Evaluation of Lipases from Wild Microbial Strains as Biocatalysts in Biodiesel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection, Isolation, and Screening of Lipolytic Microorganisms Able to Carry out the Transesterification Reaction
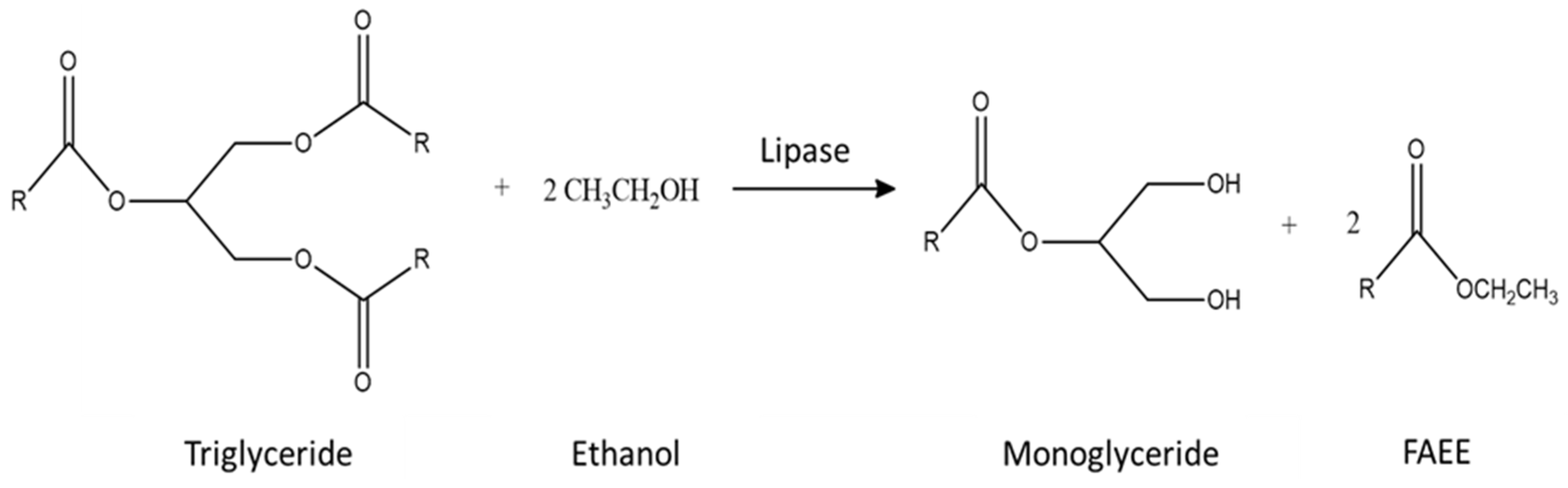
2.3. Ethanolysis Reactions
2.4. Analytical Method
2.5. Viscosity Measurements
3. Results and Discussion
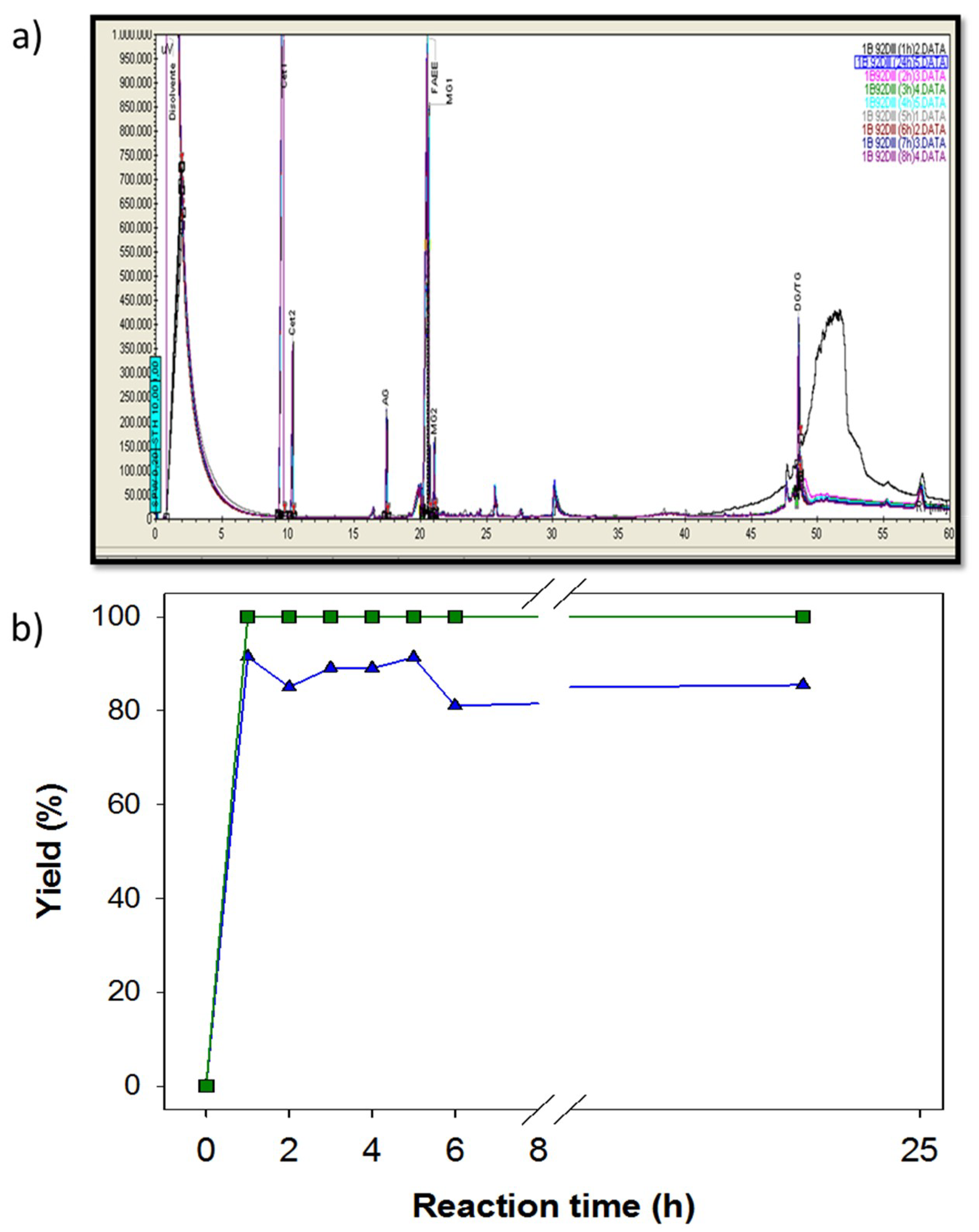
3.1. Comparative Chromatograms of Standardized Reaction Products
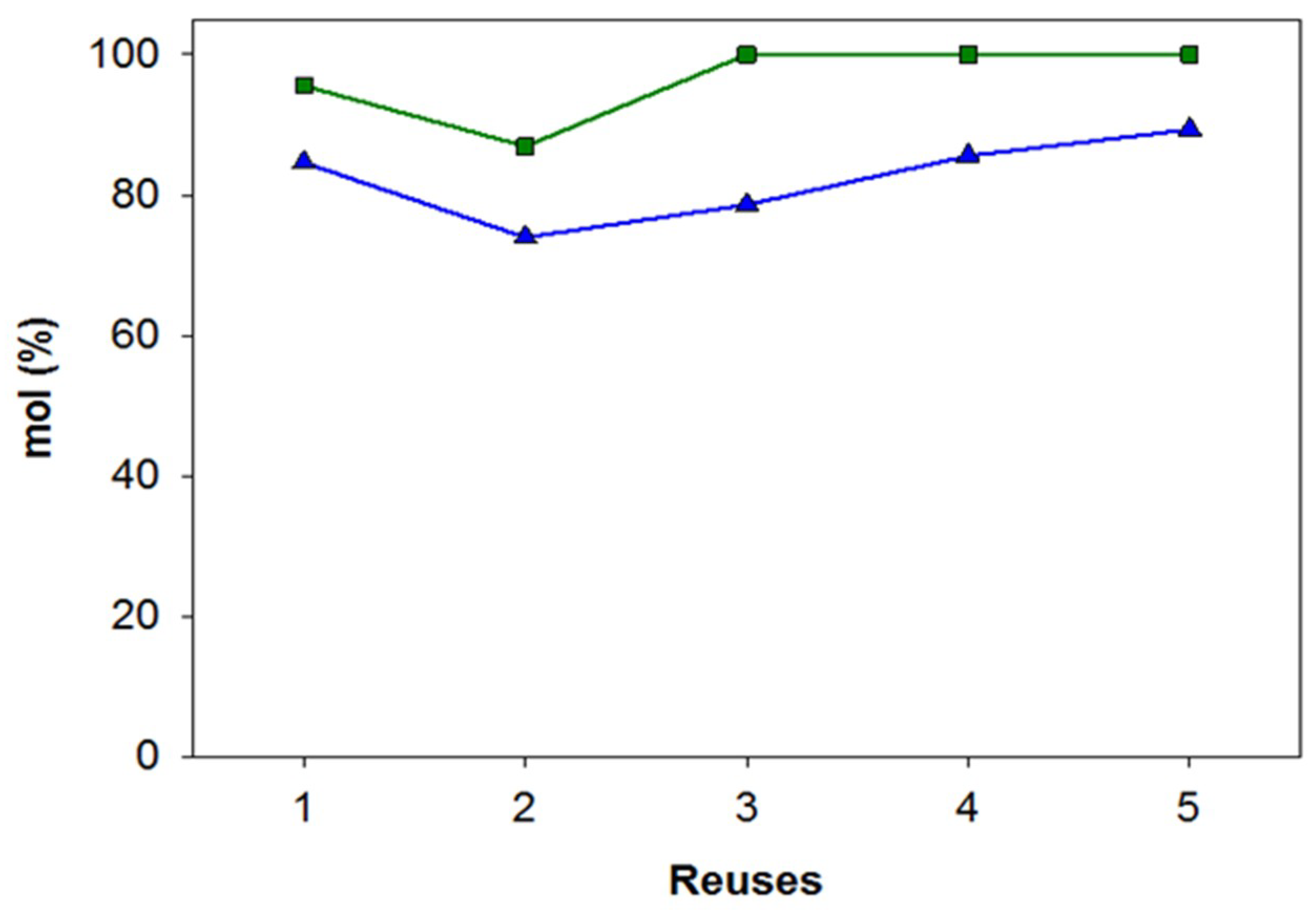
3.2. Ethanolysis Reactions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FAME | fatty acids methyl esters, components of conventional biodiesel |
| DMC | dimethyl carbonate |
| FAEE | Fatty Acid Ethyl Ester |
| PPL | porcine pancreatic lipase |
| FAE | esters of fatty acids |
| MG | monoglycerides or monoacylglycerols |
| DG | diacylglycerols |
| TG | triacylglycerols or triglycerides |
| GC | gas chromatograph |
| RT | retention times |
| p-NPP | para-nitrophenyl palmitate |
| p-NP | para-nitrophenol |
| OS | Vegetable Oil Samples/Strains |
| FS | Animal Fat Samples/Strains |
| B100 | 100% Biofuel |
References
- Chen, D.; Liu, C.J. A Current Perspective on Catalysis for New Energy Technologies. ChemCatChem 2011, 3, 423–425. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Grubler, A.; Bauer, N.; Krey, V.; Riahi, K. Future capacity growth of energy technologies: are scenarios consistent with historical evidence? Clim. Chang. 2013, 118, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Jong, S.; Antonissen, K.; Hoefnagels, R.; Lonza, L.; Wang, M.; Faaij, A.; Junginger, M. Life-cycle analysis of greenhouse gas emissions from renewable jet fuel production. Biotechnol. Biofuels 2017, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Kevin, R.; Agarwal, A.K. Production of biodiesel from high-FFA neem oil and its performance, emission and combustion characterization in a single cylinder DICI engine. Fuel Process. Technol. 2012, 97, 118–129. [Google Scholar] [CrossRef]
- Ameen, M.; Azizan, M.T.; Yusup, S.; Ramli, A.; Yasir, M. Catalytic hydrodeoxygenation of triglycerides: An approach to clean diesel fuel production. Renewable and Sustainable. Energy Rev. 2017, 80, 1072–1088. [Google Scholar] [CrossRef]
- Luna, D.; Calero, J.; Sancho, E.; Luna, C.; Posadillo, A.; Bautista, F.; Romero, A.; Berbel, J.; Verdugo, C. Technological challenges for the production of biodiesel in arid lands. J. Arid Environ. 2014, 102, 127–138. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Berbel, J.; Verdugo-Escamilla, C. An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 1437–1452. [Google Scholar] [CrossRef]
- Ganesan, D.; Rajendran, A.; Thangavelu, V. An overview on the recent advances in the transesterification of vegetable oils for biodiesel production using chemical and biocatalysts. Rev. Environ. Sci. Bio/Technol. 2009, 8, 367. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour. Technol. 2010, 101, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jung, S.M.; Park, Y.C.; Park, K. Lipase catalyzed transesterification of soybean oil using ethyl acetate, an alternative acyl acceptor. Biotechnol. Bioprocess Eng. 2007, 12, 441. [Google Scholar] [CrossRef]
- Casas, A.; Ruiz, J.R.; Ramos, M.J.; Pérez, A. Effects of Triacetin on Biodiesel Quality. Energy Fuels 2010, 24, 4481–4489. [Google Scholar] [CrossRef]
- Luna, D.; Bautista, F.M.; Caballero, V.; Campelo, J.M.; Marinas, J.M.; Romero, A.A. Method for Producing Biodiesel Using Porcine Pancreatic Lipase as an Enzymatic Catalyst. European Patent EP2050823A1, 5 September 2012. [Google Scholar]
- Verdugo, C.; Luna, D.; Posadillo, A.; Sancho, E.D.; Rodríguez, S.; Bautista, F.; Luque, R.; Marinas, J.M.; Romero, A.A. Production of a new second generation biodiesel with a low-cost lipase derived from Thermomyces lanuginosus: Optimization by response surface methodology. Catal. Today 2011, 167, 107–112. [Google Scholar] [CrossRef]
- Luna, C.; Sancho, E.; Luna, D.; Caballero, V.; Calero, J.; Posadillo, A.; Verdugo, C.; Bautista, F.M.; Romero, A.A. Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support. Energies 2013, 6, 3879–3900. [Google Scholar] [CrossRef]
- Haseeb, A.; Sia, S.; Fazal, M.; Masjuki, H. Effect of temperature on tribological properties of palm biodiesel. Energy 2010, 35, 1460–1464. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Ara, M.; Salley, S.O.; Ng, K.S. Investigation of Lubricity Characteristics of Biodiesel in Petroleum and Synthetic Fuel. Energy Fuels 2009, 23, 2229–2234. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Hu, X.; Li, C.; Zhu, X. Characterization of the lubricity of bio-oil/diesel fuel blends by high frequency reciprocating test rig. Energy 2010, 35, 283–287. [Google Scholar] [CrossRef]
- Cheenkachorn, K.; Fungtammasan, B. Biodiesel as an Additive for Diesohol. Int. J. Green Energy 2009, 6, 57–72. [Google Scholar] [CrossRef]
- Heydari-Maleney, K.; Taghizadeh-Alisaraei, A.; Ghobadian, B.; Abbaszadeh-Mayvan, A. Analyzing and evaluation of carbon nanotubes additives to diesohol-B2 fuels on performance and emission of diesel engines. Fuel 2017, 196, 110–123. [Google Scholar] [CrossRef]
- Celikten, I. The Effect of Biodiesel, Ethanol and Diesel Fuel Blends on The Performance and Exhaust Emissions in A DI Diesel Engine. Gazi Univ. J. Sci. 2011, 24, 341–345. [Google Scholar]
- Yilmaz, N.; Vigil, F.M.; Donaldson, A.B.; Darabseh, T. Investigation of CI engine emissions in biodiesel–ethanol–diesel blends as a function of ethanol concentration. Fuel 2014, 115, 790–793. [Google Scholar] [CrossRef]
- Kowalewicz, A. Eco-Diesel Engine Fuelled with Rapeseed Oil Methyl Ester and Ethanol. Part 2: Comparison of Emissions and Efficiency for Two Base Fuels: Diesel Fuel and Ester. Proc. Inst. Mech. Eng. D J. Autom. Eng. 2006, 220, 1275–1282. [Google Scholar] [CrossRef]
- Moayedallaie, S.; Mirzaei, M. Paterson, Bread improvers: Comparison of a range of lipases with a traditional emulsifier. J. Food Chem. 2010, 122, 495–499. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Production of a biodiesel-like biofuel without glycerol generation, by using Novozym 435, an immobilized Candida antarctica lipase. Bioresour. Bioprocess. 2014, 1, 11. [Google Scholar] [CrossRef]
- Luna, C.; Verdugo, C.; Sancho, E.D.; Luna, D.; Calero, J.; Posadillo, A.; Bautista, F.M.; Romero, A.A. Biocatalytic Behaviour of Immobilized Rhizopus oryzae Lipase in the 1,3-Selective Ethanolysis of Sunflower Oil to Obtain a Biofuel Similar to Biodiesel. Molecules 2014, 19, 11419–11439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar-Niño, A.; Luna, C.; Luna, D.; Marcos, A.T.; Cánovas, D.; Mellado, E. Selection and Characterization of Biofuel-Producing Environmental Bacteria Isolated from Vegetable Oil-Rich Wastes. PLoS ONE 2014, 9, e104063. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Luna, D.; Bautista, F.M.; Estevez, R.; Calero, J.; Posadillo, A.; Romero, A.A.; Sancho, E.D. Application of Enzymatic Extracts from a CALB Standard Strain as Biocatalyst within the Context of Conventional Biodiesel Production Optimization. Molecules 2017, 22, 2025. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, E.; Byron, D. Alternative Fuels in Fire Debris Analysis: Biodiesel Basics. J. Forensic Sci. 2007, 52, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yara-Varón, E.; Joli, J.E.; Torres, M.; Sala, N.; Villorbina, G.; Méndez, J.J.; Canela-Garayoa, R. Solvent-free biocatalytic interesterification of acrylate derivatives. Catal. Today 2012, 196, 86–90. [Google Scholar] [CrossRef]
- Macario, A.; Verri, F.; Diaz, U.; Corma, A.; Giordano, G. Pure silica nanoparticles for liposome/lipase system encapsulation: Application in biodiesel production. Catal. Today 2013, 204, 148–155. [Google Scholar] [CrossRef] [Green Version]



| Lipase Amount (g) | Conversion (wt %) | FAE (wt %) | DG (wt %) | TG (wt %) | Viscosity (cSt) |
|---|---|---|---|---|---|
| 0.007 | 85.7 | 77.9 | 7.9 | 14.3 | 9.4 |
| 0.010 | 100 | 96.1 | 3.9 | 0.0 | 10.1 |
| 0.020 | 100 | 97.9 | 2.1 | 0.0 | 9.8 |
| 0.030 | 90.2 | 90.0 | 0.1 | 10.0 | 11.5 |
| 0.040 | 100 | 98.0 | 2.0 | 0.0 | 10.7 |
| SAMPLE | Conversion (wt %) | FAE (wt %) | DG (wt %) | TG (wt %) | Viscosity (cSt) |
|---|---|---|---|---|---|
| OS 1 | 69.8 | 63.1 | 6.7 | 30.2 | 11.7 |
| OS 2 | 100.0 | 93.7 | 6.3 | 0.0 | 11.8 |
| OS 3 | 68.4 | 65.9 | 2.5 | 31.7 | 10.9 |
| OS 4 | 92.9 | 80.1 | 12.9 | 7.1 | 10.8 |
| OS 5 | 61.8 | 58.7 | 3.1 | 38.2 | 11.4 |
| OS 6 | 69.8 | 67.4 | 2.5 | 30.2 | 11.3 |
| OS 7 | 83.8 | 72.2 | 11.6 | 16.2 | 15.0 |
| OS 8 | 87.6 | 80.1 | 7.5 | 12.4 | 11.7 |
| OS 9 | 83.8 | 76.0 | 7.8 | 16.2 | 12.4 |
| OS 10 | 71.9 | 66.8 | 5.1 | 28.1 | 10.8 |
| OS 11 | 89.3 | 87.6 | 1.8 | 10.7 | 13.2 |
| OS 12 | 79.9 | 71.8 | 8.1 | 20.1 | 13.7 |
| OS 13 | 87.1 | 77.5 | 3.2 | 12.9 | 11.7 |
| OS 14 | 48.8 | 48.1 | 0.7 | 51.2 | 11.9 |
| OS 15 | 90.4 | 82.6 | 7.8 | 9.6 | 11.2 |
| OS 16 | 59.8 | 49.3 | 10.5 | 40.2 | 12.2 |
| OS 17 | 54.7 | 54.0 | 0.7 | 45.4 | 12.0 |
| OS 18 | 100 | 59.1 | 41.0 | 0 | 13.1 |
| OS 19 | 65.0 | 61.0 | 4.0 | 35.0 | 11.0 |
| FS 1 | 100 | 93.9 | 6.1 | 0.0 | 12.6 |
| FS 2 | 71.0 | 70.8 | 0.1 | 29.0 | 12.5 |
| FS 3 | 100 | 91.3 | 8.8 | 0 | 12.8 |
| FS 4 | 100 | 89.0 | 11.0 | 0 | 10.2 |
| FS 5 | 90.0 | 90.0 | 0 | 10.0 | 12.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna, C.; Luna, D.; Bautista, F.M.; Calero, J.; Romero, A.A.; Posadillo, A.; Sancho, E.D.; Estevez, R. Evaluation of Lipases from Wild Microbial Strains as Biocatalysts in Biodiesel Production. Separations 2018, 5, 53. https://doi.org/10.3390/separations5040053
Luna C, Luna D, Bautista FM, Calero J, Romero AA, Posadillo A, Sancho ED, Estevez R. Evaluation of Lipases from Wild Microbial Strains as Biocatalysts in Biodiesel Production. Separations. 2018; 5(4):53. https://doi.org/10.3390/separations5040053
Chicago/Turabian StyleLuna, Carlos, Diego Luna, Felipa M. Bautista, Juan Calero, Antonio A. Romero, Alejandro Posadillo, Enrique D. Sancho, and Rafael Estevez. 2018. "Evaluation of Lipases from Wild Microbial Strains as Biocatalysts in Biodiesel Production" Separations 5, no. 4: 53. https://doi.org/10.3390/separations5040053






