Review of Hydrogen Sulfide Removal from Various Industrial Gases by Zeolites
Abstract
:1. Introduction
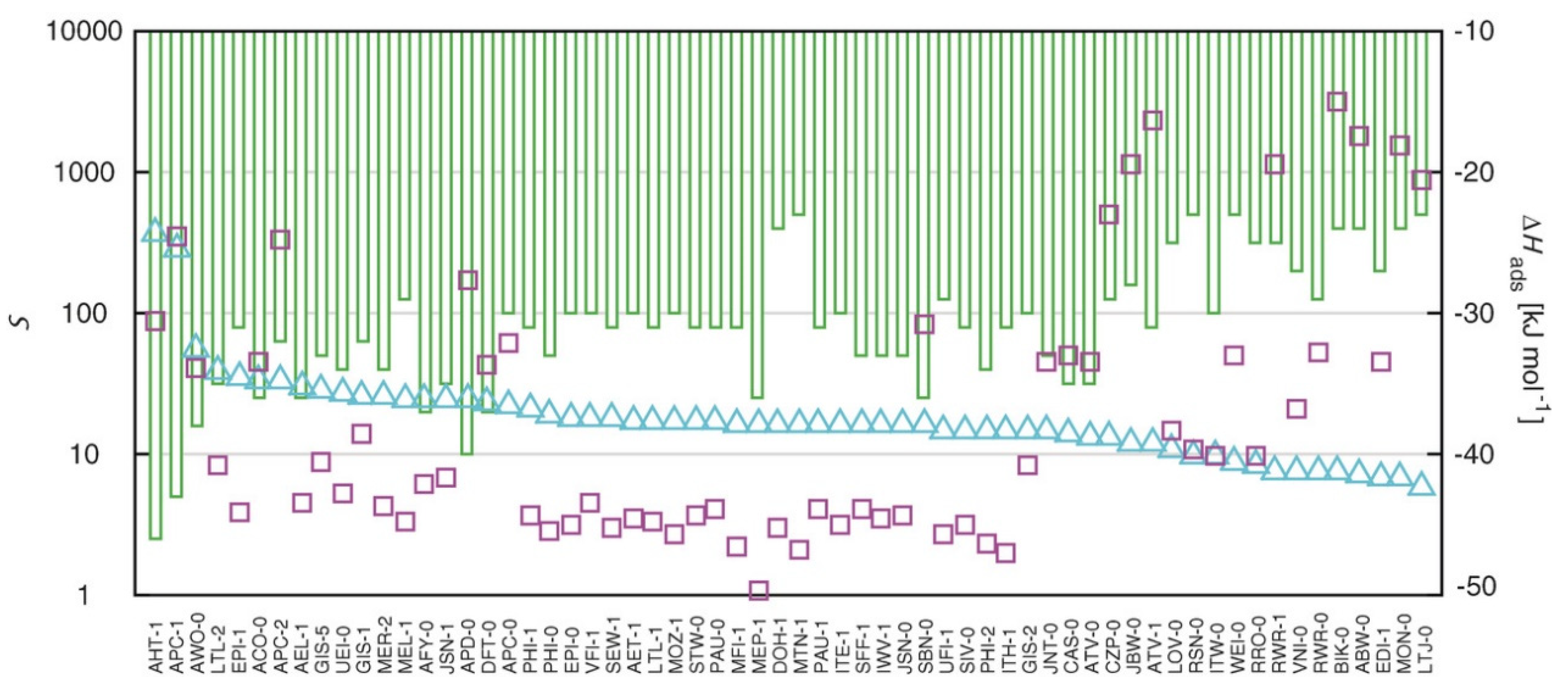
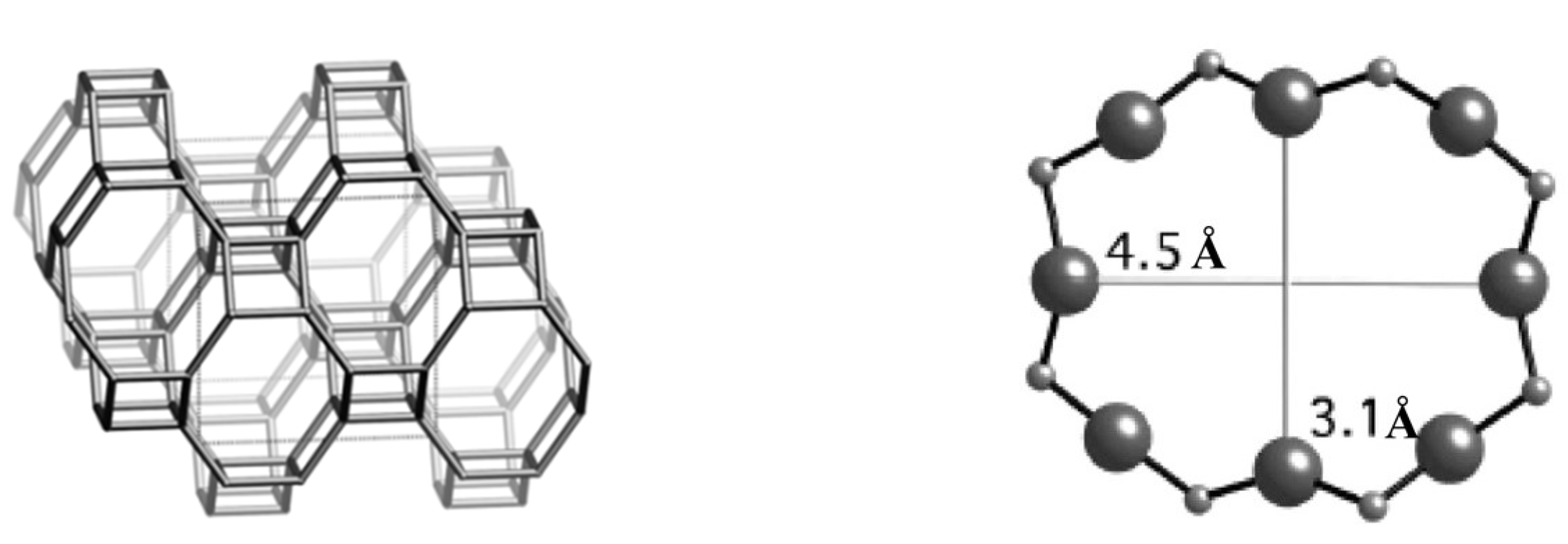
2. Physical Adsorption
3. Chemical Adsorption
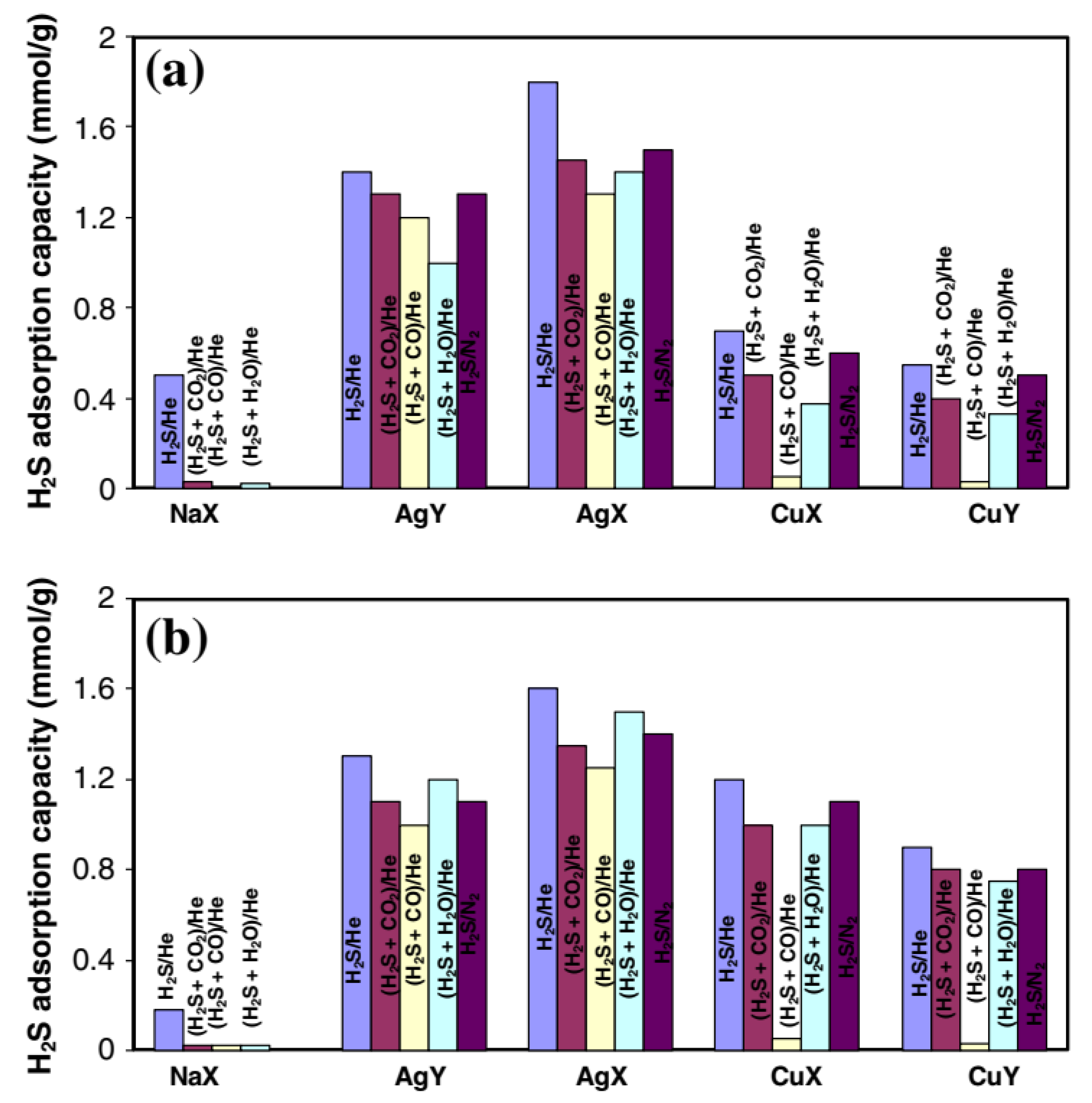
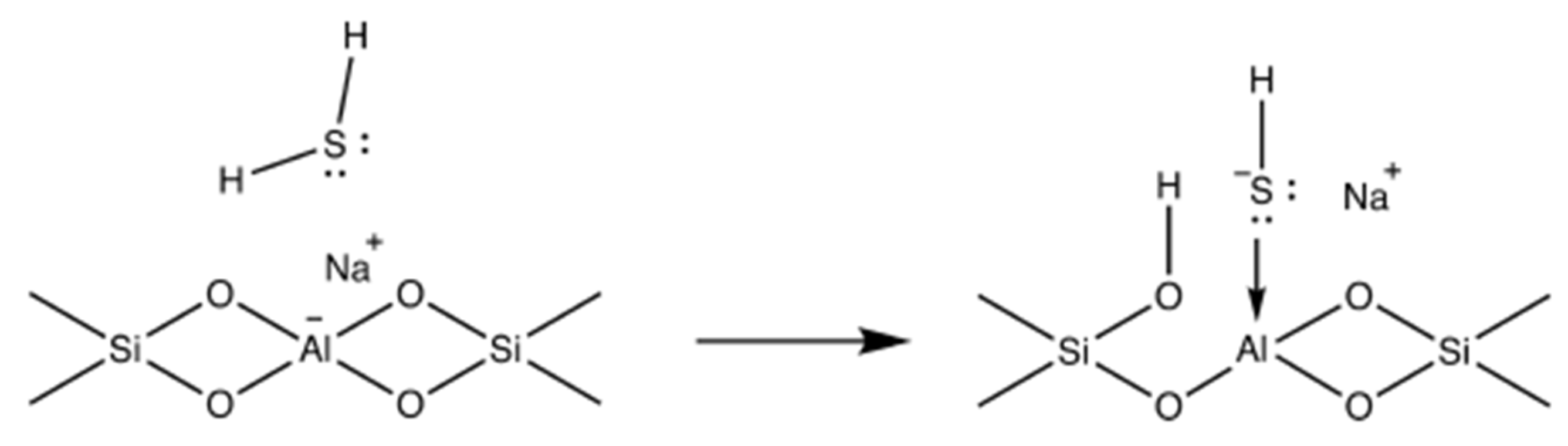
3.1. Ion-Exchange Modification
3.2. Metal Oxide Loading
4. Zeolite Membrane
5. Intracrystalline Diffusion of H2S
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Falco, G.; Montagnaro, F.; Balsamo, M.; Erto, A.; Deorsola, A.F.; Lisi, L.; Cimino, S. Synergic effect of Zn and Cu oxides dispersed on activated carbon during reactive adsorption of H2S at room temperature. Microporous Mesoporous Mater. 2018, 257, 135–146. [Google Scholar] [CrossRef]
- Peluso, A.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous Materials as H2S Adsorbents for Biogas Purification: A Review. Sep. Purif. Rev. 2019, 48, 78–89. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Gao, X.-M.; Di, X.-P.; Ouyang, Q.-Y.; Gao, P.; Qi, L.-H.; Li, C.-Y.; Zhu, C.-L. Porous Iron Molybdate Nanorods: In situ Diffusion Synthesis and Low-Temperature H2S Gas Sensing. ACS Appl. Mater. Interfaces 2013, 5, 3267–3274. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, T.L. Hydrogen Sulfide: Advances in Understanding Human Toxicity. Int. J. Toxicol. 2010, 29, 569–581. [Google Scholar] [CrossRef]
- PubChem. Hydrogen Sulfide [A/OL]. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/402 (accessed on 12 June 2022).
- Liu, G.; Huang, Z.-H.; Kang, F. Preparation of ZnO/SiO2 gel composites and their performance of H2S removal at room temperature. J. Hazard. Mater. 2012, 215-216, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, C.; Zhai, J.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Mandal, B.P.; Biswas, A.K.; Bandyopadhyay, S.S. Selective absorption of H2S from gas streams containing H2S and CO2 into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol. Sep. Purif. Technol. 2004, 35, 191–202. [Google Scholar] [CrossRef]
- Mi, J.; Liu, F.; Chen, W.; Chen, X.; Shen, L.; Cao, Y.; Au, C.; Huang, K.; Zheng, A.; Jiang, L. Design of Efficient, Hierarchical Porous Polymers Endowed with Tunable Structural Base Sites for Direct Catalytic Elimination of COS and H2S. ACS Appl. Mater. Interfaces 2019, 11, 29950–29959. [Google Scholar] [CrossRef]
- Basina, G.; Elmutasim, O.; Gaber, D.A.; Gaber, S.A.; Lu, X.; Tzitzios, V.; Vaithilingam, B.V.; Baikousi, M.; Asimakopoulos, G.; Karakassides, M.A.; et al. On the selective oxidation of H2S by heavy loaded Nanoparticles Embedded in Mesoporous Matrix (NEMMs). Appl. Catal. B Environ. 2020, 278, 119338. [Google Scholar] [CrossRef]
- Eow, J.S. Recovery of sulfur from sour acid gas: A review of the technology. Environ. Prog. 2002, 21, 143–162. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Bio/Technol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal–Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Lisi, L.; de Falco, G.; Montagnaro, F.; Balsamo, M.; Erto, A. Highlighting the effect of the support during H2S adsorption at low temperature over composite Zn-Cu sorbents. Fuel 2018, 221, 374–379. [Google Scholar] [CrossRef]
- Yu, T.; Chen, Z.; Wang, Y.; Xu, J. Synthesis of ZnO-CuO and ZnO-Co3O4 Materials with Three-Dimensionally Ordered Macroporous Structure and Its H2S Removal Performance at Low-Temperature. Processes 2021, 9, 1925. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J. Hazard. Mater. 2017, 326, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, X.; Song, C. “Molecular Basket” Sorbents for Separation of CO2 and H2S from Various Gas Streams. J. Am. Chem. Soc. 2009, 131, 5777–5783. [Google Scholar] [CrossRef]
- Okonkwo, C.N.; Okolie, C.; Sujan, A.; Zhu, G.; Jones, C.W. Role of Amine Structure on Hydrogen Sulfide Capture from Dilute Gas Streams Using Solid Adsorbents. Energy Fuels 2018, 32, 6926–6933. [Google Scholar] [CrossRef]
- Hamon, L.; Serre, C.; Devic, T.; Loiseau, T.; Millange, F.; Fe’rey, G.; Weireld, G.D. Comparative Study of Hydrogen Sulfide Adsorption in the MIL-53(Al, Cr, Fe), MIL-47(V), MIL-100(Cr), and MIL-101(Cr) Metal−Organic Frameworks at Room Temperature. J. Am. Chem. Soc. 2009, 131, 8775–8777. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Bhatt, P.M.; Adil, K.; Pillai, R.S.; Cadiau, A.; Shkurenko, A.; Maurin, G.; Liu, G.; Koros, W.J.; Eddaoudi, M. Natural gas upgrading using a fluorinated MOF with tuned H2S and CO2 adsorption selectivity. Nat. Energy 2018, 3, 1059–1066. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Kokuryo, S.; Miyake, K.; Uchida, Y.; Mizusawa, A.; Kubo, T.; Nishiyama, N. Defect engineering to boost catalytic activity of Beta zeolite on low-density polyethylene cracking. Mater. Today Sustain. 2022, 17, 100098. [Google Scholar] [CrossRef]
- Dusselier, M.; Van Wouwe, P.; Dewaele, A.; Jacobs, P.A.; Sels, B.F. Shape-selective zeolite catalysis for bioplastics production. Science 2015, 349, 78–80. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Karge, H.G.; Raskó, J. Hydrogen sulfide adsorption on faujasite-type zeolites with systematically varied Si-Al ratios. J. Colloid Interface Sci. 1978, 64, 522–532. [Google Scholar] [CrossRef]
- Maugé, F.; Sahibed-Dine, A.; Gaillard, M.; Ziolek, M. Modification of the Acidic Properties of NaY Zeolite by H2S Adsorption—An Infrared Study. J. Catal. 2002, 207, 353–360. [Google Scholar] [CrossRef]
- Salman, O.A.; Bishara, A.I. Selective separation of hydrogen sulfide by pressure-swing adsorption. Energy 1987, 12, 1275–1279. [Google Scholar] [CrossRef]
- Melo, D.M.A.; De Souza, J.R.; Melo, M.A.F.; Martinelli, A.E.; Cachima, G.H.B.; Cunha, J.D. Evaluation of the zinox and zeolite materials as adsorbents to remove H2S from natural gas. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 32–36. [Google Scholar] [CrossRef]
- Cruz, A.J.; Pires, J.; Carvalho, A.P.; Carvalho, M.B.D. Physical Adsorption of H2S Related to the Conservation of Works of Art: The Role of the Pore Structure at Low Relative Pressure. Adsorption 2005, 11, 569–576. [Google Scholar] [CrossRef]
- Bandarchian, F.; Anbia, M. Conventional hydrothermal synthesis of nanoporous molecular sieve 13X for selective adsorption of trace amount of hydrogen sulfide from mixture with propane. J. Nat. Gas Sci. Eng. 2015, 26, 1380–1387. [Google Scholar] [CrossRef]
- Tomadakis, M.M.; Heck, H.H.; Jubran, M.E.; Al-harthi, K. Pressure-Swing Adsorption Separation of H2S from CO2 with Molecular Sieves 4A, 5A, and 13X. Sep. Sci. Technol. 2011, 46, 428–433. [Google Scholar] [CrossRef]
- Heck, H.H.; Hall, M.L.; Dos Santos, R.; Tomadakis, M.M. Pressure swing adsorption separation of H2S/CO2/CH4 gas mixtures with molecular sieves 4A, 5A, and 13X. Sep. Sci. Technol. 2018, 53, 1490–1497. [Google Scholar] [CrossRef]
- Wynnyk, K.G.; Hojjati, B.; Pirzadeh, P.; Marriott, R.A. High-pressure sour gas adsorption on zeolite 4A. Adsorption 2017, 23, 149–162. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Bennett, J.M.; Grose, R.W.; Cohen, J.P.; Patton, R.L.; Kirchner, R.M.; Smith, J.V. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature 1978, 271, 512–516. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Soltanieh, M.; Bozorgzadeh, H.; Mohamadalizadeh, A. Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: Comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 2013, 19, 1045–1053. [Google Scholar] [CrossRef]
- Yaşyerli, S.; Ar, İ.; Doğu, G.; Doğu, T. Removal of hydrogen sulfide by clinoptilolite in a fixed bed adsorber. Chem. Eng. Process. Process Intensif. 2002, 41, 785–792. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Moldovan, A.; Cadar, O.; Levei, E.A.; Puskas, F.; Senila, M. Natural and Activated Zeolites as Effective Adsorbents in Drinking Water and Wastewater Treatment. In Proceedings of the International Multidisciplinary Scientific GeoConference: SGEM, Sofia, Bulgaria, 27 Jun–6 July 2020; Volume 20, pp. 281–288. [Google Scholar]
- Alonso-Vicario, A.; Ochoa-Gómez, J.R.; Gil-Río, S.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.A.; Torrecilla-Soria, J.; Domínguez, A. Purification and upgrading of biogas by pressure swing adsorption on synthetic and natural zeolites. Microporous Mesoporous Mater. 2010, 134, 100–107. [Google Scholar] [CrossRef]
- Wahono, S.K.; Prasetyo, D.J.; Jatmiko, T.H.; Suwanto, A.; Pratiwi, D.; Hernawan; Vasilev, K. Transformation of Mordenite-Clinoptilolite Natural Zeolite at Different Calcination Temperatures. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Tangerang, Indonesia, 1–2 November 2018; Volume 251, p. 012009. [Google Scholar]
- Akhalbedashvili, L.; Beruashvili, T.; Jalagania, S.; Janashvili, N.; Merabashvili, N. Adsorption of H2S from Thermal Water Using Clinoptilolite. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Prague, Czech Republic, 6–10 September 2021; Volume 906, p. 012047. [Google Scholar]
- Moradi, M.; Karimzadeh, R.; Moosavi, E.S. Modified and ion exchanged clinoptilolite for the adsorptive removal of sulfur compounds in a model fuel: New adsorbents for desulfurization. Fuel 2018, 217, 467–477. [Google Scholar] [CrossRef]
- Charpentier, J.-C. The triplet “molecular processes–product–process” engineering: The future of chemical engineering? Chem. Eng. Sci. 2002, 57, 4667–4690. [Google Scholar] [CrossRef]
- Cosoli, P.; Ferrone, M.; Pricl, S.; Fermeglia, M. Hydrogen sulphide removal from biogas by zeolite adsorption Part, I. GCMC molecular simulations. Chem. Eng. J. 2008, 145, 86–92. [Google Scholar] [CrossRef]
- Cosoli, P.; Ferrone, M.; Pricl, S.; Fermeglia, M. Hydrogen sulfide removal from biogas by zeolite adsorption. Part II. MD simulations. Chem. Eng. J. 2008, 145, 93–99. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Monte Carlo Simulations Probing the Adsorptive Separation of Hydrogen Sulfide/Methane Mixtures Using All-Silica Zeolites. Langmuir 2015, 31, 12268–12278. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Development of the Transferable Potentials for Phase Equilibria Model for Hydrogen Sulfide. J. Phys. Chem. B 2015, 119, 7041–7052. [Google Scholar] [CrossRef]
- Bai, P.; Tsapatsis, M.; Siepmann, J.I. TraPPE-zeo: Transferable Potentials for Phase Equilibria Force Field for All-Silica Zeolites. J. Phys. Chem. C 2013, 117, 24375–24387. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Identifying Optimal Zeolitic Sorbents for Sweetening of Highly Sour Natural Gas. Angew. Chem. 2016, 128, 6042–6046. [Google Scholar] [CrossRef]
- Hernández-Maldonado, A.J.; Yang, R.T.; Chinn, D.; Munson, C.L. Partially Calcined Gismondine Type Silicoaluminophosphate SAPO-43: Isopropylamine Elimination and Separation of Carbon Dioxide, Hydrogen Sulfide, and Water. Langmuir 2003, 19, 2193–2200. [Google Scholar] [CrossRef]
- Kesraoui-Ouki, S.; Cheeseman, C.R.; Perry, R. Natural zeolite utilisation in pollution control: A review of applications to metals’ effluents. J. Chem. Technol. Biotechnol. 1994, 59, 121–126. [Google Scholar] [CrossRef]
- Piéplu, A.; Saur, O.; Lavalley, J.-C.; Legendre, O.; Nédez, C. Claus Catalysis and H2S Selective Oxidation. Catal. Rev. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Kerr, G.T.; Johnson, G.C. Catalytic Oxidation of Hydrogen Sulfide to Sulfur over a Crystalline Aluminosilicate. J. Phys. Chem. 1960, 64, 381–382. [Google Scholar] [CrossRef]
- Dudzik, Z.; Ziólek, M. The specific catalytic activity of sodium faujasites in H2S oxidation. J. Catal. 1978, 51, 345–354. [Google Scholar] [CrossRef]
- Deo, A.V.; Lana, I.G.D.; Habgood, H.W. Infrared studies of the adsorption and surface reactions of hydrogen sulfide and sulfur dioxide on some aluminas and zeolites. J. Catal. 1971, 21, 270–281. [Google Scholar] [CrossRef]
- Bareschino, P.; Mancusi, E.; Forgione, A.; Pepe, F. Biogas purification on Na-X Zeolite: Experimental and numerical results. Chem. Eng. Sci. 2020, 223, 115744. [Google Scholar] [CrossRef]
- Kumar, P.; Sung, C.-Y.; Muraza, O.; Cococcioni, M.; Hashimi, S.A.; McCormick, A.; Tsapatsis, M. H2S adsorption by Ag and Cu ion exchanged faujasites. Microporous Mesoporous Mater. 2011, 146, 127–133. [Google Scholar] [CrossRef]
- Chen, X.; Shen, B.; Sun, H.; Zhan, G. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations. Microporous Mesoporous Mater. 2018, 261, 227–236. [Google Scholar] [CrossRef]
- Long, N.Q.; Vuong, H.T.; Ha, H.K.P.; Kuniawan, W.; Hinode, H.; Baba, T. Preparation, Characterization and H2S Adsorptive Removal of Ion-Exchanged Zeolite X. ASEAN Eng. J. 2016, 5, 4–14. [Google Scholar]
- Tran, H.-L.; Kuo, M.-S.; Yang, W.-D.; Huang, Y.-C. Hydrogen sulfide adsorption by thermally treated cobalt (II)-exchanged NaX zeolite. Adsorpt. Sci. Technol. 2016, 34, 275–286. [Google Scholar] [CrossRef]
- Sung, C.-Y.; Al Hashimi, S.; McCormick, A.; Tsapatsis, M.; Cococcioni, M. Density Functional Theory Study on the Adsorption of H2S and Other Claus Process Tail Gas Components on Copper- and Silver-Exchanged Y Zeolites. J. Phys. Chem. C 2012, 116, 3561–3575. [Google Scholar] [CrossRef]
- Sung, C.-Y.; Al Hashimi, S.; McCormick, A.; Cococcioni, M.; Tsapatsis, M. A DFT study on multivalent cation-exchanged Y zeolites as potential selective adsorbent for H2S. Microporous Mesoporous Mater. 2013, 172, 7–12. [Google Scholar] [CrossRef]
- Braga, M.U.C.; Perin, G.H.; De Oliveira, L.H.; Arroyo, P.A. DFT calculations for adsorption of H2S and other natural gas compounds on (Fe, Co, Ni, Cu and Zn)–Y zeolite clusters. Microporous Mesoporous Mater. 2022, 331, 111643. [Google Scholar] [CrossRef]
- Karge, H.G.; Ziółek, M.; Łaniecki, M. U.v./vis and i.r. spectroscopic study of hydrogen sulphide adsorption on faujasite-type zeolites. Zeolites 1987, 7, 197–202. [Google Scholar] [CrossRef]
- Howard, J.; Kadir, Z.A. The adsorption of H2S on some transition metal exchanged zeolites: An infrared study. Spectrochim. Acta Part A Mol. Spectrosc. 1985, 41, 825–831. [Google Scholar] [CrossRef]
- Förster, H.; Schuldt, M. Infrared spectroscopic study of the adsorption of hydrogen sulfide on zeolites NaA and NaCaA. J. Colloid Interface Sci. 1975, 52, 380–385. [Google Scholar] [CrossRef]
- Garcia, C.L.; Lercher, J.A. Adsorption of hydrogen sulfide on ZSM-5 zeolites. J. Phys. Chem. 1992, 96, 2230–2235. [Google Scholar] [CrossRef]
- Sigot, L.; Fontseré Obis, M.; Benbelkacem, H.; Germain, P.; Ducom, G. Comparing the performance of a 13X zeolite and an impregnated activated carbon for H2S removal from biogas to fuel an SOFC: Influence of water. Int. J. Hydrogen Energy 2016, 41, 18533–18541. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Germain, P. Adsorption of hydrogen sulfide (H2S) on zeolite (Z): Retention mechanism. Chem. Eng. J. 2016, 287, 47–53. [Google Scholar] [CrossRef]
- Fellmuth, P.; Lutz, W.; Bülow, M. Influence of weakly coordinated cations and basic sites upon the reaction of H2S and CO2 on zeolites. Zeolites 1987, 7, 367–371. [Google Scholar] [CrossRef]
- Lutz, W.; Suckow, M.; Bülow, M. Adsorption of hydrogen sulphide on molecular sieves: No enrichment in the presence of carbon dioxide. Gas Sep. Purif. 1990, 4, 190–196. [Google Scholar] [CrossRef]
- Bülow, M.; Lutz, W.; Suckow, M. The Mutual Transformation of Hydrogen Sulphide and Carbonyl Sulphide and Its Role for Gas Desulphurization Processes with Zeolitic Molecular Sieve Sorbents. In Studies in Surface Science and Catalysis; Dąbrowski, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 120, pp. 301–345. [Google Scholar]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in cation adsorption and cation exchange—A review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
- Kuznicki, S.M. Large-Pored Crystalline Titanium Molecular Sieve Zeolites. U.S. Patent 4,853,202A, 1 August 1989. [Google Scholar]
- Rezaei, S.; Tavana, A.; Sawada, J.A.; Wu, L.; Junaid, A.S.M.; Kuznicki, S.M. Novel Copper-Exchanged Titanosilicate Adsorbent for Low Temperature H2S Removal. Ind. Eng. Chem. Res. 2012, 51, 12430–12434. [Google Scholar] [CrossRef]
- Yazdanbakhsh, F.; Bläsing, M.; Sawada, J.A.; Rezaei, S.; Mülle, M.; Baumann, S.; Kuznicki, S.M. Copper Exchanged Nanotitanate for High Temperature H2S Adsorption. Ind. Eng. Chem. Res. 2014, 53, 11734–11739. [Google Scholar] [CrossRef]
- Rezaei, S.; Jarligo, M.O.D.; Wu, L.; Kuznicki, S.M. Breakthrough performances of metal-exchanged nanotitanate ETS-2 adsorbents for room temperature desulfurization. Chem. Eng. Sci. 2015, 123, 444–449. [Google Scholar] [CrossRef]
- Roller, D.; Bläsing, M.; Dreger, I.; Yazdanbakhsh, F.; Sawada, J.A.; Kuznicki, S.M.; Mülle, M. Removal of Hydrogen Sulfide by Metal-Doped Nanotitanate under Gasification-Like Conditions. Ind. Eng. Chem. Res. 2016, 55, 3871–3878. [Google Scholar] [CrossRef]
- Yazdanbakhsh, F.; Alizadehgiashi, M.; Bläsing, M.; Mülle, M.; Sawada, J.A.; Kuznicki, S.M. Cu–Cr–O Functionalized ETS-2 Nanoparticles for Hot Gas Desulfurization. J. Nanosci. Nanotechnol. 2016, 16, 878–884. [Google Scholar] [CrossRef]
- Westmoreland, P.R.; Harrison, D.P. Evaluation of candidate solids for high-temperature desulfurization of low-Btu gases. Environ. Sci. Technol. 1976, 10, 659–661. [Google Scholar] [CrossRef]
- Khabazipour, M.; Anbia, M. Removal of Hydrogen Sulfide from Gas Streams Using Porous Materials: A Review. Ind. Eng. Chem. Res. 2019, 58, 22133–22164. [Google Scholar] [CrossRef]
- Su, Y.-M.; Huang, C.-Y.; Chyou, Y.-P.; Svoboda, K. Sulfidation/regeneration multi-cyclic testing of Fe2O3/Al2O3 sorbents for the high-temperature removal of hydrogen sulfide. J. Taiwan Inst. Chem. Eng. 2017, 74, 89–95. [Google Scholar] [CrossRef]
- Balsamo, M.; Cimino, S.; De Falco, G.; Erto, A.; Lisi, L. ZnO-CuO supported on activated carbon for H2S removal at room temperature. Chem. Eng. J. 2016, 304, 399–407. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, L.-J.; Yang, C.; Zhang, H.-Y.; Zhang, Y.-R.; Fan, H.-L.; Huo, C. Room-temperature hydrogen sulfide removal with zinc oxide nanoparticle/molecular sieve prepared by melt infiltration. Fuel Process. Technol. 2019, 185, 26–37. [Google Scholar] [CrossRef]
- Cara, C.; Rombi, E.; Mameli, V.; Ardu, A.; Angotzi, M.S.; Niznansky, D.; Musinu, A.; Cannas, C. γ-Fe2O3-M41S Sorbents for H2S Removal: Effect of Different Porous Structures and Silica Wall Thickness. J. Phys. Chem. C 2018, 122, 12231–12242. [Google Scholar] [CrossRef]
- Cara, C.; Rombi, E.; Musinu, A.; Mameli, V.; Ardu, A.; Angotzi, M.S.; Atzori, L.; Niznansky, D.; Xin, H.; Cannas, C. MCM-41 support for ultrasmall γ-Fe2O3 nanoparticles for H2S removal. J. Mater. Chem. A 2017, 5, 21688–21698. [Google Scholar] [CrossRef]
- Pi, J.-H.; Lee, D.-H.; Lee, J.-D.; Jun, J.H.; Park, N.-K.; Ryu, S.-O.; Lee, T.-J. The study on the selective oxidation of H2S over the mixture zeolite NaX-WO3 catalysts. Korean J. Chem. Eng. 2004, 21, 126–131. [Google Scholar] [CrossRef]
- Lee, S.-K.; Jang, Y.-N.; Bae, I.-K.; Chae, S.-C.; Ryu, K.-W.; Kim, J.-K. Adsorption of Toxic Gases on Iron-Incorporated Na-A Zeolites Synthesized from Melting Slag. Mater. Trans. 2009, 50, 2476–2483. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Wei, S.; Wang, J.; Liu, Y.; Li, M.; Liu, R. Selective removal of H2S from biogas using a regenerable hybrid TiO2/zeolite composite. Fuel 2015, 157, 183–190. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Mat, R.; Somderam, S.; Aziz, A.S.A.; Mohamed, A. Hydrogen sulfide adsorption by zinc oxide-impregnated zeolite (synthesized from Malaysian kaolin) for biogas desulfurization. J. Ind. Eng. Chem. 2018, 65, 334–342. [Google Scholar] [CrossRef]
- Elmutasim, O.; Basina, G.; Shami, D.A.; Gaber, D.; Gaber, S.; Karanikolos, G.N.; Wahedi, Y.A. On the impact of copper local environment on hydrogen sulfide adsorption within microporous AlPO4-5. J. Environ. Chem. Eng. 2020, 8, 104245. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Liu, Q.; Xu, R.; Xia, H. Lattice substitution and desulfurization kinetic analysis of Zn-based spinel sorbents loading onto porous silicoaluminophosphate zeolites. J. Hazard. Mater. 2020, 383, 121151. [Google Scholar] [CrossRef]
- Gasper-Galvin, L.D.; Atimtay, A.T.; Gupta, R.P. Zeolite-Supported Metal Oxide Sorbents for Hot-Gas Desulfurization. Ind. Eng. Chem. Res. 1998, 37, 4157–4166. [Google Scholar] [CrossRef]
- Babu, V.P.; Kraftschik, B.E.; Koros, W.J. Crosslinkable TEGMC asymmetric hollow fiber membranes for aggressive sour gas separations. J. Membr. Sci. 2018, 558, 94–105. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, H.; Selyanchyn, R.; Wang, B.; Deng, L.; Dai, Z.; Xia, J. Hydrogen sulfide removal from natural gas using membrane technology: A review. J. Mater. Chem. A 2021, 9, 20211–20240. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Poshusta, J.C.; Tuan, V.A.; Pape, E.A.; Noble, R.D.; Falconer, J.L. Separation of light gas mixtures using SAPO-34 membranes. AIChE J. 2000, 46, 779–789. [Google Scholar] [CrossRef]
- Cui, Y.; Kita, H.; Okamoto, K. Preparation and gas separation performance of zeolite T membrane. J. Mater. Chem. 2004, 14, 924. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Soltanieh, M. Simultaneous separation of H2S and CO2 from CH4 by a high silica CHA-type zeolite membrane. J. Membr. Sci. 2014, 470, 159–165. [Google Scholar] [CrossRef]
- Kärger, J.; Ruthven, D.M. Diffusion in nanoporous materials: Fundamental principles, insights and challenges. New J. Chem. 2016, 40, 4027–4048. [Google Scholar] [CrossRef]
- Shen, B.; Chen, X.; Wang, H.; Xiong, H.; Bosch, E.G.T.; Lazić, I.; Cai, D.; Qian, W.; Jin, S.; Liu, X. A single-molecule van der Waals compass. Nature 2021, 592, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chu, Y.; Tang, X.; Huang, L.; Li, G.; Yi, X.; Zheng, A. Diffusion Dependence of the Dual-Cycle Mechanism for MTO Reaction inside ZSM-12 and ZSM-22 Zeolites. J. Phys. Chem. C 2017, 121, 22872–22882. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, X.; Wang, G.; Tang, X.; Li, G.; Huang, L.; Zheng, A. Roles of 8-ring and 12-ring channels in mordenite for carbonylation reaction: From the perspective of molecular adsorption and diffusion. J. Catal. 2019, 369, 335–344. [Google Scholar] [CrossRef]
- Sun, Y.; Han, S. Diffusion of N2, O2, H2S and SO2 in MFI and 4A zeolites by molecular dynamics simulations. Mol. Simul. 2015, 41, 1095–1109. [Google Scholar] [CrossRef]




| Property | Value |
|---|---|
| Molar mass | 34.08 g/mol |
| Relative gas density | 1.19 |
| Odor threshold low | 0.001 ppm |
| Triple point | 187.6 K |
| Explosive range | 4.5~46% |
| Kinetic diameter | 0.36 nm |
| Dipole moment | 0.97 D |
| Odor | Rotten eggs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Chen, Z.; Liu, Z.; Xu, J.; Wang, Y. Review of Hydrogen Sulfide Removal from Various Industrial Gases by Zeolites. Separations 2022, 9, 229. https://doi.org/10.3390/separations9090229
Yu T, Chen Z, Liu Z, Xu J, Wang Y. Review of Hydrogen Sulfide Removal from Various Industrial Gases by Zeolites. Separations. 2022; 9(9):229. https://doi.org/10.3390/separations9090229
Chicago/Turabian StyleYu, Tao, Zhuo Chen, Zhendong Liu, Jianhong Xu, and Yundong Wang. 2022. "Review of Hydrogen Sulfide Removal from Various Industrial Gases by Zeolites" Separations 9, no. 9: 229. https://doi.org/10.3390/separations9090229





