Enhancement of Thermal Stability and Selectivity by Introducing Aminotriazine Comonomer to Poly(Octadecyl Acrylate)-Grafted Silica as Chromatography Matrix
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
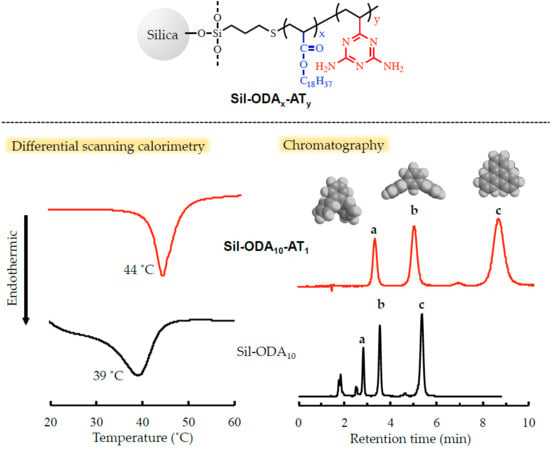
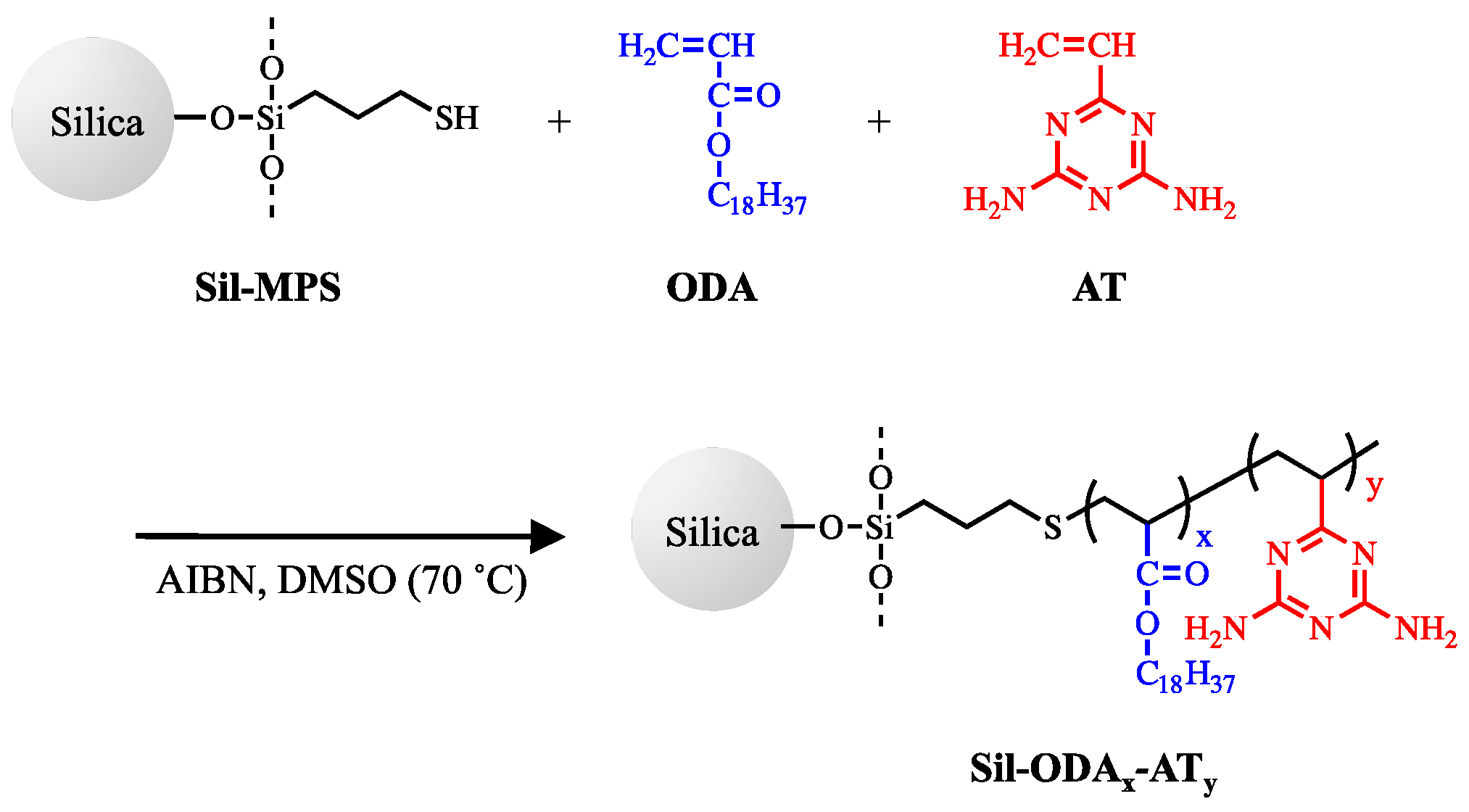
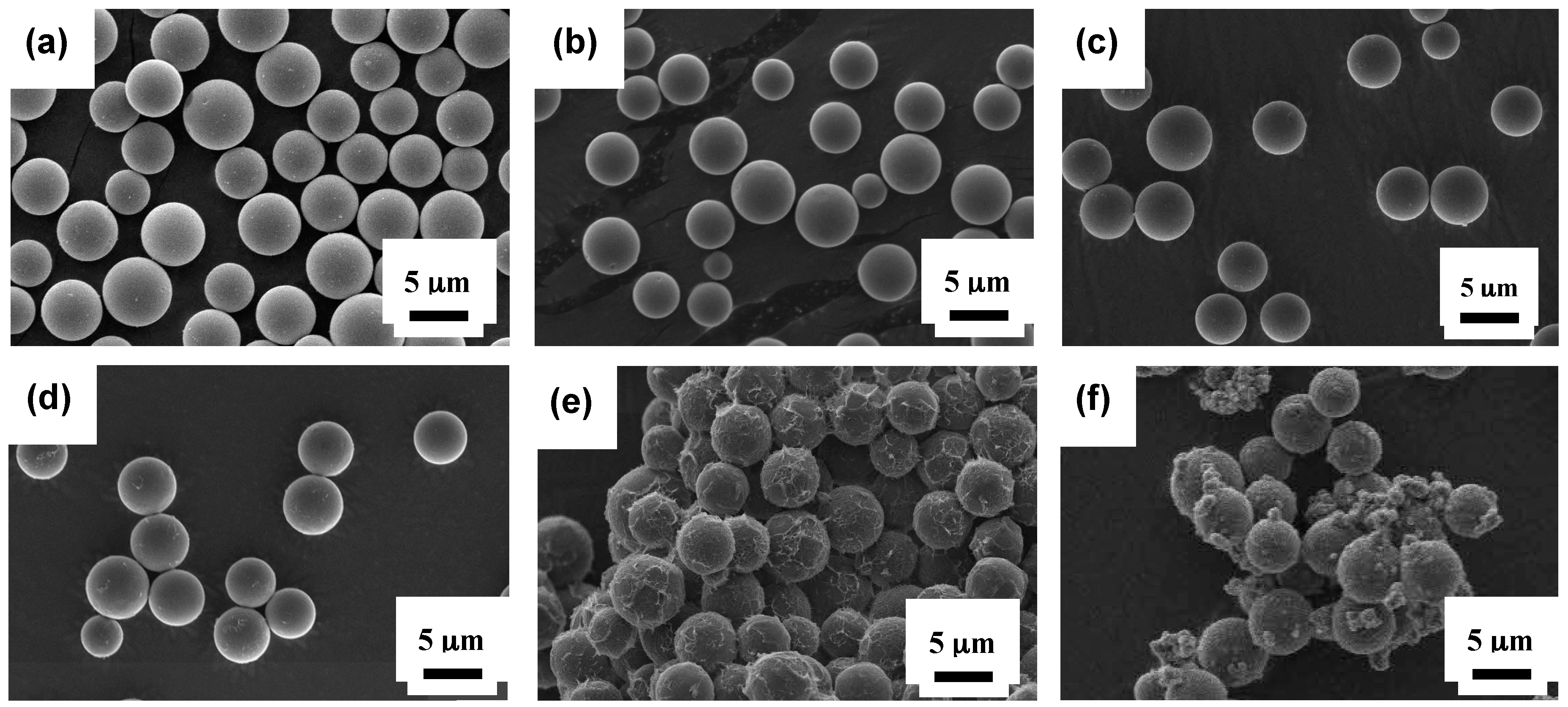
3.1. Characterization of Grafted Copolymers on Silica Surface
3.2. Evaluation of Side-Chain Orientation in Grafted Polymeric Phase
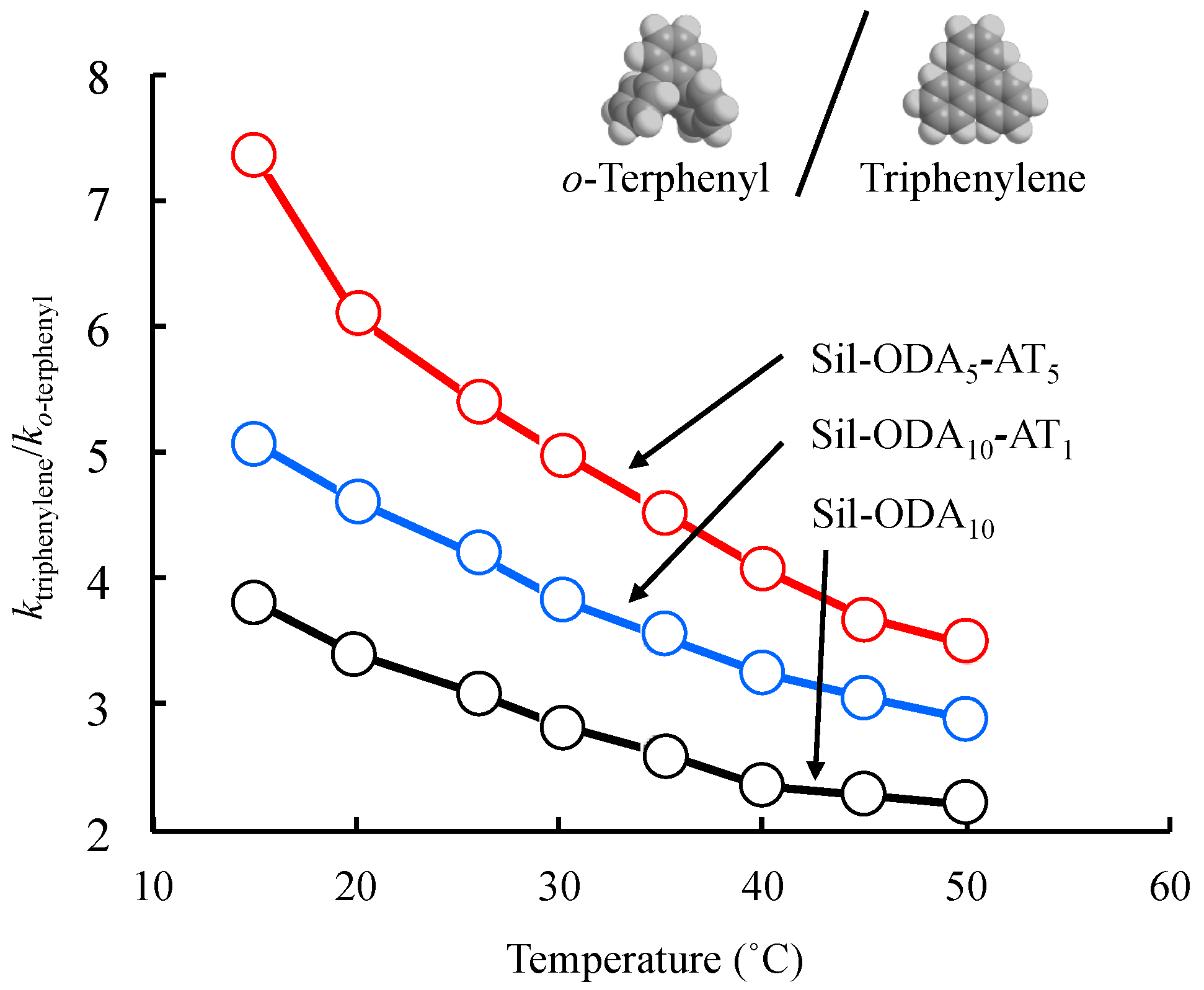
3.3. HPLC
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kato, K.; Uchida, E.; Kang, E.-T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Vogt, A.P. Macromolecular engineering through click chemistry and other efficient transformations. Macromolecules 2009, 43, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, L.; Shi, X.; Xu, Y.; Song, B.; Chen, H. Block copolymer modified surfaces for conjugation of biomacromolecules with control of quantity and activity. Langmuir 2013, 29, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Broughton, R.M.; Huang, T.S.; Bozack, M.; Worley, S.D. Polymeric antimicrobial N-halamine-surface modification of stainless steel. Ind. Eng. Chem. Res. 2017, 56, 11773–11781. [Google Scholar] [CrossRef]
- Mallik, A.K.; Takafuji, M.; Ihara, H. Molecular-shape selectivity tuned by donor-acceptor type copolymers as organic phase in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 7433–7439. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Kumazaki, M.; Kanazawa, H.; Kobayashi, J.; Kikuci, A.; Akiyama, Y.; Annaka, M.; Okano, T. Thermoresponsive polymer brush surfaces with hydrophobic groups for all-aqueous chromatography. ACS Appl. Mater. Interfaces 2010, 2, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Kobayashi, J.; Kikuchi, A.; Akiyama, Y.; Kanazawa, H.; Okano, T. High stability of thermoresponsive polymer-brush-grafted silica beads as chromatography matrices. ACS Appl. Mater. Interfaces 2012, 4, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, Y.; Kanazashi, R.; Ayano, E.; Okano, T.; Kanazawa, H. Temperature-responsive molecular recognition chromatography using phenylalanine and tryptophan derived polymer modified silica beads. Analyst 2016, 141, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Bo, C.M.; Wang, C.; Wei, Y.M. Preparation and evaluation of diblock copolymer-grafted silica by sequential surface initiated-atom transfer radical polymerization for reverse-phase/ion-exchange mixed-mode chromatography. J. Sep. Sci. 2017, 40, 4700–4708. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Nagaoka, S.; Tanaka, H.; Sakaki, S.; Hirayama, C. Lipid membrane analogue-immobilized silica gels for separation with molecular recognition. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 2967–2984. [Google Scholar] [CrossRef]
- Hirayama, C.; Ihara, H.; Mukai, T. Lipid membrane analogs. Specific retention behavior in comb-shaped telomer-immobilized porous silica gels. Macromolecules 1992, 25, 6375–6376. [Google Scholar] [CrossRef]
- Takafuji, M.; Fukui, M.; Ansarian, H.R.; Derakhshan, M.; Shundo, A.; Ihara, H. Conformational effect of silica-supported poly(octadecyl acrylate) on molecular-shape selectivity of polycyclic aromatic hydrocarbons in RP-HPLC. Anal. Sci. 2004, 20, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.P.; Breedveld, V.; Weck, M. Complementary hydrogen-bonded thermoreversible polymer networks with tunable properties. Macromolecules 2008, 41, 3429–3438. [Google Scholar] [CrossRef]
- Gao, H.; Wang, N.; Hu, X.; Nan, W.; Han, Y.; Liu, W. Double hydrogen-bonding pH-sensitive hydrogels retaining high-strengths over a wide pH range. Macromol. Rapid Commun. 2013, 34, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, Y.; Gao, F.; Zhai, X.; Sun, M.; Lu, W.; Cao, Z.; Liu, W. High strength multifunctional multiwalled hydrogel tubes: Ion-triggered shape memory, antibacterial, and anti-inflammatory efficacies. ACS Appl. Mater. Interfaces 2015, 7, 16865–16872. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Ban, T.; Gotoh, S.; Hishiya, T.; Komiyama, M. Hydrogen bonding in water by poly(vinyldiaminotriazine) for the molecular recognition of nucleic acid bases and their derivatives. Macromolecules 1998, 31, 371–377. [Google Scholar] [CrossRef]
- Asanuma, H.; Ban, T.; Gotoh, S.; Hishiya, T.; Komiyama, M. Precise recognition of nucleotides and their derivatives through hydrogen bonding in water by poly(vinyldiaminotriazine). Supramol. Sci. 1998, 5, 405–410. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Tsai, H.-T. Complementary multiple hydrogen-bonding interactions increase the glass transition temperatures to PMMA copolymer mixtures. Macromolecules 2009, 42, 4701–4711. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Y.; Bai, T.; Liu, W. Robust MeO2MA/vinyl-4,6-diamino-1,3,5-triazine copolymer hydrogels-mediated reverse gene transfection and thermo-induced cell detachment. Biomaterials 2011, 32, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Mallik, A.K.; Rahman, M.M.; Czaun, M.; Takafuji, M.; Ihara, H. A new route for preparation of high-density organic phase to high selective HPLC for polycyclic aromatic hydrocarbons by atom-transfer radical polymerization of octadecyl acrylate on silica. Chem. Lett. 2007, 36, 1460–1461. [Google Scholar] [CrossRef]
- Mallik, A.K.; Qiu, H.; Takafuji, M.; Ihara, H. Selectivity enhancement for the separation of tocopherols and steroids by integration of highly ordered weak interaction sites along the polymer main chain. Anal. Bioanal. Chem. 2012, 404, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, T.; Ihara, H.; Sakaki, S.; Shosenji, H.; Hirayama, C. Chromatographic separation of geometrical isomers using highly oriented polymer-immobilized silica gels. J. Chromatogr. A 1994, 672, 237–241. [Google Scholar] [CrossRef]
- Ihara, H.; Fukumoto, T.; Hirayama, C. Molecular length recognition of polyaromatics using lipid membrane analogue-immobilized silica gels. Anal. Sci. 1993, 9, 711–713. [Google Scholar] [CrossRef]
- Jinno, K.; Kawasaki, K. Correlation between the tetention data of polycyclic aromatic hydrocarbons and several description in reversed-phase HPLC. Chromatgraphia 1983, 17, 445–449. [Google Scholar] [CrossRef]
- Wise, S.A.; Bonnett, W.J.; Guenther, F.R.; May, W.E. A relationship between reversed-phase C18 liquid chromatographic retention and the shape of polycyclic aromatic hydrocarbons. J. Chromatogr. Sci. 1981, 19, 457–465. [Google Scholar] [CrossRef]
- Tanaka, N.; Tokuda, Y.; Iwaguchi, K.; Araki, M. Effect of stationary phase structure on retention and selectivity in reversed-phase liquid chromatography. J. Chromatogr. A 1982, 239, 761–772. [Google Scholar] [CrossRef]

| Silica Composites | Molar Ratio | EA | TGA | ||||
|---|---|---|---|---|---|---|---|
| x | y | C (%) | N (%) | C/N | Loss (%) | Polymer (%) | |
| Sil-MPS | - | - | 03.81 | 0 | - | 11.3 | - |
| Sil-ODA10-AT1 | 10 | 1 | 21.75 | 00.64 | 33.98 | 32.7 | 21.4 |
| Sil-ODA5-AT5 | 5 | 5 | 19.55 | 04.09 | 04.78 | 34.7 | 23.4 |
| Sil-AT10 | 0 | 10 | 20.68 | 23.11 | 00.89 | 28.4 | 17.1 |
| PAHs as Elutes | Sil-ODA5-AT5 | Sil-ODA10-AT1 | Sil-ODA10 | ||||
|---|---|---|---|---|---|---|---|
| k | α | k | α | k | α | ||
| o-Terphenyl | Non-planar | 0.39 | 1 | 1.30 | 1 | 0.97 | 1 |
| m-Terphenyl | Non-planar | 0.88 | 2.27 | 2.47 | 1.90 | 1.48 | 1.52 |
| p-Terphenyl | Non-planar | 1.34 | 3.48 | 3.76 | 2.89 | 1.77 | 1.83 |
| Triphenylene | Planar | 1.93 | 5.01 | 4.98 | 3.83 | 2.76 | 2.84 |
| cis-Stilbene | Non-planar | 0.11 | 1 | 1.01 | 1 | 0.80 | 1 |
| trans-Stilbene | Planar | 0.24 | 2.26 | 1.64 | 1.62 | 1.15 | 1.43 |
| Triphenylene | Planar | 1.93 | 1 | 4.98 | 1 | 2.76 | 1 |
| Benzo[α]anthracene | Planar | 2.42 | 1.25 | 6.16 | 1.24 | 3.39 | 1.23 |
| Naphthacene | Planar | 4.38 | 2.27 | 12.5 | 2.51 | 6.34 | 2.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
K. Mallik, A.; Noguchi, H.; Han, Y.; Kuwahara, Y.; Takafuji, M.; Ihara, H. Enhancement of Thermal Stability and Selectivity by Introducing Aminotriazine Comonomer to Poly(Octadecyl Acrylate)-Grafted Silica as Chromatography Matrix. Separations 2018, 5, 15. https://doi.org/10.3390/separations5010015
K. Mallik A, Noguchi H, Han Y, Kuwahara Y, Takafuji M, Ihara H. Enhancement of Thermal Stability and Selectivity by Introducing Aminotriazine Comonomer to Poly(Octadecyl Acrylate)-Grafted Silica as Chromatography Matrix. Separations. 2018; 5(1):15. https://doi.org/10.3390/separations5010015
Chicago/Turabian StyleK. Mallik, Abul, Hiroki Noguchi, Yige Han, Yutaka Kuwahara, Makoto Takafuji, and Hirotaka Ihara. 2018. "Enhancement of Thermal Stability and Selectivity by Introducing Aminotriazine Comonomer to Poly(Octadecyl Acrylate)-Grafted Silica as Chromatography Matrix" Separations 5, no. 1: 15. https://doi.org/10.3390/separations5010015