Hollow Fibre Membrane-Protected Molecularly Imprinted Microsolid-Phase Extraction (HFM-Protected-MI-MSPE) of Triazines from Soil Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Polymer Preparation
2.3. Sample Preparation
2.4. Preparation of MIP Membranes
2.5. Hollow-Fibre Membrane-Protected Molecularly Imprinted Microsolid-Phase Extraction (HFM-Protected-MI-MSPE)
2.6. HPLC Analysis
3. Results and Discussion
3.1. Optimization of HFM-Protected-MI-MSPE in Soil Samples
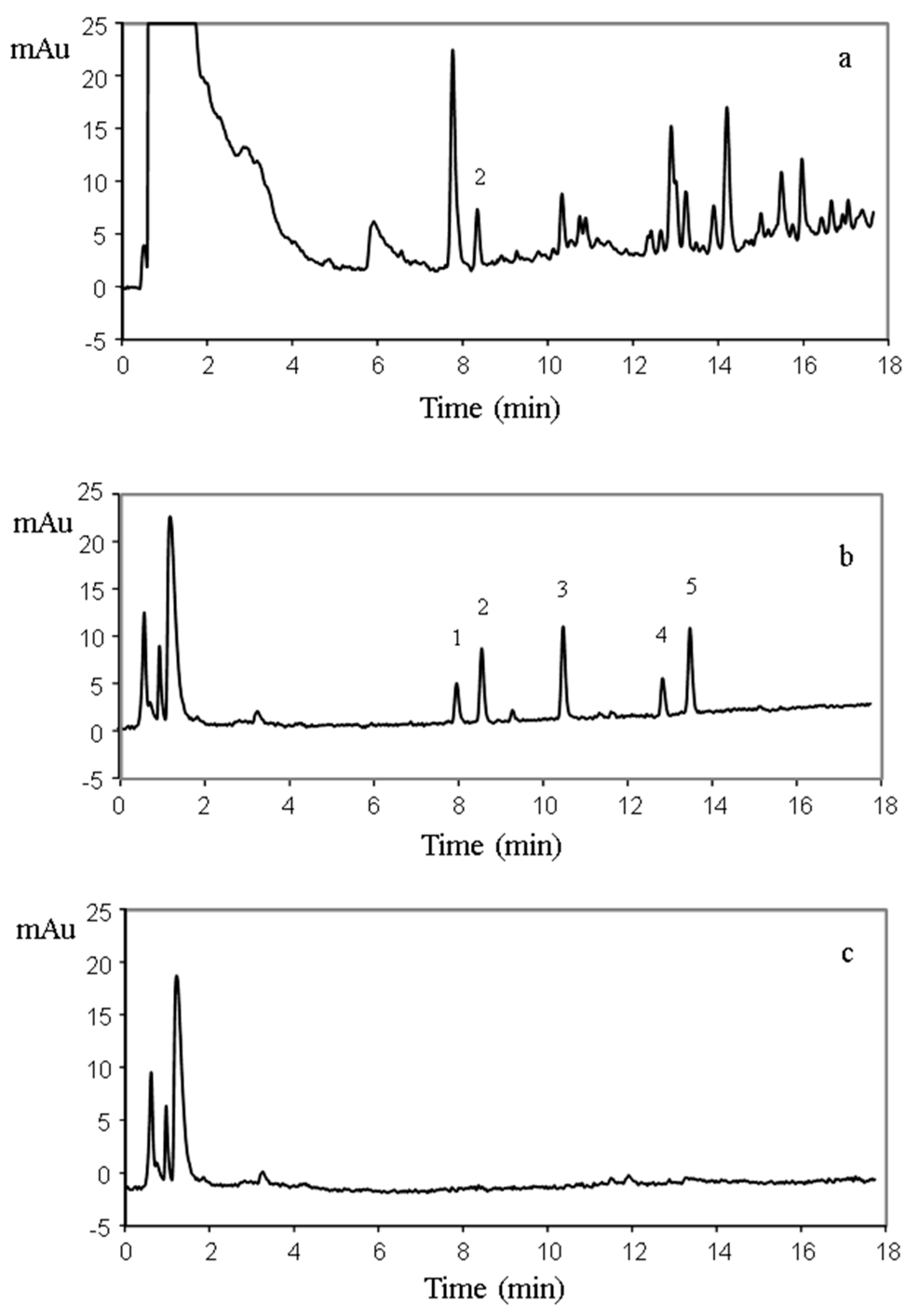
3.2. Analytical Performance and Application of the HFM-Protected-MI-MSPE of Triazines in Soil Samples
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Smith, R.M. Before the injection—Modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid-phase microextraction with thermal-desorption using fused-silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, J.; Zhang, K.; Lian, H.; Li, G. Novel applications of molecularly-imprinted polymers in sample preparation. TrAC Trends Anal. Chem. 2013, 43, 37–52. [Google Scholar] [CrossRef]
- Martin-Esteban, A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. TrAC Trends Anal. Chem. 2013, 45, 169–181. [Google Scholar] [CrossRef]
- Martín-Esteban, A.; Sellergren, B. The use of molecularly imprinted polymers in sampling and sample preparation. In Handbook of Sample Preparation; Pawliszyn, J., Lord, H.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 445–473. ISBN 978-0-470-09934-6. [Google Scholar]
- Koster, E.H.M.; Crescenzi, C.; den Hoedt, W.; Ensing, K.; de Jong, G.J. Fibers coated with molecularly imprinted polymers for solid-phase microextraction. Anal. Chem. 2001, 73, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, F.G.; Turiel, E.; Martin-Esteban, A. Molecularly imprinted polymers for solid-phase extraction and solid-phase microextraction: Recent developments and future trends. J. Chromatogr. A 2007, 1152, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Tadeo, J.L.; Martin-Esteban, A. Molecularly imprinted polymeric fibers for solid-phase microextraction. Anal. Chem. 2007, 79, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Djozan, D.; Baheri, T. Preparation and evaluation of solid-phase microextraction fibers based on monolithic molecularly imprinted polymers for selective extraction of diacetylmorphine and analogous compounds. J. Chromatogr. A 2007, 1166, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Hu, Y.; Li, G. Liquid–liquid–solid microextraction based on membrane-protected molecularly imprinted polymer fiber for trace analysis of triazines in complex aqueous samples. J. Chromatogr. A 2009, 1216, 8304–8311. [Google Scholar] [CrossRef] [PubMed]
- Barahona, F.; Turiel, E.; Martin-Esteban, A. Supported liquid membrane-protected molecularly imprinted fibre for solid-phase microextraction of thiabendazole. Anal. Chim. Acta 2011, 694, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, L.Y.; Tian, M.K.; Zhou, X.M.; Zhang, Y.P. Hollow-fiber membrane tube embedded with a molecularly imprinted monolithic bar for the microextraction of triazine pesticides. Anal. Methods 2014, 6, 602–608. [Google Scholar] [CrossRef]
- Diaz-Alvarez, M.; Barahona, F.; Turiel, E.; Martin-Esteban, A. Supported liquid membrane-protected molecularly imprinted beads for micro-solid phase extraction of sulfonamides in environmental waters. J. Chromatogr. A 2014, 1357, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Diaz-Alvarez, M.; Martin-Esteban, A. Supported liquid membrane-protected molecularly imprinted beads for the solid phase micro-extraction of triazines from environmental waters. J. Chromatogr. A 2016, 1432, 1–6. [Google Scholar] [CrossRef] [PubMed]
- European Union Risk Assesment Report and Directive 2008/105/EC. Annex VII. PNEC Values and Hazard Information for Candidate Substances. 2009. Available online: https://circabc.europa.eu/webdav/CircaBC/env/wfd/Library/framework_directive/thematic_documents/priority_substances/supporting_substances/monitoring-based/07_Annex%20VII_PNEC_Candidate-substances.pdf (accessed on 15 January 2018).
- Wang, J.F.; Cormack, P.A.G.; Sherrington, D.C.; Khoshdel, E. Monodisperse, molecularly imprinted polymer microspheres prepared by precipitation polymerization for affinity separation applications. Angew. Chem. Int. Ed. 2003, 42, 5336–5338. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Brunete, C.; Albero, B.; Tadeo, J.L. Determination of Pesticides in soil. In Analysis of Pesticides in Food and Environmental Samples; Tadeo, J.L., Ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2008; ISBN 9780849375521. [Google Scholar]
- Turiel, E.; Martin-Esteban, A.; Fernandez, P.; Perez-Conde, C.; Camara, C. Molecular recognition in a propazine-imprinted polymer and its application to the determination of triazines in environmental samples. Anal. Chem. 2001, 73, 5133–5141. [Google Scholar] [CrossRef] [PubMed]

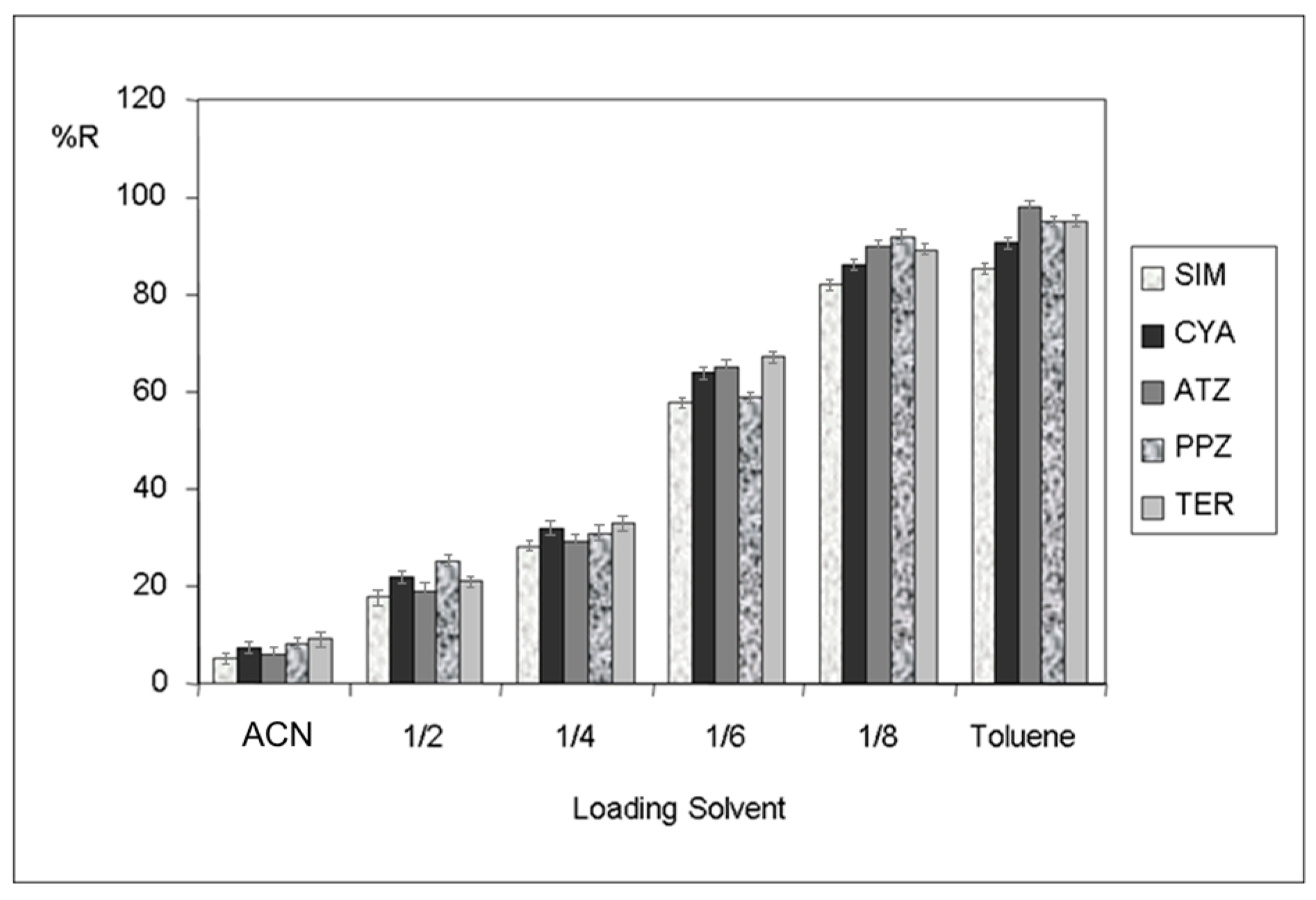

| Murcia | Valencia | Madrid | |||||||
|---|---|---|---|---|---|---|---|---|---|
| %R | RSD (%) 1 | LOD 2 | %R | RSD (%) 1 | LOD 2 | %R | RSD (%) 1 | LOD 2 | |
| SIM | 77.3 | 9.2 | 3.7 | 72.8 | 9.0 | 3.4 | 76.1 | 8.9 | 2.9 |
| CYA | 85.9 | 8.9 | 1.9 | 94.6 | 7.1 | 1.5 | 101.1 | 6.4 | 1.3 |
| ATR | 102.3 | 10.0 | 1.5 | 82.5 | 9.5 | 1.4 | 85.8 | 5.8 | 1.2 |
| PPZ | 93.7 | 9.5 | 3.6 | 86.7 | 9.0 | 3.6 | 92.4 | 7.3 | 3.4 |
| TER | 99.2 | 10.7 | 1.6 | 96.2 | 7.4 | 1.2 | 84.3 | 8.2 | 1.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Álvarez, M.; Martín-Esteban, A.; Turiel, E. Hollow Fibre Membrane-Protected Molecularly Imprinted Microsolid-Phase Extraction (HFM-Protected-MI-MSPE) of Triazines from Soil Samples. Separations 2018, 5, 8. https://doi.org/10.3390/separations5010008
Díaz-Álvarez M, Martín-Esteban A, Turiel E. Hollow Fibre Membrane-Protected Molecularly Imprinted Microsolid-Phase Extraction (HFM-Protected-MI-MSPE) of Triazines from Soil Samples. Separations. 2018; 5(1):8. https://doi.org/10.3390/separations5010008
Chicago/Turabian StyleDíaz-Álvarez, Myriam, Antonio Martín-Esteban, and Esther Turiel. 2018. "Hollow Fibre Membrane-Protected Molecularly Imprinted Microsolid-Phase Extraction (HFM-Protected-MI-MSPE) of Triazines from Soil Samples" Separations 5, no. 1: 8. https://doi.org/10.3390/separations5010008




