 | 25 | 1.12 |
| 7 | 8.5 | 8 | 25 | 2.11 |
| 8 | 9.5 | 8 | 25 | 2.08 |
| 9 | 9.0 | 5.5 | 20 | 1.60 |
| 10 | 9.0 | 5.5 | 20 | 1.79 |
| 11 | 9.0 | 5.5 | 20 | 1.78 |
| Analytes | Method | Detection Time (min) | Separation Medium | LODs | Ref. |
|---|---|---|---|---|---|
| 12 sulfonylurea compounds including Metsulfuron-methyl and Nicosulfuron | CZE-UV (λ: 239 nm) | NS (25) | 50 mM acetate buffer, pH 4.76 | 1.0 µg·kg−1 soil (SPE included through C18 column) | [19] |
| MSM (29) | |||||
| 12 sulfonylurea compounds including Metsulfuron-methyl and Nicosulfuron | LC-UV (λ: 226 nm) | NS (13) | mobile phase: acetonitrile–0.01M H3PO4 (gradient elution program) | 1.0 µg·kg−1 soil (SPE included through C18 column) | [19] |
| MSM (21) | |||||
| 17 sulfonylurea compounds including Metsulfuron-methyl Nicosulfuron and degradation products | CZE-UV (λ: 239 nm) | NS (12.9) | 25 mM acetic acid and 25 mM sodium acetate, pH 4.76, and 1.86 M acetonitrile in water | 0.1 µg·mL−1 (NS and MSM) | [20] |
| MSM (14.8) | |||||
| 8 sulfonylurea compounds including Metsulfuron-methyl and Nicosulfuron | CZE-UV (λ: 254 nm) | NS (6) | 5 mM ammonium acetate-acetonitrile (75:25), pH 5 | - | [21] |
| MSM (8) | |||||
| 10 sulfonylurea compounds including Metsulfuron-methyl and Nicosulfuron | LC-ESI-MS | NS (10.7) | mobile phase: acetonitrile/methanol–0.2% (v/v) acetic acid in water (gradient elution program) | 3.5 µg·mL−1 (NS), 1.5 µg·mL−1 (MSM) | [22] |
| MSM (11.5) | |||||
| 30 sulfonylurea compounds including Metsulfuron-methyl and Nicosulfuron | LC-MS2 | NS (14.2) | Mobile phase: acetonitrile–0.1% formic acid (gradient elution program) | 6.10−7µg·mL−1 (NS) 3.10−7µg·mL−1 (MSM) (SPE included through Oasis HLB cartridges) | [23] |
| MSM (15.5) | |||||
| Nicosulfuron and metsulfuron-methyl | CZE-UV (λ: 231 nm) | NS (9.1) | 25 mM sodium borate, 3% v/v methanol, pH 8.5 | 0.044 µg·mL−1 (NS) 0.034 µg·mL−1 (MSM) | This work |
| MSM (9.8) |
| Sample Added (µg·mL−1) | Found (µg·mL−1) * ± SD | RSD (%) | Recovery (%) | |
|---|---|---|---|---|
| Groundwater | ||||
| NS | 0.1 | 0.092 ± 0.002 | 2.2 | 92.0 |
| 0.5 | 0.455 ± 0.007 | 1.5 | 92.0 | |
| MSM | 0.1 | 0.082 ± 0.002 | 2.4 | 82.0 |
| 0.5 | 0.499 ± 0.004 | 0.8 | 99.8 | |
| Surface | ||||
| NS | 0.1 | 0.102 ± 0.006 | 5.8 | 102 |
| 0.5 | 0.471 ± 0.003 | 0.6 | 94.2 | |
| MSM | 0.1 | 0.097 ± 0.003 | 3.1 | 97.0 |
| 0.5 | 0.500 ± 0.009 | 1.8 | 100 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, V.; Di Anibal, C.V.; Lista, A.G. Screening and Evaluation of Variables for Determination of Sulfonylurea Herbicides in Water Samples by Capillary Zone Electrophoresis. Separations 2016, 3, 22. https://doi.org/10.3390/separations3030022
Springer V, Di Anibal CV, Lista AG. Screening and Evaluation of Variables for Determination of Sulfonylurea Herbicides in Water Samples by Capillary Zone Electrophoresis. Separations. 2016; 3(3):22. https://doi.org/10.3390/separations3030022
Chicago/Turabian StyleSpringer, Valeria, Carolina V. Di Anibal, and Adriana G. Lista. 2016. "Screening and Evaluation of Variables for Determination of Sulfonylurea Herbicides in Water Samples by Capillary Zone Electrophoresis" Separations 3, no. 3: 22. https://doi.org/10.3390/separations3030022