Definition of the Pnictogen Bond: A Perspective
Abstract
:1. Preface
2. Definition and Recommendations
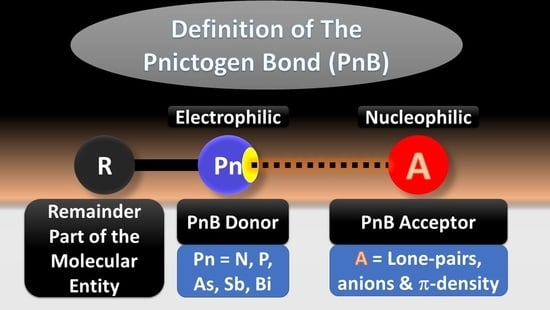
- A pnictogen bond occurs in chemical systems when there is evidence of a net attractive interaction between an electrophilic region associated with a pnictogen atom in a molecular entity and a nucleophilic region in another, or the same molecular entity.
- Note 1: A pnictogen bond is usually represented by three dots in the geometric motif R–Pn···A, where Pn is the PnB donor, representing any pnictogen atom (possibly hypervalent) that has an electrophilic region on it; R is the remaining part of the molecular entity R–Pn containing the PnB donor; A is a PnB acceptor, which may or may not represent a molecular entity, but that has at least one nucleophilic region.
- Note 2: An electrophilic site on the PnB donor Pn generally refers to the lowest electron density region, while a nucleophilic site on the PnB acceptor A usually refers to the highest electron density region, and the resulting interactions formed between the two entities exhibit different directional features and complementarity.
- Note 3: At an equilibrium configuration, PnB donors Pn exhibit the ability to act as electron density acceptors, and PnB acceptors A exhibit the ability to act as electron density donors.
- Note 4: A pnictogen bond may occur within a neutral molecule [12,14] or between two neutral molecules in close proximity [12,13]; it can also occur between a neutral molecule with a PnB donor Pn and an anion containing A [15]; between a PnB donor in a molecular cation and a nucleophile (or negative π-density) A on a neutral molecule [16]; between an electron-poor delocalized region (positive π-density) as the PnB donor Pn and nucleophile A (or negative π-density) on the acceptor entity; or between two molecular entities of opposite charge polarity (i.e., an ion pair)) with a PnB donor and a PnB acceptor [7,17,18].
- Note 6: Because of its variable electrostatic character, a pnictogen atom in a molecular entity may engage in a number of interactions that lead to the appearance of a variety electronic and geometric features [6,7,11,12,13,14,19]. The term pnictogen bond should not be used for attractive interactions in which the pnictogen atom (frequently nitrogen and sometimes phosphorous) functions as a nucleophile.
- Note 7: The electrophilic and nucleophilic characteristics of a bound pnictogen atom and its PnB forming ability may be found by searching for the local minima and maxima of the potential on the electrostatic surface of the molecular entity [6,7,11,12,13,14,20,21,22,23,24,25,26,27,28]. The electrophilic region on the surface of the bound pnictogen atom along the outermost extension of the R–Pn covalent or coordinate bond in an isolated monomeric entity is often (but not always) represented by a local maximum of the potential and may be used to search for pnictogen bonds between it and the nucleophilic regions on atoms in the entities with which it interacts [6,7,11,12,13,14,20,21,22,23,24,25,26,27,28].
- Note 8: Two pnictogen atoms in two different molecular entities may be involved in an attractive engagement to form a pnictogen bond, in which case, one of the pnictogen atoms must act as a pnictogen bond donor, and that in the partner molecular entity must act as a PnB acceptor, such as in NO2HP···NH3 [29].
- Note 9: The pnictogen bond should be viewed as an attractive interaction between PnB donor site Pn and PnB acceptor site A of opposite charge polarity (Pnδ+ and Aδ−), resulting in a coulombic interaction between them; the charge polarity δ+ and δ− symbolically refers to the local charge polarity on the interacting regions on Pn and A, respectively.
- Note 10: The pnictogen bond should follow the Type-II topology of non-covalent bonding interactions; a Type-II interaction, R–Pn···A, is often linear or quasi-linear (but may be non-linear) and satisfies Note 9.
3. Some Common Pnictogen Bond Donors and Acceptors
- –
- A pnictogen in a trihalide: PnX3 (Pn = N, P, As, Sb, Bi; X = halide).
- –
- –
- The Nβ of covalently bonded azides, such as –Nα = Nβ = Nγ (as in 2,4,6-triazidoborazine (H3B3N12) [35], 5-diazonio-4-(2H-tetrazol-5-yl)-1,2,3-triazol-1-ide (C3HN9) [36]), and 2,2,4,4,6,6-hexaazido-2,4,6-triphospha-1,3,5-triazine (P3N21) [37], or nitrogen in the diazonio fragment, such as in –Nα = Nβ (as in 4-diazonio-3,5-dinitropyrazol-1-ide (C3N6O4) [38] and in diazonionaphthalen-1-olate (C10H6N2O) [39].
- –
- The nitrogen in ammonium, diammonium, and (chain and arene) derivatives of ammonium (for example, NH4+, NH3NH32+, NH3NH2+, CH3NH3+, [CnH2n+1NH3]+ (n = 2, 3, …, 18), and [NH3(CH2)mNH3]2+ (m = 2, 3, …, 8) [7]).
- –
- –
- –
- The phosphorous in phosphoryl halides (POF3, POCl3, and POBr3) [11]; phosphorus(V) triazides OP(N3)3 and SP(N3)3 [52]; diphosphorus tetraiodide P2I4, phosphorus tricyanide, P(CN)3, and 4,4′,4″-phosphinetriyltripyridine [53]; and disphospha-functionalised naphthalenes (such as Nap(PCl2)2 Nap(PBr2)2 and Nap(PI)2 (Nap = naphthalene-1,8-diyl) [54]) and phosphorus diisocyanate chloride P(CO)2 [55], etc.
- –
- Phosphorous in derivatives of halo-substituted phosphazenes (viz. cyclo-tetrakis(difluorophosphazene) F8N4P4 [56], decafluorocyclo-pentaphosphazene F10N5P5 [57], hexachloro-cyclo-triphosphazene Cl6N3P3 [58], cyclo-tetrakis(phosphorus(V) nitride dichloride) Cl8N4P4 [59], nonachlorohexahydroheptaazahexaphosphaphenalene Cl9N7P6 [60], tris(dibromophosphazene) Br6N3P3 [61], octabromocyclo-tetraphosphazene Br8N4P4, [62], etc.).
- –
- Phosphorous in phosphorus oxides (phosphorus(V) oxide P2O5 [63], tetraphosphorus(III) oxide P4O6 [64], tetraphosphorus(III,IV) heptaoxide P4O7 [65], phosphorus(II) oxide P4O8 [66], tetraphosphorus(II,III) nonaoxide oxide P4O9 [67], tetraphosphorus(V) oxide P4O10 [68], phosphorus ozonide P4O18 [69], etc.).
- –
- Pnictogen in halide-, amino-, imidazole-, oxy-, and thio-substituted heavier pnictogen derivatives, in diaryl halido-substituted bismuthanes (e.g., C24H34BiI [70]), and in BiMe3Cl2, AsMe3, SbMe3, BiMe3, etc.).
- –
- –
- The antimony in bis(dimethylstibanyl)sulfane [76], bis(dimethylstibanyl)oxane [76], (trimethyl-stibino)-dimethyl-stibonium [77], trichloro-dipyridine-antimony [78], triphenyl-bis(p-tolylacetato)-antimony [79], bis(3-methoxyphenylacetate)-triphenyl-antimony [79], bis(acetato-O)-(2,6-bis(t-butoxymethyl)phenyl-C)-antimony(III) [80], bis(trichloro-antimony) [81], etc.
- –
- Arene-substituted pnictogen derivatives, including the bismuth in triphenyl-bismuth Bi(C6H5)3 and pyridine dipyrrolide complexes, C43H37BiIN3, etc.
- –
- A positive π system (species featuring a double or triple bond (e.g., midpoint of the N≡N bond in N2; P in P2; Bi in Bi2; N in NO2) of neutral and cationic entities).
- –
- A lone pair on an atom in a molecule. There are almost limitless possibilities, for example, the N in pyridines or amines, or even in N2; the O in H2O, CO, CO2, an ether, or a carbonyl group, or a phosphorus oxide; covalently bonded halogens in molecules; As in AsMe3; a chalcogen in a heterocycle such as a thio-, seleno-, and tellurophene derivatives as well as fused polycyclic derivatives thereof; furoxans, 2,5-thiadiazoles N-oxides, sulfoxide, aryl sulfoxides, and tellurazoles N-oxides; derivatives of macrocyclic crown-ethers such as 18-crown-6, 15-crown-5 and 21-crown-7, etc.
- –
- Many anions, such as halide anions; NO3−; CF3SO3−; BF4−; tetraphenylborate C24H20B−; ClO4−; 5-oxotetrazole CHN4O−; I3−; Br3−; N3−; BF4−; AuCl4−; PF6−; AsF6−; pentazolide N5−; 5,5′-bistetrazolates C2N82−; p-tosylate C7H7SO3−; polyatomic oxyanions such as C2O42−; GaCl4−; ZnCl42−; ReO4−; AsCl4−, SbCl4−; BiCl4−; etc.
- –
- A (negative) π system (species featuring a double or triple bond) and arene moieties of any kind, such as the centroid of the arenes and the midpoints of molecular As2 and N in NO3−, etc.
4. Examples of Chemical Systems Featuring Pnictogen Bonding
5. A List of Characteristic Features
- The separation distance between the PnB donor atom Pn and the nucleophilic site of PnB acceptor A tends to be smaller than the sum of the van der Waals radii of the respective interacting atomic basins [6,7,11,12,13,14] and larger than the sum of their covalent bond radii [2,3,4]; the deviation of the former is likely since the known van der Waals radii of atoms are only accurate with ±0.2 Å [13,113,114];
- The PnB donor site on Pn tends to approach the PnB acceptor site A along the outer extension of a σ covalent or coordinate bond, and the angular deviation from the extension is often more pronounced in PnBs [6,7,11,12,13,14] than in halogen bonds, as in ChBs [4], with the latter possibly being due to the involvement of secondary interactions;
- The angle of interaction, ∠R–Pn···A, tends to be linear or quasi-linear when the approach of the electrophile on Pn is along the σ covalent/coordinate bond extension, but this can be non-linear or have a bent shape when the pnictogen bond occurs between an electron density-deficient (electrophilic) π-type orbital of the bonded pnictogen atom and the nucleophilic region on A [6,7,11,12,13,14] or when secondary interactions are involved;
- The distance of the R–Pn covalent bond opposite to the PnB in a molecular adduct is typically longer than that in the isolated (unbound) PnB donor;
- The infrared absorption and Raman scattering observables of both R–Pn and A are affected by PnB formation; the vibrational frequency of the R–Pn bond may be red-shifted or blue-shifted depending on the extent of the interactions involved compared to the frequency of the same bond in the isolated molecular entity; new vibrational modes associated with the formation of the Pn···A intermolecular pnictogen bond should also be characteristically observed [116,117], as observed for ChBs;
- The UV–vis absorption bands of the PnB donor chromophore may experience a shift to longer wavelengths [128];
- At least some transfer of charge density from the frontier PnB acceptor orbital to the frontier PnB donor orbital may occur [15,129,130]; when the transfer of electron charge density between them is significant, the formation of a dative coordinate interaction is likely [131]; the occurrence of the IUPAC-recommended phenomena for HBs (see Criteria E1 and Characteristic C5 of Ref. [2]) is also applicable to XBs [132,133,134,135] and ChBs [136,137,138];
- The PnB strength typically decreases with a given acceptor A, as the electronegativity of Pn increases in the order Bi < Sb < As < P < N, and the electron withdrawing ability of R decreases;
- The PnB bond strength increases for a specific PnB acceptor A and the remaining R, as the polarizability of the pnictogen atoms in the molecular entities increases (Bi > Sb > As > P > N) [15]. This is the same as what is observed for the halogen derivative forming XB (I > Br > Cl > F) [145,146] and the chalcogen derivatives forming ChB (Te > Se > S > O) [147]. However, if secondary interactions (e.g., a hydrogen bond, halogen bond, chalcogen bond, tetrel bond, etc.) are simultaneously involved with either the PnB donor or PnB acceptor, the order of interaction strength may also be altered;
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Girolami, G.S. Origin of the Terms Pnictogen and Pnictide. J. Chem. Ed. 2009, 86, 1200. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Shing Ho, P.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, R.G.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Nagle, J.K. Atomic polarizability and electronegativity. J. Am. Chem. Soc. 1990, 112, 4741–4747. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. The Nitrogen Bond, or The Nitrogen-centered Pnictogen Bond: The Covalently Bound Nitrogen Atom in Molecular Entities and Crystals as a Pnictogen Bond Donor. Compounds 2022, 2, 80–110. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond. Int. J. Mol. Sci. 2022; in press. [Google Scholar]
- Varadwaj, P.R.; Cukrowski, I.; Marques, H.M. DFT-X3LYP Studies on the Coordination Chemistry of Ni2+. Part 1: Six Coordinate [Ni(NH3)n(H2O)6-n]2+ Complexes. J. Phys. Chem. A 2008, 112, 10657–10666. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Marques, H.M. The physical chemistry of coordinated aqua-, ammine-, and mixed-ligand Co2+ complexes: DFT studies on the structure, energetics, and topological properties of the electron density. Phys. Chem. Chem. Phys. 2010, 12, 2126–2138. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Cukrowski, I.; Perry, C.B.; Marques, H.M. A Density Functional Theory and Quantum Theory of Atoms-in-Molecules Analysis of the Stability of Ni(II) Complexes of Some Amino Alcohol Ligands. J. Phys. Chem. A 2011, 115, 6629–6640. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. The Phosphorous Bond, or the Phosphorous-Centered Pnictogen Bond: The Covalently Bound Phosphorous Atom in Molecular Entities and Crystals as a Pnictogen Bond Donor. Molecules 2022, 27, 1487. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Pnictogen Bond: The Covalently Bound Arsenic Atom in Molecular Entities in Crystals as a Pnictogen Bond Donor. Molecules 2022, 27, 3421. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Stibium Bond or the Antimony-Centered Pnictogen Bond: The Covalently Bound Antimony Atom in Molecular Entities in Crystal Lattices as a Pnictogen Bond Donor. Int. J. Mol. Sci. 2022, 23, 4674. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. The Capacity of Covalently Bound Bismuth in Crystal Lattices and Nanomaterials to Act as a Pnictogen Bond Donor. arXiv 2022, arXiv:2209.07319v1. [Google Scholar] [CrossRef]
- de Azevedo Santos, L.; Hamlin, T.A.; Ramalho, T.C.; Bickelhaupt, F.M. The pnictogen bond: A quantitative molecular orbital picture. Phys. Chem. Chem. Phys. 2021, 23, 13842–13852. [Google Scholar] [CrossRef] [PubMed]
- Sharutin, V.V.; Sharutina, O.K.; Senchurin, V.S.; Pel’kov, P.A. Osmium complexes [Ph4Sb⋯DMSO]2[OsBr6] and [p-Tol4Sb⋯DMSO][p-Tol4Sb][OsBr6]: Synthesis and structure. Russ. J. Inorg. Chem. 2016, 61, 183–187. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Andreev, P.V. Tetra(para-Tolyl)antimony aroxides (4-MeC6H4)4SbOAr (Ar = C6H3Cl2-2,6, C6H3(NO2)2-2,4, and C6H2(NO2)3-2,4,6): Syntheses and structures. Russ. J. Coord. Chem. 2016, 42, 449–454. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Senchurin, V.S.; Sharutina, O.K.; Pakusina, A.P.; Fastovets, O.A. Synthesis and structure of tetra-p-tolylantimony complexes [(4-MeC6H4)4Sb]2+[Hg2I6]2−, [(4-MeC6H4)4Sb]2+[HgI4]2−, [(4-MeC6H4)4Sb]3+[Sb3I12]2−, and [(4-MeC6H4)4Sb]+[ReO4]−. Russ. J. Inorg. Chem. 2011, 56, 558–570. [Google Scholar] [CrossRef]
- Kumar, V.; Scilabra, P.; Politzer, P.; Terraneo, G.; Daolio, A.; Fernandez-Palacio, F.; Murray, J.S.; Resnati, G. Tetrel and Pnictogen Bonds Complement Hydrogen and Halogen Bonds in Framing the Interactional Landscape of Barbituric Acids. Cryst. Growth Des. 2021, 21, 642–652. [Google Scholar] [CrossRef]
- Frontera, A.; Bauza, A. On the Importance of Pnictogen and Chalcogen Bonding Interactions in Supramolecular Catalysis. Int. J. Mol. Sci. 2021, 22, 12550. [Google Scholar] [CrossRef]
- Weiner, P.K.; Langridge, R.; Blaney, J.M.; Schaefer, R.; Kollman, P.A. Electrostatic potential molecular surfaces. Proc. Natl. Acad. Sci. USA 1982, 79, 3754–3758. [Google Scholar] [CrossRef] [Green Version]
- Gadre, S.R.; Kulkarni, S.A.; Shrivastava, I.H. Molecular electrostatic potentials: A topographical study. J. Chem. Phys. 1992, 96, 5253–5260. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. WIREs Comput. Mol. Sci. 2017, 7, e1326. [Google Scholar] [CrossRef]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. Can Combined Electrostatic and Polarization Effects Alone Explain the F⋯F Negative-Negative Bonding in Simple Fluoro-Substituted Benzene Derivatives? A First-Principles Perspective. Computation 2018, 6, 51. [Google Scholar] [CrossRef]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Is the Fluorine in Molecules Dispersive? Is Molecular Electrostatic Potential a Valid Property to Explore Fluorine-Centered Non-Covalent Interactions? Molecules 2019, 24, 379. [Google Scholar] [CrossRef]
- Varadwaj, P.R. Does Oxygen Feature Chalcogen Bonding? Molecules 2019, 24, 3166. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Yamashita, K. Do surfaces of positive electrostatic potential on different halogen derivatives in molecules attract? like attracting like! J. Comput. Chem. 2018, 39, 343–350. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Description of pnicogen bonding with the help of vibrational spectroscopy —The missing link between theory and experiment. Chem. Phys. Lett. 2014, 614, 136–142. [Google Scholar] [CrossRef]
- CSD 5.43; Cambridge Crystallographic Data Centre (CCDC): Cambridge, UK, 2022.
- Hellenbrandt, M. The Inorganic Crystal Structure Database (ICSD)—Present and Future. Crystallogr. Rev. 2004, 10, 17–22. [Google Scholar] [CrossRef]
- Inorganic Chemistry Structure Database (ICSD). Available online: https://icsd.products.fiz-karlsruhe.de/en (accessed on 25 January 2022).
- Batail, P.; Louer, M.; Grandjean, D.; Dudragne, F.; Michaud, C. Etude structurale de fluoroamines aromatiques. III. Structure cristalline et moleculaire du (N,N-difluoroamino) dinitro-2,4 benzene, C6H3O4N3F2. Acta Cryst. B 1976, 32, 2780–2786. [Google Scholar] [CrossRef]
- Butcher, R.J.; Gilardi, R.; Baum, K.; Trivedi, N.J. The structural chemistry of energetic compounds containing geminal-difluoramino groups. Thermochim. Acta 2002, 384, 219–227. [Google Scholar] [CrossRef]
- Paine, R.T.; Koestle, W.; Borek, T.T.; Wood, G.L.; Pruss, E.A.; Duesler, E.N.; Hiskey, M.A. Synthesis, Characterization, and Explosive Properties of the Nitrogen-Rich Borazine [H3N3B3(N3)3]. Inorg. Chem. 1999, 38, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Krumm, B.; Pflüger, C. Isolation of a Moderately Stable but Sensitive Zwitterionic Diazonium Tetrazolyl-1,2,3-triazolate. J. Org. Chem. 2016, 81, 6123–6127. [Google Scholar] [CrossRef] [PubMed]
- Göbel, M.; Karaghiosoff, K.; Klapötke, T.M. The First Structural Characterization of a Binary P–N Molecule: The Highly Energetic Compound P3N21. Angew. Chem. Int. Ed. 2006, 45, 6037–6040. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Peng, P.; Su, H.; Li, S.; Pang, S. Synthesis and characterization of three pyrazolate inner diazonium salts: Green, powerful and stable primary explosives. New J. Chem. 2017, 41, 9244–9249. [Google Scholar] [CrossRef]
- Kitamura, M.; Sakata, R.; Tashiro, N.; Ikegami, A.; Okauchi, T. Synthesis of Diazonaphthoquinones from Naphthols by Diazo-Transfer Reaction. Bull. Chem. Soc. Jpn. 2015, 88, 824–833. [Google Scholar] [CrossRef]
- Fischer, D.; Klapötke, T.M.; Stierstorfer, J. Salts of Tetrazolone–Synthesis and Properties of Insensitive Energetic Materials. Propellants Explos. Pyrotech. 2012, 37, 156–166. [Google Scholar] [CrossRef]
- Arias Ugarte, R.; Devarajan, D.; Mushinski, R.M.; Hudnall, T.W. Antimony(v) cations for the selective catalytic transformation of aldehydes into symmetric ethers, α,β-unsaturated aldehydes, and 1,3,5-trioxanes. Dalton Trans. 2016, 45, 11150–11161. [Google Scholar] [CrossRef]
- Park, G.; Gabbaï, F.P. Redox-controlled chalcogen and pnictogen bonding: The case of a sulfonium/stibonium dication as a preanionophore for chloride anion transport. Chem. Sci. 2020, 11, 10107–10112. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Gubanova, Y.O.; Eltsov, O.S. Dihydroxybenzoic acids as polydentate ligands in phenylantimony (V) complexes. Inorg. Chim. Acta 2019, 494, 211–215. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Pakusina, A.P.; Egorova, I.V.; Platonova, T.P.; Gerasimenko, A.V.; Gerasimenko, E.A.; Zakharov, L.N.; Fukin, G.K. Synthesis and Structure of Tetraphenylstibonium and Tetraphenylphosphonium Hydrogen Sulfates. Russ. J. Gen. Chem. 2003, 73, 536–540. [Google Scholar] [CrossRef]
- Matano, Y. Synthesis, Structure, and Reactions of Triaryl(methyl)bismuthonium Salts. Organometallics 2000, 19, 2258–2263. [Google Scholar] [CrossRef]
- Matano, Y.; Suzuki, T.; Shinokura, T.; Imahori, H. Mesityltriphenylbismuthonium tetrafluoroborate as an efficient bismuth(V) oxidant: Remarkable steric effects on reaction rates and chemoselectivities in alcohol oxidation. Tetrahedron Lett. 2007, 48, 2885–2888. [Google Scholar] [CrossRef]
- Park, G.; Brock, D.J.; Pellois, J.-P.; Gabbai, F.P. Heavy pnictogenium cations as transmembrane anion transporters in vesicles and erythrocytes. Chem 2019, 5, 2215–2227. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Charpiot, B.; Dau, E.T.H.; Motherwell, W.B.; Pascard, C.; Pichon, C. Structural Studies of Crystalline Pentacalent Organobismuth Compounds. Helv. Chim. Acta 1984, 67, 586–599. [Google Scholar] [CrossRef]
- Suzuki, H.; Ikegami, T.; Azuma, N. Unexpected formation of highly stabilized tetrakis-(2-alkoxyphenyl)bismuthonium salts in the oxidation of tris-(2-alkoxyphenyl)bismuthanes with iodosylbenzene. J. Chem. Soc. Perkin Trans. 1997, 1, 1609–1616. [Google Scholar] [CrossRef]
- Benz, M.; Klapötke, T.M.; Krumm, B.; Lommel, M.; Stierstorfer, J. Nitrocarbamoyl Azide O2NN(H)C(O)N3: A Stable but Highly Energetic Member of the Carbonyl Azide Family. J. Am. Chem. Soc. 2021, 143, 1323–1327. [Google Scholar] [CrossRef]
- Wu, B.; Yang, L.; Zhai, D.; Ma, C.; Pei, C. Facile synthesis of 4-amino-3,5-dinitropyrazolated energetic derivatives via 4-bromopyrazole and their performances. FirePhysChem 2021, 1, 76–82. [Google Scholar] [CrossRef]
- Zeng, X.; Bernhardt, E.; Beckers, H.; Willner, H. Synthesis and Characterization of the Phosphorus Triazides OP(N3)3 and SP(N3)3. Inorg. Chem. 2011, 50, 11235–11241. [Google Scholar] [CrossRef]
- Dubován, L.; Pöllnitz, A.; Silvestru, C. Tri(3-pyridyl)- and Tri(4-pyridyl)-phosphine Chalcogenides and Their Complexes with ZnTPP (TPP = Tetraphenylporphyrinate). Eur. J. Inorg. Chem. 2016, 2016, 1521–1527. [Google Scholar] [CrossRef]
- Kilian, P.; Slawin, A.M.Z.; Woollins, J.D. Naphthalene-1,8-diyl Bis(Halogenophosphanes): Novel Syntheses and Structures of Useful Synthetic Building Blocks. Chem. Eur. J. 2003, 9, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lu, B.; Song, C.; Zhu, B.; Wang, L.; Bernhardt, E.; Zeng, X. Synthesis and characterization of phosphorous(iii) diisocyanate and triisocyanate. Dalton Trans. 2021, 50, 3299–3307. [Google Scholar] [CrossRef] [PubMed]
- McGeachin, H.M.; Tromans, F.R. 942. Phosphonitrilic derivatives. Part VII. The crystal structure of tetrameric phosphonitrilic fluoride. J. Chem. Soc. 1961, 4777–4783. [Google Scholar] [CrossRef]
- Hartsuiker, J.G.; Wagner, A.J. Crystal structure of compounds with (N–P)n rings. Part 12. Decafluorocyclopentaphosphazene. J. Chem. Soc. Dalton Trans. 1978, 1425–1430. [Google Scholar] [CrossRef]
- Schlueter, A.W.; Jacobson, R.A. The crystal structure of pentameric phosphorus nitride dichloride, (PNCl2)5. J. Chem. Soc. A 1968, 2317–2325. [Google Scholar] [CrossRef]
- Ketelaar, J.A.A.; de Vries, T.A. The crystal structure of tetra phosphonitrile chloride, p4N4Cl8. Rec. Trav. Chim. Pays-Bas 1939, 58, 1081–1099. [Google Scholar] [CrossRef]
- Harrison, W.; Trotter, J. Crystal and molecular structure of nitrilohexaphosphonitrilic chloride [2,2,3a,5,5,6a,8,8,9a-nonachloro-2,2,5,5,8,8-hexahydro-1,3,4,6,7,9,9b-hepta-aza-2,3a,5,6a,8,9a-hexaphospha(3a,6a,9a,-PV)phenalene]. J. Chem. Soc. Dalton Trans. 1972, 623–626. [Google Scholar] [CrossRef]
- Bode, H. Kristallstruktur der Triphosphor-nitrilhalogenide. Angew Chem. 1949, 61, 438–439. [Google Scholar]
- Zoer, H.; Wagner, A.J. The crystal structure of compounds with (N-P)n rings. IX. Octabromocyclotetraphosphazene, N4P4Br8. Acta Cryst. B 1972, 28, 252–257. [Google Scholar] [CrossRef]
- de Decker, H.C.J.; Mac Gillavry, C.H. Die Krystallstruktur des Flüchtigen Metastabilen Phosphorpentoxyds. Recl. Trav. Chim. Pays-Bas 1941, 60, 153–175. [Google Scholar] [CrossRef]
- Jansen, M.; Voss, M.; Deiseroth, H.-J. Struktureigenschaften der Phosphoroxide im festen Aggregatzustand. Angew. Chem. 1981, 93, 1023–1024. [Google Scholar] [CrossRef]
- Jost, K.H.; Schneider, M. Structure of phosphorus(III,V) oxide P4O7. Acta Cryst. B 1981, 37, 222–224. [Google Scholar] [CrossRef]
- Beagley, B.; Cruickshank, D.W.J.; Hewitt, T.G.; Jost, K.H. Molecular structures of P4O6 and P4O8. Trans. Faraday Soc. 1969, 65, 1219–1230. [Google Scholar] [CrossRef]
- Luer, B.; Jansen, M. Crystal structure refinement of tetraphosphorous nonaoxide, P4O9. Zeit. Krist. 1991, 197, 247–248. [Google Scholar] [CrossRef]
- Cruickshank, D. Refinements of structures containing bonds between Si, P, S or Cl and O or N. VI. P2O5, form III. Acta Cryst. 1964, 17, 679–680. [Google Scholar] [CrossRef]
- Dimitrov, A.; Ziemer, B.; Hunnius, W.-D.; Meisel, M. The First Ozonide of a Phosphorus Oxide—Preparation, Characterization, and Structure of P4O18. Angew. Chem. Int. Ed. 2003, 42, 2484–2486. [Google Scholar] [CrossRef]
- Dunaj, T.; Dollberg, K.; Ritter, C.; Dankert, F.; von Hänisch, C. 2,6-Diisopropylphenyl-Substituted Bismuth Compounds: Synthesis, Structure, and Reactivity. Eur. J. Inorg. Chem. 2021, 2021, 870–878. [Google Scholar] [CrossRef]
- Betz, R.; Klüfers, P.; Reichvilser, M.M.; Roeßner, F.W. The Structures of Methylenebis(dichloroarsane) and Methylenebisarsonic Acid—A Combined Theoretical and Experimental Study. Z. Anorg. Allg. Chem. 2008, 634, 696–700. [Google Scholar] [CrossRef]
- Allen, D.W.; Coppola, J.C.; Kennard, O.; Mann, F.G.; Motherwell, W.D.S.; Watson, D.G. Preparation, reactions, and structure of 5,10-epoxy-, 5,10-epithio-, 5,10-episeleno-, and 5,10-epitelluro-5,10-dihydroarsanthren. J. Chem. Soc. C 1970, 810–815. [Google Scholar] [CrossRef]
- Kihara, H.; Tanaka, S.; Imoto, H.; Naka, K. Phenyldiquinolinylarsine as a Nitrogen-Arsenic-Nitrogen Pincer Ligand. Eur. J. Inorg. Chem. 2020, 2020, 3662–3665. [Google Scholar] [CrossRef]
- Burford, N.; Parks, T.M.; Bakshi, P.K.; Cameron, T.S. The First Cycloaddition Reactions of Dimeric Arsenium Cations. Angew. Chem. Int. Ed. Engl. 1994, 33, 1267–1268. [Google Scholar] [CrossRef]
- DeGraffenreid, A.J.; Feng, Y.; Wycoff, D.E.; Morrow, R.; Phipps, M.D.; Cutler, C.S.; Ketring, A.R.; Barnes, C.L.; Jurisson, S.S. Dithiol Aryl Arsenic Compounds as Potential Diagnostic and Therapeutic Radiopharmaceuticals. Inorg. Chem. 2016, 55, 8091–8098. [Google Scholar] [CrossRef] [PubMed]
- Breunig, H.J.; Lork, E.; Rösler, R.; Becker, G.; Mundt, O.; Schwarz, W. Common Features in the Crystal Structures of the Compounds Bis(dimethylstibanyl)oxane and -sulfane, and the Minerals Valentinite and Stibnite (Grauspießglanz). Z. Anorg. Allg. Chem. 2000, 626, 1595–1607. [Google Scholar] [CrossRef]
- Althaus, H.; Breunig, H.J.; Lork, E. Crystal structure of [Me3Sb–SbMe2]2[(MeSbBr3)2], a trimethylstibine adduct of the dimethylstibenium ion or a stibinostibonium salt? Chem. Commun. 1999, 1971–1972. [Google Scholar] [CrossRef]
- Davydova, E.I.; Virovets, A.; Peresypkina, E.; Pomogaeva, A.V.; Timoshkin, A.Y. Crystal structures of antimony(III) chloride complexes with pyridine. Polyhedron 2019, 158, 97–101. [Google Scholar] [CrossRef]
- Duffin, R.N.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Comparative stability, cytotoxicity and anti-leishmanial activity of analogous organometallic Sb(V) and Bi(V) acetato complexes: Sb confirms potential while Bi fails the test. J. Inorg. Biochem. 2018, 189, 151–162. [Google Scholar] [CrossRef]
- Machuča, L.; Dostál, L.; Jambor, R.; Handlíř, K.; Jirásko, R.; Růžička, A.; Císařová, I.; Holeček, J. Intramolecularly coordinated organoantimony(III) carboxylates. J. Organomet. Chem. 2007, 692, 3969–3975. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Landers, A.G.; Dahlstrom, P.; Zubieta, J. Novel antimony cluster displaying a quadruply bridging chloride. X-Ray crystal structure of {[Fe(η-C5H5)2]2[Sb4Cl12O]}2·2C6H6. J. Chem. Soc. Chem. Commun. 1979, 143–144. [Google Scholar] [CrossRef]
- Henne, F.D.; Dickschat, A.T.; Hennersdorf, F.; Feldmann, K.O.; Weigand, J.J. Synthesis of Selected Cationic Pnictanes [LnPnX3–n]n+ (L = Imidazolium-2-yl; Pn = P, As; n = 1–3) and Replacement Reactions with Pseudohalogens. Inorg. Chem. 2015, 54, 6849–6861. [Google Scholar] [CrossRef]
- Terzis, A.; Ioannou, P.V. On the Reaction of Dithioarsonites, L-As(SPh)2 (L = Ar, R), with Octasulfur in the Presence of Triethylamine as an Activator. The Crystal Structure of the Sesquisulfide (2-O2N-C6H4-As)2S3. Z. Für Anorg. Und Allg. Chem. 2004, 630, 278–285. [Google Scholar] [CrossRef]
- Willey, G.R.; Aris, D.R.; Errington, W. Crown ether complexation of p-block metal halides: Synthesis and structural characterisation of [InI2(dibenzo-24-crown-8)(H2O)][InI4], [(SnBr4)2(dibenzo-24-crown-8)]·MeCN, [(SbCl3)2(dibenzo-24-crown-8)]·MeCN, [(BiCl3)2(dibenzo-24-crown-8)]·MeCN and [(SbBr3)2(dibenzo-24-crown-8)]. Inorg. Chim. Acta 2000, 300–302, 1004–1013. [Google Scholar] [CrossRef]
- Rogers, R.D.; Bond, A.H.; Aguinaga, S.; Reyes, A. Complexation chemistry of bismuth(III) halides with crown ethers and polyethylene glycols. Structural manifestations of a stereochemically active lone pair. J. Am. Chem. Soc. 1992, 114, 2967–2977. [Google Scholar] [CrossRef]
- Alcock, N.W.; Ravindran, M.; Willey, G.R. Crown ether complexes of Bi. Synthesis and crystal and molecular structures of BiCl3·12-crown-4 and 2BiCl3·18-crown-6. J. Chem. Soc. Chem. Commun. 1989, 1063–1065. [Google Scholar] [CrossRef]
- Alcock, N.W.; Ravindran, M.; Willey, G.R. Preparations and Structural Correlations for the Complexes if MIII Halides (M = As, Sb, Bi) with Crown Ethers: Structures of AsCl3.12-Crown-4, AsCl3.15-Crown-5, SbCl3.12-Crown-4, and BiCl3.15-Crown-5 an an Evaluation of Relative Binding Strengths for Crown Ligands. Acta Crystallogr. B 1993, 49, 507–514. [Google Scholar]
- Wagner, B.; Heine, J. (15-crown-5)BiI3 as a Building Block for Halogen Bonded Supramolecular Aggregates. Z. Anorg. Allg. Chem. 2021, 647, 663–666. [Google Scholar] [CrossRef]
- Hall, M.; Sowerby, D.B. Donor properties of triphenylantimony dihalides: Preparation and crystal structures of Ph3SbCl2·SbCl3 and [Ph3SbCl][SbCl6]. J. Chem. Soc. Dalton Trans. 1983, 1095–1099. [Google Scholar] [CrossRef]
- Bricklebank, N.; Godfrey, S.M.; Lane, H.P.; McAuliffe, C.A.; Pritchard, R.G.; Moreno, J.-M. Synthesis and structural characterisation of R3AsX2 compounds (R = Me, Ph, p-FC6H4 or p-MeOC6H4; X2= Br2, I2 or IBr); dependency of structure on R, X and the solvent of preparation. J. Chem. Soc. Dalton Trans. 1995, 3873–3879. [Google Scholar] [CrossRef]
- Schäfer, R.; Einholz, W.; Keller, W.; Eulenberger, G.; Haubold, W. A Direct Route to Halogenated Arsaborane Clusters: Crystal Structure of 3,4,5,6-Tetrachloro-1,2-diarsa-closo-hexaborane(4). Chem. Ber. 1995, 128, 735–736. [Google Scholar] [CrossRef]
- Pluntze, A.M.; Bukovsky, E.V.; Lacroix, M.R.; Newell, B.S.; Rithner, C.D.; Strauss, S.H. Deca-B-fluorination of diammonioboranes. Structures and NMR characterization of 1,2-, 1,7-, and 1,12-B12H10(NH3)2 and 1,2-, 1,7-, and 1,12-B12F10(NH3)2. J. Fluor. Chem. 2018, 209, 33–42. [Google Scholar] [CrossRef]
- Bruce, M.I.; Zaitseva, N.N.; Skelton, B.W.; White, A.H. Synthesis of carbido and related derivatives from calcium carbide and ruthenium carbonyl clusters. J. Chem. Soc. Dalton Trans. 2002, 3879–3885. [Google Scholar] [CrossRef]
- Salmerón-Valverde, A.; Bernès, S. Crystal growth and characterization of solvated organic charge-transfer complexes built on TTF and 9-dicyanomethylenefluorene derivatives. CrystEngComm 2015, 17, 6227–6235. [Google Scholar] [CrossRef]
- Gonsior, M.; Krossing, I.; Müller, L.; Raabe, I.; Jansen, M.; van Wüllen, L. PX4+, P2X5+, and P5X2+ (X=Br, I) Salts of the Superweak Al(OR)4− Anion [R=C(CF3)3]. Chem. Eur. J. 2002, 8, 4475–4492. [Google Scholar] [CrossRef]
- Li, D.; Schwabedissen, J.; Stammler, H.-G.; Mitzel, N.W.; Willner, H.; Zeng, X. Dichlorophosphanyl isocyanate–spectroscopy, conformation and molecular structure in the gas phase and the solid state. Phys. Chem. Chem. Phys. 2016, 18, 26245–26253. [Google Scholar] [CrossRef] [PubMed]
- Gonsior, M.; Krossing, I. Preparation of stable AsBr4+ and I2As–PI3+ salts. Why didn’t we succeed to prepare AsI4+ and As2X5+? A combined experimental and theoretical study. Dalton Trans. 2005, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.G.B.; Kisenyi, J.M.; Willey, G.R. Studies of dioxamide and dithio-oxamide metal complexes. Part 1. Crystal and molecular structures of SbCl3L1.5(L =NN′-diethyldithio-oxamide) and uncomplexed L. J. Chem. Soc. Dalton Trans. 1982, 1729–1732. [Google Scholar] [CrossRef]
- Breunig, H.J.; Lork, E.; Raţ, C. The Complex BiCl3 · CH3C6H5. Z. Naturforsch. B 2007, 62, 1224–1226. [Google Scholar] [CrossRef]
- Ohshita, J.; Yamaji, K.; Ooyama, Y.; Adachi, Y.; Nakamura, M.; Watase, S. Synthesis, Properties, and Complex Formation of Antimony- and Bismuth-Bridged Bipyridyls. Organometallics 2019, 38, 1516–1523. [Google Scholar] [CrossRef]
- Larsen, S.; Vinzents, P.; Dahl, O. 3′,3″3‴-Phosphinetriyltripropionitrile, C9H12N3P, at 100 K. Acta Cryst. C 1983, 39, 1280–1282. [Google Scholar] [CrossRef]
- Ivlev, S.I.; Conrad, M.; Hoelzel, M.; Karttunen, A.J.; Kraus, F. Crystal Structures of α- and β-Nitrogen Trifluoride. Inorg. Chem. 2019, 58, 6422–6430. [Google Scholar] [CrossRef]
- Bjorvatten, T.; Hassel, O.; Lindheim, A. Crystal Structure of the Addition Compound SbI3:3S8. Acta Chem. Scand. 1963, 17, 689–702. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Senchurin, V.S.; Somov, N.V. Iridium complexes [p-Tol4Sb]+[p-Tol4Sb(DMSO)]+[IrBr6]2– and [p-Tol4Sb(DMSO)]+[IrBr4(DMSO)2]−: Synthesis and structure. Russ. J. Inorg. Chem. 2016, 61, 969–974. [Google Scholar] [CrossRef]
- Dornhaus, F.; Lerner, H.-W.; Bolte, M. Diphenylphosphenium bromide. Acta Crystal. E 2005, 61, o448–o449. [Google Scholar] [CrossRef]
- Ilyin, I.Y.; Konchenko, S.N.; Virovets, A.V.; Kuratieva, N.V.; Pushkarevsky, N.A. Synthesis and structures of the first triiodoarsenate(III) anion, EtAsI3−, and zwitterions, (HPy)2As2I6. Polyhedron 2014, 67, 115–121. [Google Scholar] [CrossRef]
- Schorpp, M.; Tamim, R.; Krossing, I. Oxidative addition, reduction and reductive coupling: The versatile reactivity of subvalent gallium cations. Dalton Trans. 2021, 50, 15103–15110. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.; Raţ, C.I.; Silvestru, A.; Rüffer, T.; Lang, H.; Mehring, M. Heterocyclic bismuth(III) compounds with transannular S→Bi interactions. An experimental and theoretical approach. J. Organomet. Chem. 2016, 806, 5–11. [Google Scholar] [CrossRef]
- Musina, E.I.; Galimova, M.F.; Musin, R.R.; Dobrynin, A.B.; Gubaidullin, A.T.; Litvinov, I.A.; Karasik, A.A.; G. Sinyashin, O. A Series of Cu2I2 Complexes of 10-(Aryl)phenoxarsines: Synthesis and Structural Diversity. ChemistrySelect 2017, 2, 11755–11761. [Google Scholar] [CrossRef]
- Rodina, L.L.; Azarova, X.V.; Medvedev, J.J.; Semenok, D.V.; Nikolaev, V.A. Novel photochemical reactions of carbocyclic diazodiketones without elimination of nitrogen—A suitable way to N-hydrazonation of C–H-bonds. Beilstein J. Org. Chem. 2018, 14, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.L.; Olinger, B.; Cromer, D.T. Structures and phase diagrams of N2 and CO to 13 GPa by x-ray diffraction. J. Chem. Phys. 1986, 84, 2837–2845. [Google Scholar] [CrossRef]
- Gougoutas, J.Z. BOSPUU: Naphthalene-2-diazonium-3-carboxylate Monohydrate. Available online: https://www.ccdc.cam.ac.uk (accessed on 24 August 2022).
- Politzer, P.; Murray, J.S. The use and misuse of van der Waals radii. Struct. Chem. 2021, 32, 623–629. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Sánchez-Sanz, G.; Trujillo, C.; Solimannejad, M.; Alkorta, I.; Elguero, J. Orthogonal interactions between nitryl derivatives and electron donors: Pnictogen bonds. Phys. Chem. Chem. Phys. 2013, 15, 14310–14318. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.R.; Sankaran, K. P⋯N type pnicogen bonding in phosphorus trichloride–pyridine adduct: A matrix isolation infrared, DFT and ab initio study. J. Mol. Str. 2020, 1217, 128408. [Google Scholar] [CrossRef]
- Feller, M.; Lux, K.; Kornath, A. Crystal Structure and Spectroscopic Investigations of POF3. Z. Anorg. Allg. Chem. 2014, 640, 53–56. [Google Scholar] [CrossRef]
- Bader, R.F. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Shukla, R.; Chopra, D. Characterization of N---O non-covalent interactions involving σ-holes: “Electrostatics” or “dispersion”. Phys. Chem. Chem. Phys. 2016, 18, 29946–29954. [Google Scholar] [CrossRef] [PubMed]
- Minkin, V.I. Glossary of terms used in theoretical organic chemistry. Pure Appl. Chem. 1999, 71, 1919–1981. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Matveychuk, Y.V.; Mukhitdinova, S.E.; Sobalev, S.A.; Khrenova, M.G.; Tsirelson, V.G. The common trends for the halogen, chalcogen, and pnictogen bonds via sorting principles and local bonding properties. Theor. Chem. Acc 2020, 139, 26. [Google Scholar] [CrossRef]
- Thomas, S.P.; Dikundwar, A.G.; Sarkar, S.; Pavan, M.S.; Pal, R.; Hathwar, V.R.; Row, T.N.G. The Relevance of Experimental Charge Density Analysis in Unraveling Noncovalent Interactions in Molecular Crystals. Molecules 2022, 27, 3690. [Google Scholar] [CrossRef]
- Sarkar, S.; Pavan, M.S.; Guru Row, T.N. Experimental validation of ‘pnicogen bonding’ in nitrogen by charge density analysis. Phys. Chem. Chem. Phys. 2015, 17, 2330–2334. [Google Scholar] [CrossRef]
- Narth, C.; Maroun, Z.; Boto, R.A.; Chaudret, R.; Bonnet, M.-L.; Piquemal, J.-P.; Contreras-García, J. A complete NCI perspective: From new bonds to reactivity. In Applications of Topological Methods in Molecular Chemistry; Esmail, A., Remi, C., Christine, L., Bernard, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 22, pp. 491–527. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Lefebvre, C.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Piquemal, J.-P.; Hénon, E. The Independent Gradient Model: A New Approach for Probing Strong and Weak Interactions in Molecules from Wave Function Calculations. ChemPhysChem 2018, 19, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Moaven, S.; Andrews, M.C.; Polaske, T.J.; Karl, B.M.; Unruh, D.K.; Bosch, E.; Bowling, N.P.; Cozzolino, A.F. Triple-Pnictogen Bonding as a Tool for Supramolecular Assembly. Inorg. Chem. 2019, 58, 16227–16235. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Kraka, E.; Cremer, D. Strength of the Pnicogen Bond in Complexes Involving Group Va Elements N, P, and As. J. Phys. Chem. A 2015, 119, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E. Systematic Coupled Cluster Study of Noncovalent Interactions Involving Halogens, Chalcogens, and Pnicogens. J. Phys. Chem. A 2017, 121, 9544–9556. [Google Scholar] [CrossRef]
- Mokrai, R.; Barrett, J.; Apperley, D.C.; Benkő, Z.; Heift, D. Tweaking the Charge Transfer: Bonding Analysis of Bismuth(III) Complexes with a Flexidentate Phosphane Ligand. Inorg. Chem. 2020, 59, 8916–8924. [Google Scholar] [CrossRef]
- Brammer, L. Halogen bonding, chalcogen bonding, pnictogen bonding, tetrel bonding: Origins, current status and discussion. Faraday Discuss. 2017, 203, 485–507. [Google Scholar] [CrossRef]
- Inscoe, B.; Rathnayake, H.; Mo, Y. Role of Charge Transfer in Halogen Bonding. J. Phys. Chem. A 2021, 125, 2944–2953. [Google Scholar] [CrossRef]
- Holthoff, J.M.; Weiss, R.; Rosokha, S.V.; Huber, S.M. “Anti-electrostatic” Halogen Bonding between Ions of Like Charge. Chem. Eur. J. 2021, 27, 16530–16542. [Google Scholar] [CrossRef]
- Řezáč, J.; de la Lande, A. On the role of charge transfer in halogen bonding. Phys. Chem. Chem. Phys. 2017, 19, 791–803. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Effects of Charge and Substituent on the S···N Chalcogen Bond. J. Phys. Chem. A 2014, 118, 3183–3192. [Google Scholar] [CrossRef]
- Oliveira, V.; Cremer, D.; Kraka, E. The Many Facets of Chalcogen Bonding: Described by Vibrational Spectroscopy. J. Phys. Chem. A 2017, 121, 6845–6862. [Google Scholar] [CrossRef] [PubMed]
- Aljameedi, K.; Karton, A.; Jayatilaka, D.; Thomas, S.P. Bond orders for intermolecular interactions in crystals: Charge transfer, ionicity and the effect on intramolecular bonds. IUCrJ 2018, 5, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Mokrai, R.; Barrett, J.; Apperley, D.C.; Batsanov, A.S.; Benkő, Z.; Heift, D. Weak Pnictogen Bond with Bismuth: Experimental Evidence Based on Bi−P Through-Space Coupling. Chem. Eur. J. 2019, 25, 4017–4024. [Google Scholar] [CrossRef] [PubMed]
- Benz, S.; Poblador-Bahamonde, A.I.; Low-Ders, N.; Matile, S. Catalysis with Pnictogen, Chalcogen, and Halogen Bonds. Angew. Chem. Int. Ed. 2018, 57, 5408–5412. [Google Scholar] [CrossRef] [PubMed]
- Zahn, S.; Frank, R.; Hey-Hawkins, E.; Kirchner, B. Pnicogen Bonds: A New Molecular Linker? Chem. Eur. J. 2011, 17, 6034–6038. [Google Scholar] [CrossRef]
- Hill, W.E.; Silva-Trivino, L.M. Preparation and Characterization of Di(tertiary phosphines) with Electronegative Substituents. 1, Symmetrical Derivatives. Inorg. Chem. 1978, 17, 2495–2498. [Google Scholar] [CrossRef]
- Hill, W.E.; Silva-Trivino, L.M. Preparation and Characterization of Di(tertiary phosphines) with Electronegative Substituents. 2. Unsymmetrical Derivatives. Inorg. Chem. 1979, 18, 361–364. [Google Scholar] [CrossRef]
- Xu, Y.; Szell, P.M.J.; Kumar, V.; Bryce, D.L. Solid-state NMR spectroscopy for the analysis of element-based non-covalent interactions. Coord. Chem. Rev. 2020, 411, 213237. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Jin, B.-Y. Fluorines in tetrafluoromethane as halogen bond donors: Revisiting address the nature of the fluorine’s σ-hole. Int. J. Quantum Chem. 2015, 115, 453–470. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M. Halogen Bonding: A Halogen-Centered Noncovalent Interaction Yet to Be Understood. Inorganics 2019, 7, 40. [Google Scholar] [CrossRef]
- Scheiner, S. Participation of S and Se in hydrogen and chalcogen bonds. CrystEngComm 2021, 23, 6821–6837. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Hnyk, D. Dihalogen and Pnictogen Bonding in Crystalline Icosahedral Phosphaboranes. Crystals 2018, 8, 390. [Google Scholar] [CrossRef] [Green Version]
- Mahmudov, K.T.; Gurbanov, A.V.; Aliyeva, V.A.; Resnati, G.; Pombeiro, A.J.L. Pnictogen bonding in coordination chemistry. Coord. Chem. Rev. 2020, 418, 213381. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Janjić, G.V.; Zarić, S.D. σ-Hole interactions of covalently-bonded nitrogen, phosphorus and arsenic: A survey of crystal structures. Crystals 2014, 4, 12–31. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Deyà, P.M.; Frontera, A. Halogen bonding versus chalcogen and pnicogen bonding: A combined Cambridge structural database and theoretical study. CrystEngComm 2013, 15, 3137–3144. [Google Scholar] [CrossRef]
- Lee, L.M.; Tsemperouli, M.; Poblador-Bahamonde, A.I.; Benz, S.; Sakai, N.; Sugihara, K.; Matile, S. Anion Transport with Pnictogen Bonds in Direct Comparison with Chalcogen and Halogen Bonds. J. Am. Chem. Soc. 2019, 141, 810–814. [Google Scholar] [CrossRef]
- Humeniuk, H.V.; Gini, A.; Hao, X.; Coelho, F.; Sakai, N.; Matile, S. Pnictogen-Bonding Catalysis and Transport Combined: Polyether Transporters Made In Situ. JACS Au 2021, 1, 1588–1593. [Google Scholar] [CrossRef]
- Gini, A.; Paraja, M.; Galmés, B.; Besnard, C.; Poblador-Bahamonde, A.I.; Sakai, N.; Frontera, A.; Matile, S. Pnictogen-bonding catalysis: Brevetoxin-type polyether cyclizations. Chem. Sci. 2020, 11, 7086–7091. [Google Scholar] [CrossRef]
- Paraja, M.; Gini, A.; Sakai, N.; Matile, S. Pnictogen-Bonding Catalysis: An Interactive Tool to Uncover Unorthodox Mechanisms in Polyether Cascade Cyclizations. Chem. Eur. J. 2020, 26, 15471–15476. [Google Scholar] [CrossRef]
- Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. Halogen in Materials Design: Revealing the Nature of Hydrogen Bonding and Other Non-Covalent Interactions in the Polymorphic Transformations of Methylammonium Lead Tribromide Perovskite. Mater. Chem. Today 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Varadwaj, P.R.; Varadwaj, A.; Marques, H.M.; Yamashita, K. Significance of hydrogen bonding and other noncovalent interactions in determining octahedral tilting in the CH3NH3PbI3 hybrid organic-inorganic halide perovskite solar cell semiconductor. Sci. Rep. 2019, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. σ-holes and π-holes: Similarities and differences. J. Comp. Chem. 2018, 39, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Wysokiński, R.; Scheiner, S. On the ability of pnicogen atoms to engage in both σ and π-hole complexes. Heterodimers of ZF2C6H5 (Z = P, As, Sb, Bi) and NH3. J. Mol. Model. 2019, 25, 152. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zeng, Y.; Meng, L.; Zhang, X. Theoretical insights into the [pi]-hole interactions in the complexes containing triphosphorus hydride (P3H3) and its derivatives. Acta Cryst. B 2017, 73, 195–202. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varadwaj, A.; Varadwaj, P.R.; Marques, H.M.; Yamashita, K. Definition of the Pnictogen Bond: A Perspective. Inorganics 2022, 10, 149. https://doi.org/10.3390/inorganics10100149
Varadwaj A, Varadwaj PR, Marques HM, Yamashita K. Definition of the Pnictogen Bond: A Perspective. Inorganics. 2022; 10(10):149. https://doi.org/10.3390/inorganics10100149
Chicago/Turabian StyleVaradwaj, Arpita, Pradeep R. Varadwaj, Helder M. Marques, and Koichi Yamashita. 2022. "Definition of the Pnictogen Bond: A Perspective" Inorganics 10, no. 10: 149. https://doi.org/10.3390/inorganics10100149