Determination of the Dissociation Constants of 16 Active Ingredients in Medicinal Herbs Using a Liquid–Liquid Equilibrium Method
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Materials
2.2. Preparation of Medicinal Material Extract
2.2.1. Preparation of Caulis Sinomenii Extract
2.2.2. Preparation of Semen Aesculi Extract
2.2.3. Preparation of the Flos Lonicerae, Radix Scutellariae, and Radix Astragali Extracts
2.3. Liquid–Liquid Equilibrium Extraction Experiment
2.3.1. Liquid–Liquid Equilibrium Experiment of Caulis Sinomenii
2.3.2. Liquid–Liquid Equilibrium Experiment Using Semen Aesculi
2.3.3. Liquid–Liquid Equilibrium Experiment Using Flos Lonicerae
2.3.4. Liquid–Liquid Equilibrium Experiment Using Radix Scutellariae
2.3.5. Liquid–Liquid Equilibrium Experiment Using Radix Astragali
2.4. Analysis Method
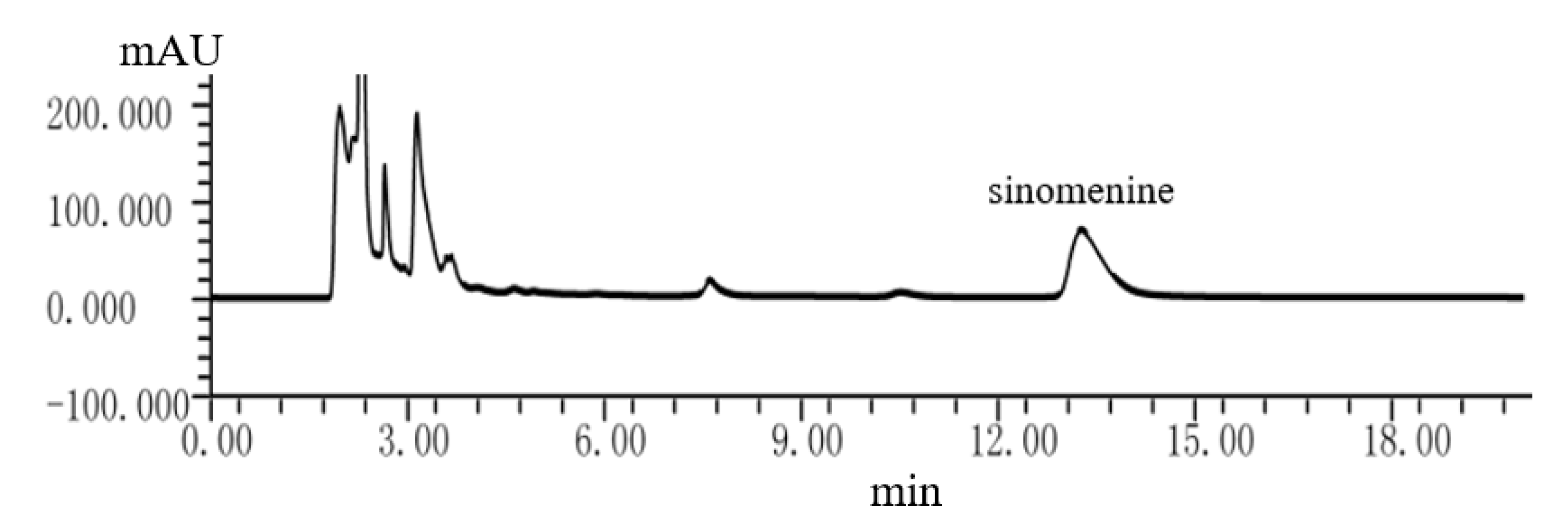
2.4.1. Analysis Method of Sinomenine in Caulis Sinomenii
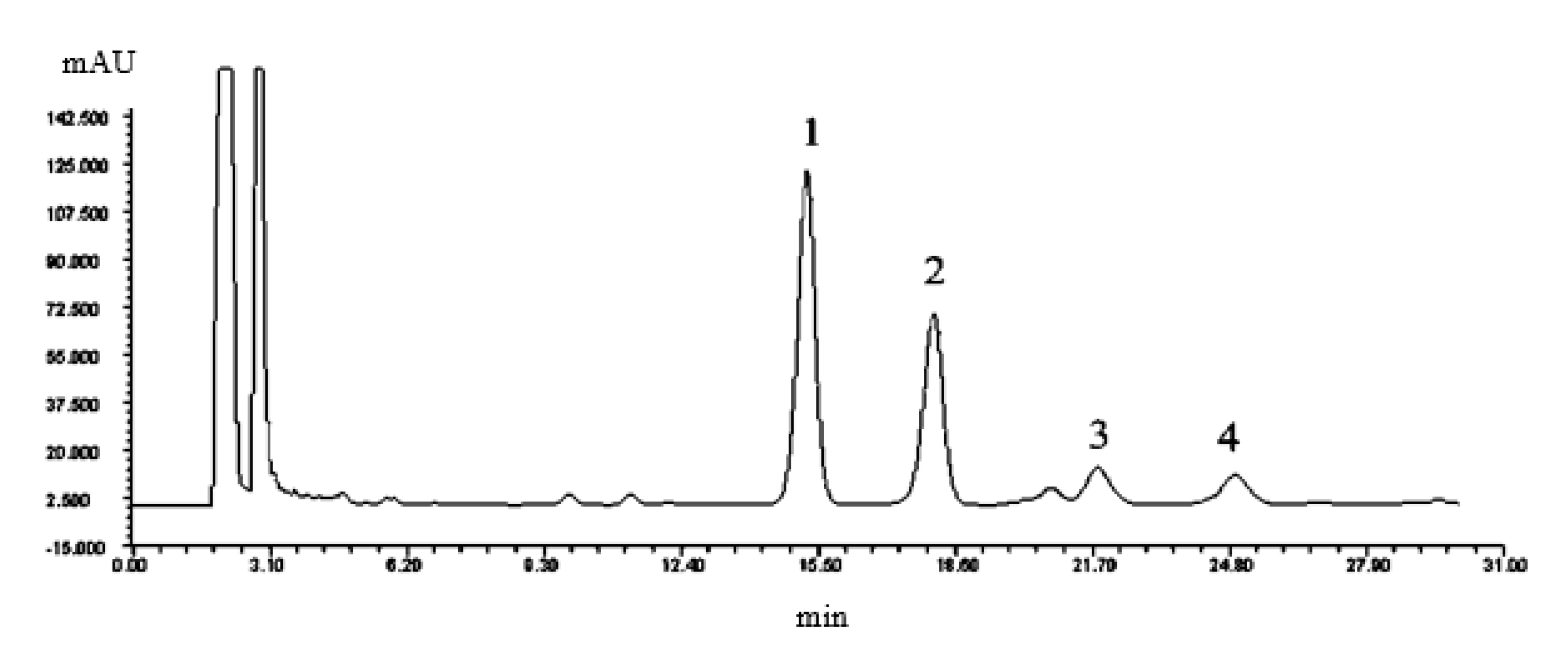
2.4.2. Analysis Method of Aescins in Semen Aesculi
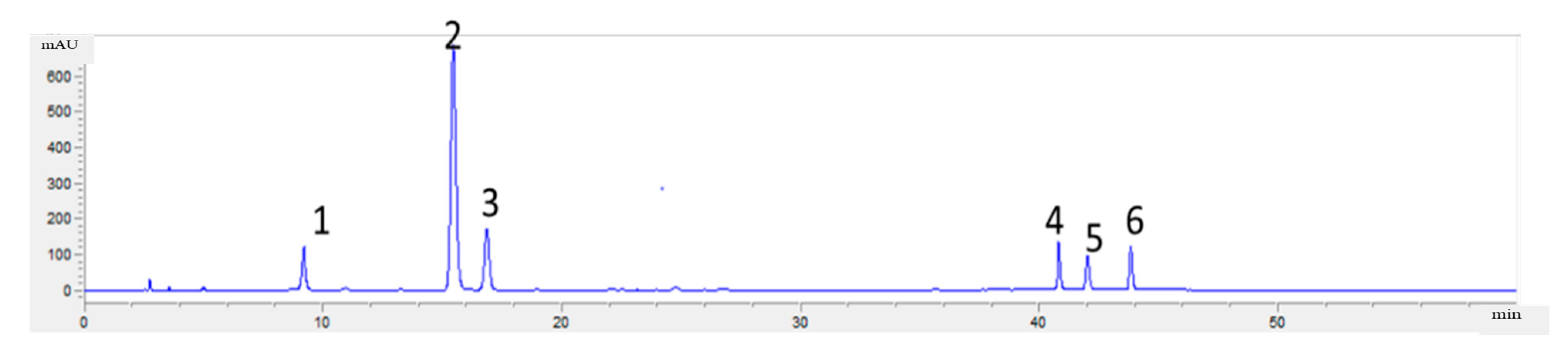
2.4.3. Analysis Method of Phenolic Acids in Flos Lonicerae
2.4.4. Analysis Method of Flavonoids in Radix Scutellariae
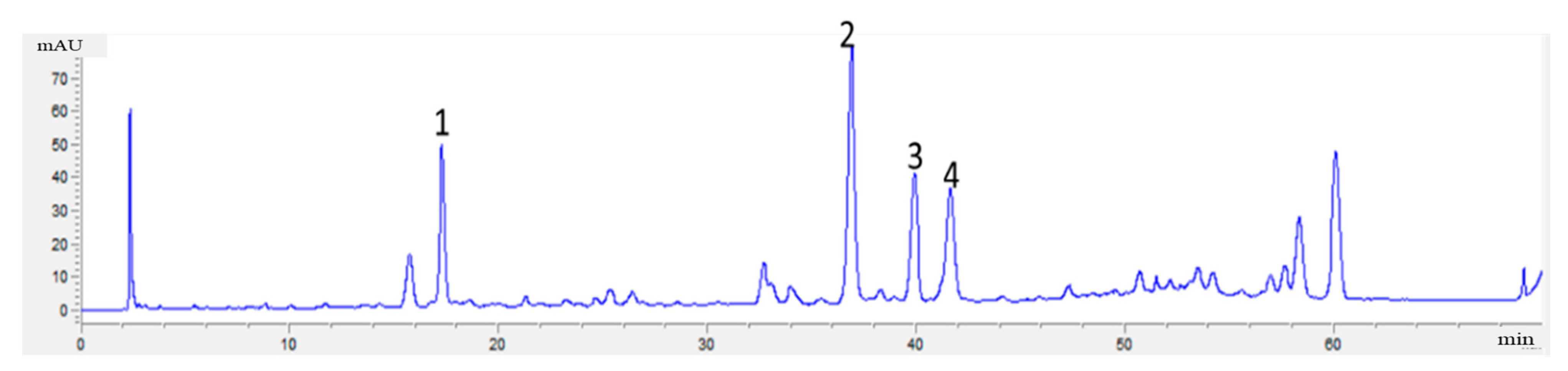
2.4.5. Analysis Method of Flavonoids in Radix Astragali
2.5. Data Processing
2.5.1. pKa Fitting of the Active Ingredients in Semen Aesculi, Flos Lonicerae, Radix Scutellariae, and Radix Astragali
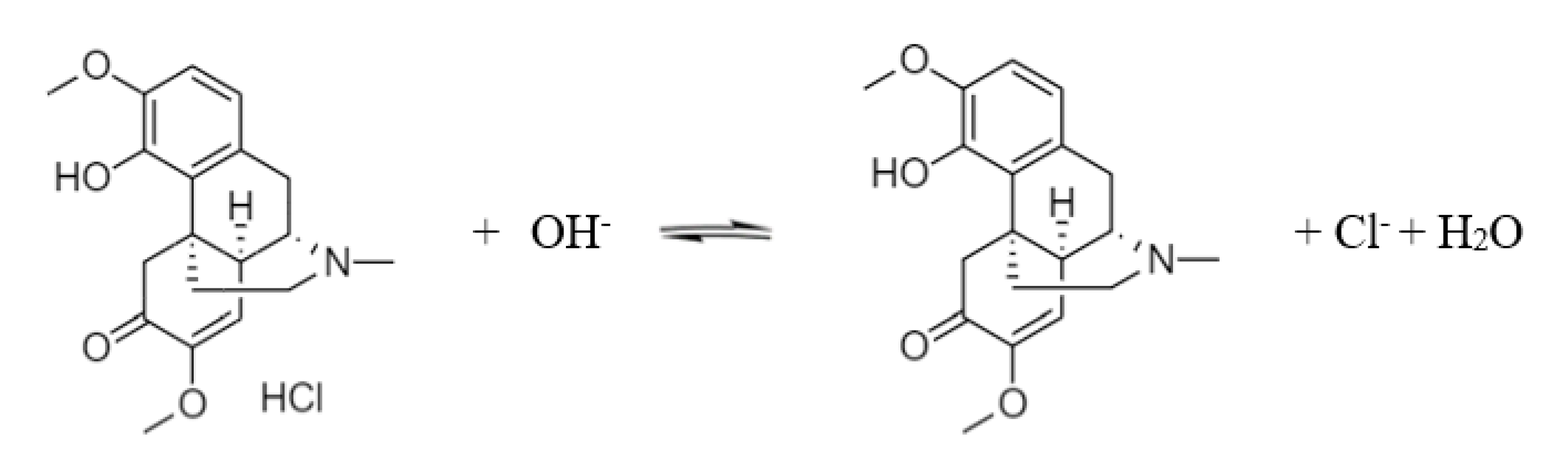
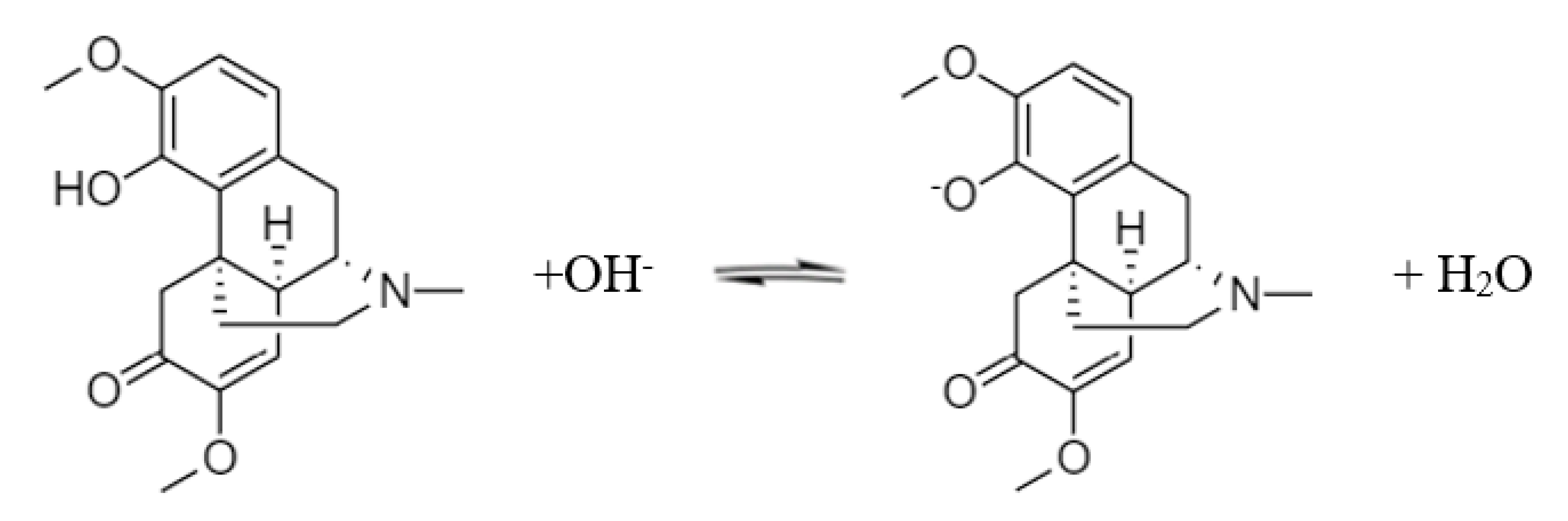
2.5.2. pKa Fitting of Sinomenine
3. Results and Discussion
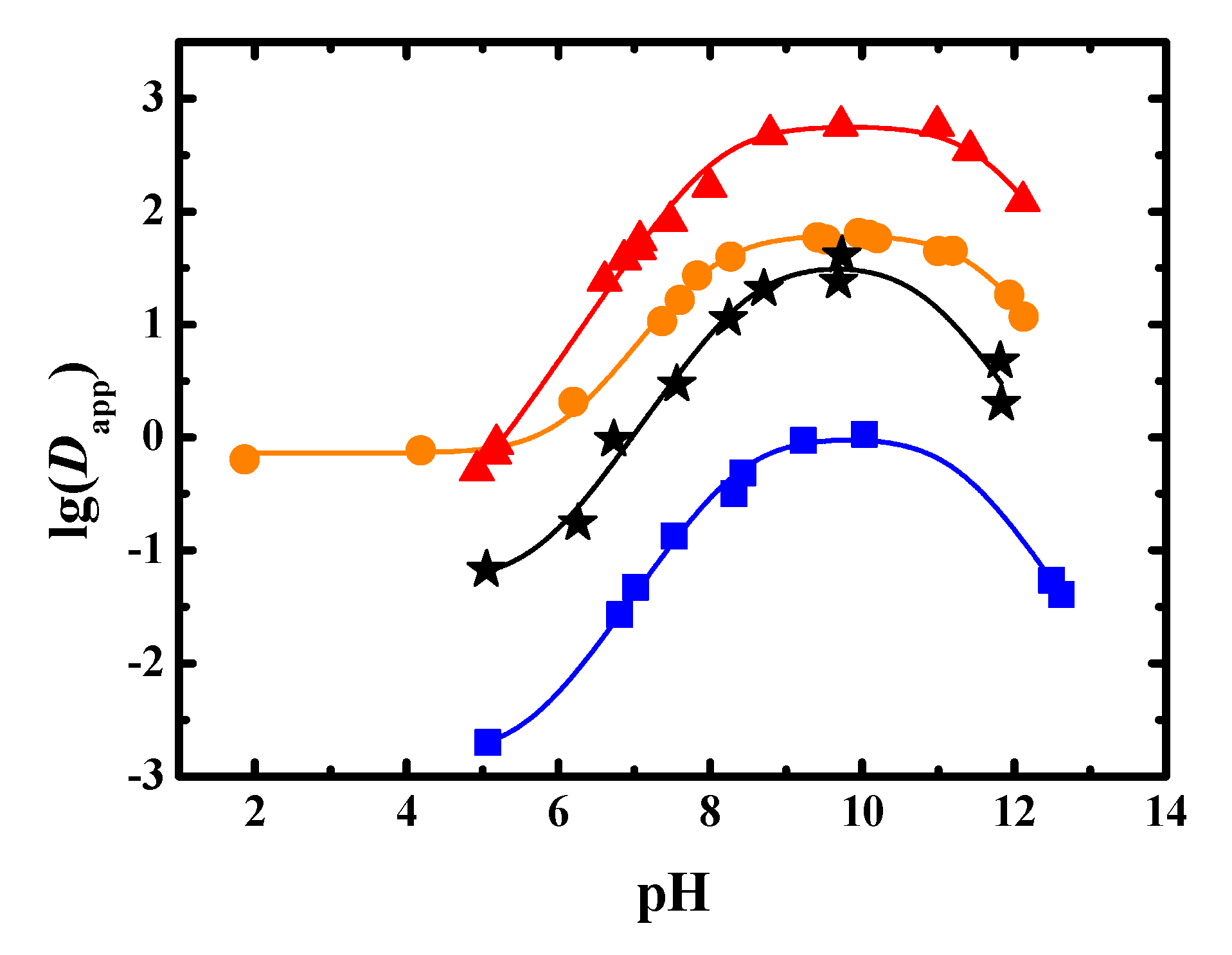
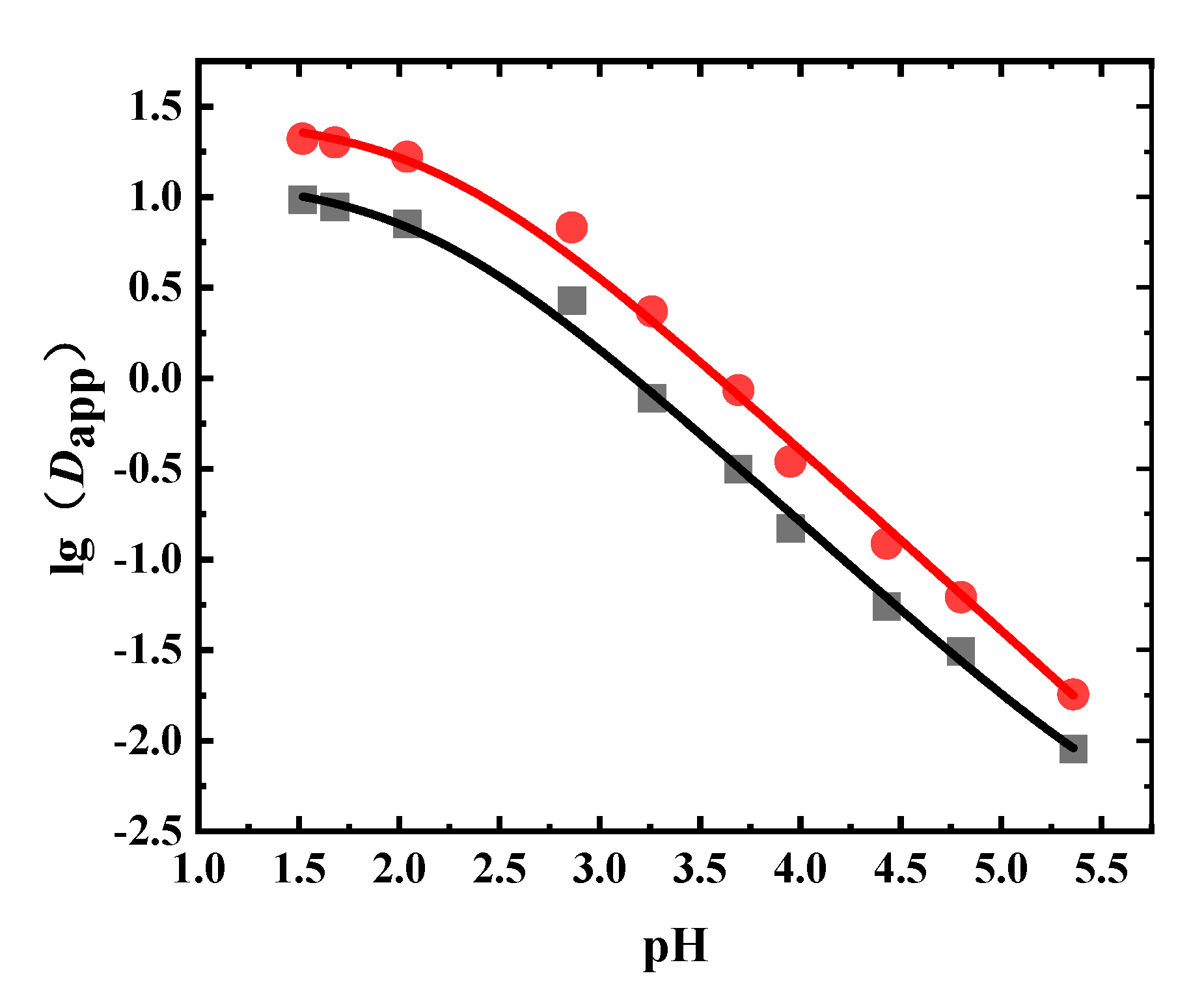
3.1. Dapp and pKa of Sinomenine
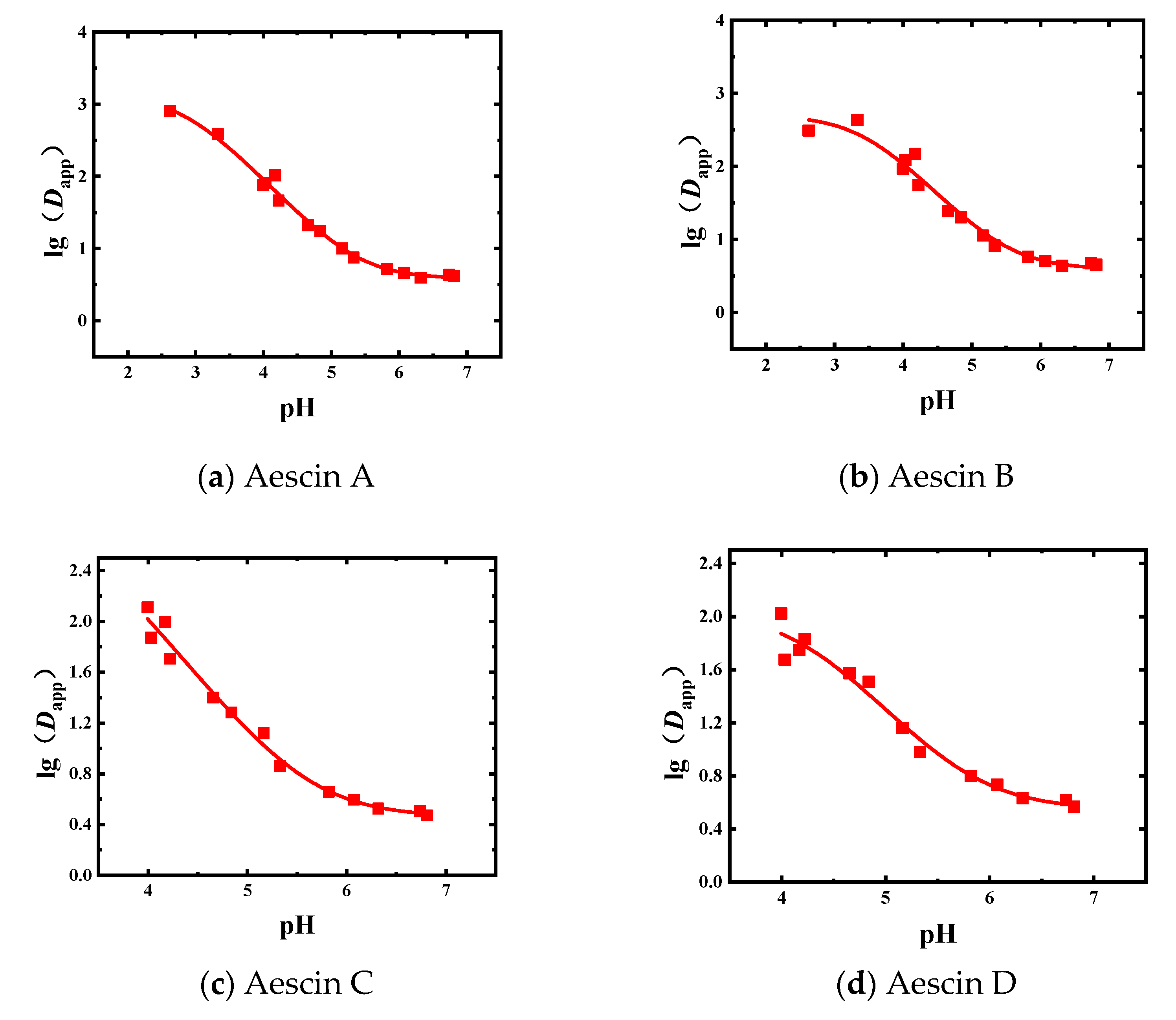
3.2. Dapp and pKa of Aescins
3.3. Dapp and pKa of the Phenolic Acids in Flos Lonicerae
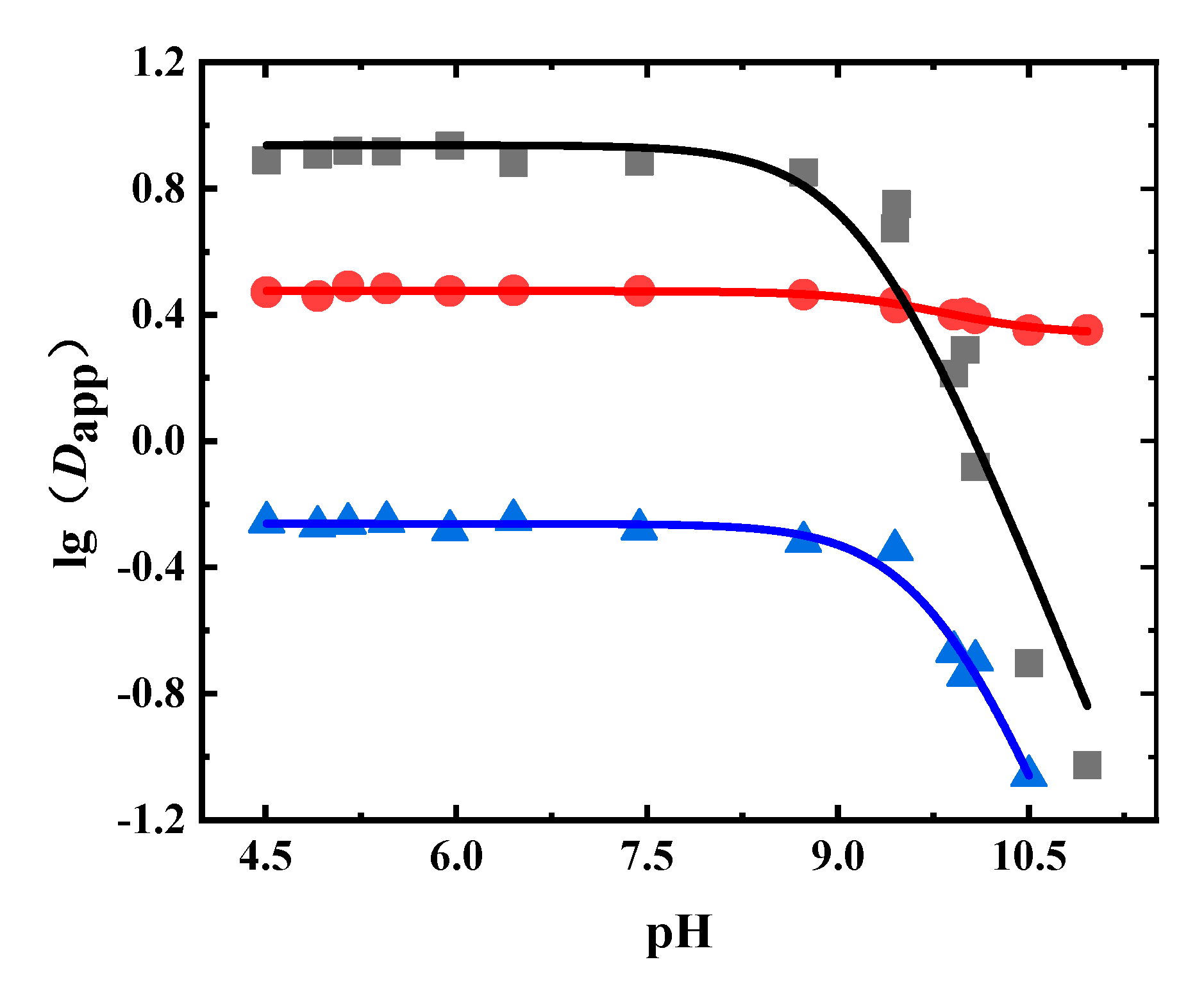
3.4. Dapp and pKa of Flavonoids in Radix Scutellariae
3.5. Dapp and pKa of Glycosides in Radix Astragali
3.6. Comparison between the Experimental Values and Literature Values
3.7. Suitable Mobile Phase for the HPLC Analysis of Each Ingredient
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1-Butanol | Trichloromethane | Methyl Isobutyl Ketone | 1-Octanol | ||||
|---|---|---|---|---|---|---|---|
| pH | Dapp | pH | Dapp | pH | Dapp | pH | Dapp |
| 1.871 | 0.636 | 4.925 | 0.510 | 5.071 | 0.002 | 5.053 | 0.068 |
| 4.188 | 0.769 | 5.156 | 0.717 | 6.804 | 0.027 | 6.258 | 0.175 |
| 6.197 | 2.073 | 5.180 | 0.871 | 7.015 | 0.047 | 6.729 | 0.961 |
| 7.366 | 10.70 | 6.609 | 24.36 | 7.520 | 0.134 | 7.562 | 2.981 |
| 7.596 | 16.54 | 6.862 | 37.69 | 8.309 | 0.317 | 8.236 | 11.28 |
| 7.826 | 27.13 | 7.062 | 46.37 | 8.429 | 0.483 | 8.703 | 20.63 |
| 8.264 | 39.87 | 7.071 | 55.50 | 9.226 | 0.939 | 9.688 | 24.05 |
| 9.413 | 59.42 | 7.471 | 82.10 | 10.03 | 1.067 | 9.730 | 40.90 |
| 9.523 | 56.52 | 7.990 | 165.5 | 12.50 | 0.054 | 11.82 | 4.769 |
| 9.954 | 64.25 | 8.788 | 481.2 | 12.63 | 0.041 | 11.83 | 2.005 |
| 10.08 | 62.42 | 9.723 | 574.3 | - | - | - | - |
| 10.20 | 57.92 | 10.99 | 571.7 | - | - | - | - |
| 11.00 | 44.59 | 11.42 | 348.2 | - | - | - | - |
| 11.19 | 44.88 | 12.11 | 123.6 | - | - | - | - |
| 11.94 | 18.30 | - | - | - | - | - | - |
| 12.13 | 11.74 | - | - | - | - | - | - |
| pH | Aescin A | Aescin B | Aescin C | Aescin D |
|---|---|---|---|---|
| 2.624 | 801.4 | 309.0 | - | - |
| 3.332 | 383.5 | 431.1 | - | - |
| 3.995 | 75.03 | - | 130.0 | 105.5 |
| 4.030 | 80.79 | 121.3 | 74.50 | - |
| 4.171 | - | 148.2 | 99.03 | - |
| 4.223 | 46.28 | 55.71 | 50.88 | 67.76 |
| 4.654 | 21.11 | 24.25 | 25.16 | 37.42 |
| 4.839 | 17.48 | 20.06 | 19.16 | 32.24 |
| 5.162 | 10.04 | 11.25 | 13.23 | 14.47 |
| 5.331 | 7.489 | 8.220 | 7.279 | 9.521 |
| 5.819 | 5.210 | 5.745 | 4.554 | 6.298 |
| 6.073 | 4.608 | 5.036 | 3.940 | 5.390 |
| 6.317 | 3.942 | 4.349 | 3.357 | 4.269 |
| 6.737 | 4.321 | 4.712 | 3.207 | 4.121 |
| 6.811 | 4.185 | 4.501 | 2.954 | 3.685 |
| pH | Neochlorogenic Acid | Chlorogenic Acid | Cryptochlorogenic Acid | Isochlorogenic Acid B | Isochlorogenic Acid A | Isochlorogenic Acid C |
|---|---|---|---|---|---|---|
| 4.910 | 0.054 | 0.254 | 0.112 | 2.527 | 10.89 | 15.80 |
| 4.530 | 0.128 | 0.581 | 0.270 | 5.800 | 25.00 | 37.02 |
| 4.200 | 0.245 | 1.104 | 0.524 | 12.24 | 49.84 | 73.58 |
| 3.690 | 0.552 | 2.474 | 1.241 | 29.51 | 112.3 | 181.5 |
| 3.200 | 0.935 | 4.201 | 2.228 | 54.28 | 181.7 | 326.9 |
| 2.730 | 1.339 | 5.905 | 3.326 | 83.89 | 259.9 | 506.8 |
| 2.350 | 1.314 | 6.009 | 3.444 | 87.93 | 281.2 | 545.1 |
| 1.860 | 1.435 | 6.852 | 3.971 | 97.22 | 278.2 | 594.8 |
| 1.660 | 1.699 | 7.606 | 4.507 | 106.7 | 300.3 | 669.1 |
| 1.520 | 1.572 | 7.310 | 4.337 | 100.0 | 271.6 | 632.7 |

| pH | Baicalin | Wogonoside |
|---|---|---|
| 5.360 | 0.009 | 0.018 |
| 4.800 | 0.031 | 0.062 |
| 4.430 | 0.055 | 0.122 |
| 3.950 | 0.148 | 0.347 |
| 3.690 | 0.315 | 0.861 |
| 3.260 | 0.777 | 2.337 |
| 2.860 | 2.678 | 6.800 |
| 2.040 | 7.073 | 16.67 |
| 1.680 | 8.796 | 19.99 |
| 1.520 | 9.658 | 20.98 |
| pH | Isomucronulatol 7-O-Glucoside | Astraisoflavan-7-O-β-D-Glucoside | Calycosin-7-Glucoside |
|---|---|---|---|
| 10.96 | 0.094 | 2.249 | - |
| 10.500 | 0.198 | 2.245 | 0.087 |
| 10.08 | 0.828 | 2.449 | 0.202 |
| 10.00 | 1.947 | 2.538 | 0.181 |
| 9.910 | 1.635 | 2.513 | 0.215 |
| 9.460 | 5.632 | 2.645 | 1.030 |
| 9.450 | 4.724 | 2.753 | 0.452 |
| 8.730 | 7.099 | 2.914 | 0.480 |
| 7.440 | 7.681 | 2.990 | 0.526 |
| 6.450 | 7.594 | 3.001 | 0.564 |
| 5.950 | 8.639 | 2.987 | 0.522 |
| 5.450 | 8.294 | 3.042 | 0.556 |
| 5.150 | 8.320 | 3.094 | 0.549 |
| 4.910 | 8.095 | 2.882 | 0.537 |
| 4.510 | 7.764 | 2.964 | 0.554 |
References
- Alexovic, M.; Dotsikas, Y.; Bober, P.; Sabo, J. Achievements in robotic automation of solvent extraction and related approaches for bioanalysis of pharmaceuticals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1092, 402–421. [Google Scholar] [CrossRef]
- Machado, T.C.; Kuminek, G.; Cardoso, S.G.; Rodriguez-Hornedo, N. The role of pH and dose/solubility ratio on cocrystal dissolution, drug supersaturation and precipitation. Eur. J. Pharm. Sci. 2020, 152, 105422. [Google Scholar] [CrossRef]
- Santos Lins, P.V.; Henrique, D.C.; Ide, A.H.; da Silva Duarte, J.L.; Dotto, G.L.; Yazidi, A.; Sellaoui, L.; Erto, A.; de Paiva e Silva Zanta, C.L.; Meili, L. Adsorption of a non-steroidal anti-inflammatory drug onto MgAl/LDH-activated carbon composite-Experimental investigation and statistical physics modeling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124217. [Google Scholar] [CrossRef]
- Queiroz, A.L.P.; Wood, B.; Faisal, W.; Farag, F.; Garvie-Cook, H.; Glennon, B.; Vucen, S.; Crean, A.M. Application of percolation threshold to disintegration and dissolution of ibuprofen tablets with different microcrystalline cellulose grades. Int. J. Pharm. 2020, 589, 119838. [Google Scholar] [CrossRef]
- Wiedenbeck, E.; Gebauer, D.; Cölfen, H. Potentiometric titration method for the determination of solubility limits and pK(a) values of weak organic acids in water. Anal. Chem. 2020, 92, 9511–9515. [Google Scholar] [CrossRef]
- Celebier, M.; Kocak, E.; Dogan, A.; Altinoz, S.; Basci, N.E. Investigating the physicochemical properties of phenazopyridine hydrochloride using high-performance liquid chromatography and UV-visible spectrophotometry. J. Res. Pharm. 2018, 22, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Su, X.; Bo, T.; Zhang, X.; Liu, H.; Li, K. Determination of dissociation constants of ten alkaloids by capillary zone electrophoresis. J. Sep. Sci. 2003, 26, 549–554. [Google Scholar] [CrossRef]
- Huo, H.; Li, T.; Zhang, L. pK(a) determination of oxysophocarpine by reversed-phase high performance liquid chromatography. Springerplus 2013, 2, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, A.J. Ionization-constants of aqueous ammonia from 25 to 250 °C and to 2000 bar. J. Solut. Chem. 1982, 11, 649–664. [Google Scholar] [CrossRef]
- Gong, X.; Huang, S.; Jiao, R.; Pan, J.; Li, Y.; Qu, H. The determination of dissociation constants for active ingredients from herbal extracts using a liquid liquid equilibrium method. Fluid Phase Equilibria 2016, 409, 447–457. [Google Scholar] [CrossRef]
- Madej, K.; Kozka, G.; Winiarski, M.; Piekoszewski, W. A Simple, Fast, and Green Oil Sample Preparation Method for Determination of Cannabidioloic Acid and Cannabidiol by HPLC-DAD. Separations 2020, 7, 60. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, Q.; Li, J. Research progress on anti-inflammatory and anti-tumor effects of sinomenine. Chin. Pharmacol. Bull. 2015, 31, 1040–1043. [Google Scholar]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2020, 19, 1–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Li, P.; Liu, S.; Liu, Q.; Li, Y.; Sun, Y.; He, C.; Xiao, P. Traditional uses, ten-years research progress on phytochemistry and pharmacology, and clinical studies of the genus Scutellaria. J. Ethnopharmacol. 2021, 265, 113198. [Google Scholar] [CrossRef]
- Lyu, Q.; Zhao, W.; Wang, S.; Teng, J.; Xin, D.; Li, J.; Kong, X. Effect of Astragali Radix Membranaceus in promoting blood circulation and its modern pharmacology research. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 215–224. [Google Scholar]
- Cao, Y.; Zhang, C.; Zhu, M.; Yang, G. Comparison of Different Extraction Methods of β-aescin A, B from Three Different Aesculi Semens in Wudang Area. J. Hubei Univ. Med. 2018, 37, 161–163. [Google Scholar]
- Wang, L.N.; Liu, H.Y.; Zhang, J.; Li, J.; Zhang, Y.Q. Simultaneous determination of eight Bioactive components in lonicerae japonicae flos by quantitative analysis of multi-components by single marker. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 57–61. [Google Scholar]
- Zhu, J.; Wang, Z.; Zhang, Q.; Niu, J.; Li, F. A quantitative method for simultaneous assay of four flavones with one marker in Radix Scutellariae. China J. Chin. Mater. Med. 2009, 34, 3229–3234. [Google Scholar]
- Chen, X.; Li, Q.; Tan, X.; Wang, P.; Bi, K. RP-HPLC simultaneous determination of six flavonoids in Radix Astragali. Chin. J. Pharm. Anal. 2009, 29, 1115–1118. [Google Scholar]
- Ma, Y.; Jin, Y.; Wang, Y.; Wang, R.; Lu, X.; Kong, D.; Xu, W. The discovery of a novel and selective inhibitor of PTP1B over TCPTP: 3D QSAR pharmacophore modeling, virtual screening, synthesis, and biological evaluation. Chem. Biol. Drug Des. 2014, 83, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, F.; Zhou, Y.; Mou, M.; Lu, Y.; Chen, K.; Xue, J.; Luo, Y.; Fu, J.; He, X.; et al. INTEDE: Interactome of drug-metabolizing enzymes. Nucleic Acids Res. 2021, 49, D1233–D1243. [Google Scholar] [CrossRef]
- Chokshi, A.B.; Chhabria, M.T.; Desai, P.R. Rational Discovery of Novel Squalene Synthase Inhibitors through Pharmacophore Modelling. Curr. Comput. Aided Drug Des. 2018, 14, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gao, H. Pharmacophore-based drug design for the identification of novel butyrylcholinesterase inhibitors against Alzheimer’s disease. Phytomed. Int. J. Phytother. Phytopharm. 2019, 54, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, S.; Gu, H.; Wu, Z.; Nyagblordzro, M.; Feng, G.; He, X. In vitro-in vivo predictive dissolution-permeation-absorption dynamics of highly permeable drug extended-release tablets via drug dissolution/absorption simulating system and pH alteration. AAPS PharmSciTech 2018, 19, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S. Degradation Mechanism and Mateiral Behaviors Analysis of the Inclusion Complexes of Sinomenine to Cyclodextrins. Master’s Thesis, Jishou University, Jishou, China, 2012. [Google Scholar]
- Xu, H.; Xu, Y. Simultaneous Determination of Three Phenolic Acid in Pugongying Granules by High Performance Liquid Chromatography-Mass Spectrometry. Chemistry 2008, 71, 415–419. [Google Scholar]
- Moridani, M.Y.; Scobie, H.; Jamshidzadeh, A.; Salehi, P.; O’Brien, P.J. Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: Glutathione conjugate formation. Drug Metab. Dispos. 2001, 29, 1432–1439. [Google Scholar]
- Tang, B.; Huang, Y.; Ma, X.; Liao, X.; Wang, Q.; Xiong, X.; Li, H. Multispectroscopic and docking studies on the binding of chlorogenic acid somers to human serum albumin: Effects of esteryl position on affinity. Food Chem. 2016, 212, 434–442. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, G.; Wang, X.; Kim, H.Y.; Zhao, S.; Sun, W.; Tong, S.; Lim, S.S. Retention mechanism of pH-peak-focusing in countercurrent chromatographic separation of baicalin and wogonoside fromScutellaria baicalensisGeorgi. J. Sep. Sci. 2020, 43, 3806–3815. [Google Scholar] [CrossRef]
- Guo, Z.; Chang, J.; Wang, W. Study on Reversed-Phase High Performance Liquid Chromatography Separation Condition and Determination Method of Organic Acids. Chin. J. Chromatogr. 2001, 19, 260. [Google Scholar]
- Yan, H.; Ma, S. RP-HPLC determination of sinomenine in Caulis Sinomenii. Chin. J. Pharm. Anal. 2006, 26, 201–203. [Google Scholar]
- Guo, J.; Xu, W.; Yang, X. Quantified analysis of triterpenoid saponins in Semen Aesculi by HPLC. Chin. Tradit. Herb. Drugs 2007, 38, 767–770. [Google Scholar]
- Li, M.; Wang, Y.; Meng, J.; Fu, X.; Bi, Y.; Wang, Z.; Xiao, W. Determination of eight components in Lonicerae Japonicae Flos by HPLC. Chin. Tradit. Herb. Drugs 2014, 45, 1006–1010. [Google Scholar]



| Number | Medicinal Extracts | Organic Extractant | Equilibrium Temperature |
|---|---|---|---|
| 1 | Caulis Sinomenii | Trichloromethane | 30 °C |
| 2 | 1-Butanol | 30 °C | |
| 3 | Methyl isobutyl ketone | 30 °C | |
| 4 | 1-Octanol | 30 °C | |
| 5 | Semen Aesculi | 1-Butanol | 30 °C |
| 6 | Flos Lonicerae | 1-Octanol | 30 °C |
| 7 | Radix Scutellariae | 1-Octanol | 30 °C |
| 8 | Radix Astragali | 1-Octanol | 30 °C |
| Variable | 1-Butanol | Trichloromethane | Methyl Isobutyl Ketone | 1-Octanol |
|---|---|---|---|---|
| d0 | −0.14 ± 0.03 | −0.92 ± 0.35 | −2.81 ± 0.10 | −1.27 ± 0.20 |
| d1 | 1.80 ± 0.02 | 2.76 ± 0.06 | 0.01 ± 0.06 | 1.55 ± 0.12 |
| d2 | −20 | −20 | −20 | −20 |
| pKa,1 | 8.01 ± 0.04 | 8.10 ± 0.08 | 8.40 ± 0.08 | 8.53 ± 0.18 |
| pKa,2 | 11.52 ± 0.05 | 11.59 ± 0.15 | 11.25 ± 0.08 | 10.81 ± 0.19 |
| R2adj | 0.996 | 0.992 | 0.992 | 0.969 |
| Aescin | d0 | d1 | pKa | R2adj |
|---|---|---|---|---|
| Aescin A | 3.14 ± 0.13 | 0.58 ± 0.04 | 2.83 ± 0.14 | 0.989 |
| Aescin B | 2.70 ± 0.13 | 0.59 ± 0.07 | 3.42 ± 0.16 | 0.970 |
| Aescin C | 2.96 ± 0.78 | 0.46 ± 0.05 | 3.10 ± 0.84 | 0.982 |
| Aescin D | 2.06 ± 0.11 | 0.54 ± 0.06 | 4.24 ± 0.18 | 0.970 |
| Phenolic Acid | d0 | d1 | pKa | R2adj |
|---|---|---|---|---|
| Chlorogenic acid | 0.85 ± 0.01 | −1.50 ± 0.34 | 3.44 ± 0.03 | 0.997 |
| Neochlorogenic acid | 0.19 ± 0.01 | −2.57 ± 0.96 | 3.46 ± 0.04 | 0.997 |
| Cryptochlorogenic acid | 0.62 ± 0.01 | −2.08 ± 0.63 | 3.33 ± 0.04 | 0.997 |
| Isochlorogenic acid A | 2.46 ± 0.01 | −20 | 3.50 ± 0.02 | 0.999 |
| Isochlorogenic acid B | 2.01 ± 0.01 | −20 | 3.31 ± 0.02 | 0.999 |
| Isochlorogenic acid C | 2.80 ± 0.01 | −20 | 3.31 ± 0.02 | 0.999 |
| Flavanoid | d0 | d1 | pKa | R2adj |
|---|---|---|---|---|
| Baicalin | 1.10 ± 0.06 | −2.68 ± 0.35 | 2.10 ± 0.08 | 0.996 |
| Wogonoside | 1.44 ± 0.07 | −20 | 2.17 ± 0.08 | 0.997 |
| Glycoside | d0 | d1 | pKa | R2adj |
|---|---|---|---|---|
| Isomucronulatol 7-O-glucoside | 0.94 ± 0.06 | −20 | 9.19 ± 0.10 | 0.940 |
| Astraisoflavan-7-O-β-D-glucoside | 0.48 ± 0.01 | 0.34 ± 0.01 | 9.76 ± 0.10 | 0.964 |
| Calycosin-7-glucoside | −0.26 ± 0.01 | −20 | 9.78 ± 0.03 | 0.981 |
| Active Ingredients | Literature Values | Prediction Values Using Software | Results of This Work |
|---|---|---|---|
| Sinomenine | pKa,1: 7.98 [25] pKa,2: 11.2 [26] | pKa,1: 7.45 pKa,2: 7.67 | pKa,1: 8.01, 8.10, 8.40, 8.53 pKa,2: 11.52, 11.59, 11.25, 10.81 |
| Aescin A | - | 2.77 | 2.83 ± 0.14 |
| Aescin B | - | 2.77 | 3.42 ± 0.16 |
| Aescin C | - | 2.77 | 3.10 ± 0.84 |
| Aescin D | - | 2.77 | 4.24 ± 0.18 |
| Chlorogenic acid | 3.59 [27] 3.58 [28] 3.90 [29] | 2.66 | 3.44 ± 0.03 |
| Neochlorogenic acid | 3.91 [29] | 2.66 | 3.46 ± 0.04 |
| Cryptochlorogenic acid | 4.07 [29] | 2.66 | 3.33 ± 0.04 |
| Isochlorogenic acid A | - | 2.66 | 3.50 ± 0.02 |
| Isochlorogenic acid B | - | 2.66 | 3.31 ± 0.02 |
| Isochlorogenic acid C | - | 2.66 | 3.31 ± 0.02 |
| Isomucronulatol 7-O-glucoside | - | 11.9 | 9.19 ± 0.10 |
| Astraisoflavan-7-O-β-D-glucoside | - | 11.9 | 9.76 ± 0.10 |
| Calycosin-7-glucoside | - | 10.4 | 9.78 ± 0.03 |
| Baicalin | - | 2.86 | 2.10 ± 0.08 |
| Wogonoside | 3.99 [30] | 2.86 | 2.17 ± 0.08 |
| Active Ingredient | Prediction Values Using Software | Results of This Work |
|---|---|---|
| Sinomenine | 1.674 | 1.55 ± 0.12 |
| Chlorogenic acid | −0.340 | 0.85 ± 0.01 |
| Neochlorogenic acid | −0.340 | 0.19 ± 0.01 |
| Cryptochlorogenic acid | −0.340 | 0.62 ± 0.01 |
| Isochlorogenic acid A | 1.687 | 2.46 ± 0.01 |
| Isochlorogenic acid B | 1.687 | 2.01 ± 0.01 |
| Isochlorogenic acid C | 1.687 | 2.80 ± 0.01 |
| Isomucronulatol 7-O-glucoside | 1.249 | 0.94 ± 0.06 |
| Astraisoflavan-7-O-β-D-glucoside | 1.249 | 0.48 ± 0.01 |
| Calycosin-7-glucoside | 0.436 | −0.26 ± 0.01 |
| Baicalin | 0.608 | 1.10 ± 0.06 |
| Wogonoside | 0.833 | 1.44 ± 0.07 |
| Active Ingredients | Suitable Mobile Phase for HPLC Analysis | pH Range of Mobile Phase |
|---|---|---|
| Sinomenine in Caulis Sinomenii | Methanol phosphate buffer (0.005 mol/L disodium hydrogen phosphate solution, 0.005 mol/L sodium hydrogen phosphate adjusted to pH 8.0, and 1% triethylamine adjusted to pH 9.0) (55:45) [32] | 9.0 |
| Aescins in Semen Aesculi | Acetonitrile–water–phosphoric acid (123:277:7) [33] | 1.5 |
| Phenolic acids in Flos Lonicerae | Methanol (A) and 0.1% phosphoric acid aqueous solution (B) using the following gradient program: 0–20 min, 12–30% A; 20–60 min, 30–50% A [34] | 2.0–2.7 |
| Flavonoids in Radix Scutellariae | 0.2% phosphoric acid-water (A) and methanol (B) using the following gradient program: 45–45% B, 0–10 min; 45–70% B, 10–55 min [19] | 2.2–2.8 |
| Glycosides in Radix Astragali | 0.05% formic acid–water (A) and acetonitrile (B) using the following gradient program: 15–29% B, 0–40 min; 29–40% B, 40–50 min; 40–40% B, 50–60 min; 40–90% B, 60–70 min [20] | 2.8–3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zheng, B.; Wu, J.; Lv, W.; Lin, P.; Gong, X. Determination of the Dissociation Constants of 16 Active Ingredients in Medicinal Herbs Using a Liquid–Liquid Equilibrium Method. Separations 2021, 8, 49. https://doi.org/10.3390/separations8040049
Wang W, Zheng B, Wu J, Lv W, Lin P, Gong X. Determination of the Dissociation Constants of 16 Active Ingredients in Medicinal Herbs Using a Liquid–Liquid Equilibrium Method. Separations. 2021; 8(4):49. https://doi.org/10.3390/separations8040049
Chicago/Turabian StyleWang, Wanying, Baixiu Zheng, Jiahao Wu, Weisong Lv, Peiying Lin, and Xingchu Gong. 2021. "Determination of the Dissociation Constants of 16 Active Ingredients in Medicinal Herbs Using a Liquid–Liquid Equilibrium Method" Separations 8, no. 4: 49. https://doi.org/10.3390/separations8040049
APA StyleWang, W., Zheng, B., Wu, J., Lv, W., Lin, P., & Gong, X. (2021). Determination of the Dissociation Constants of 16 Active Ingredients in Medicinal Herbs Using a Liquid–Liquid Equilibrium Method. Separations, 8(4), 49. https://doi.org/10.3390/separations8040049






