Heteroleptic Zn(II)–Pentaiodobenzoate Complexes: Structures and Features of Halogen–Halogen Non-Covalent Interactions in Solid State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 1
2.2. Synthesis of 2
2.3. X-ray Diffractometry
2.4. Powder X-ray Diffractometry
2.5. Computational Details
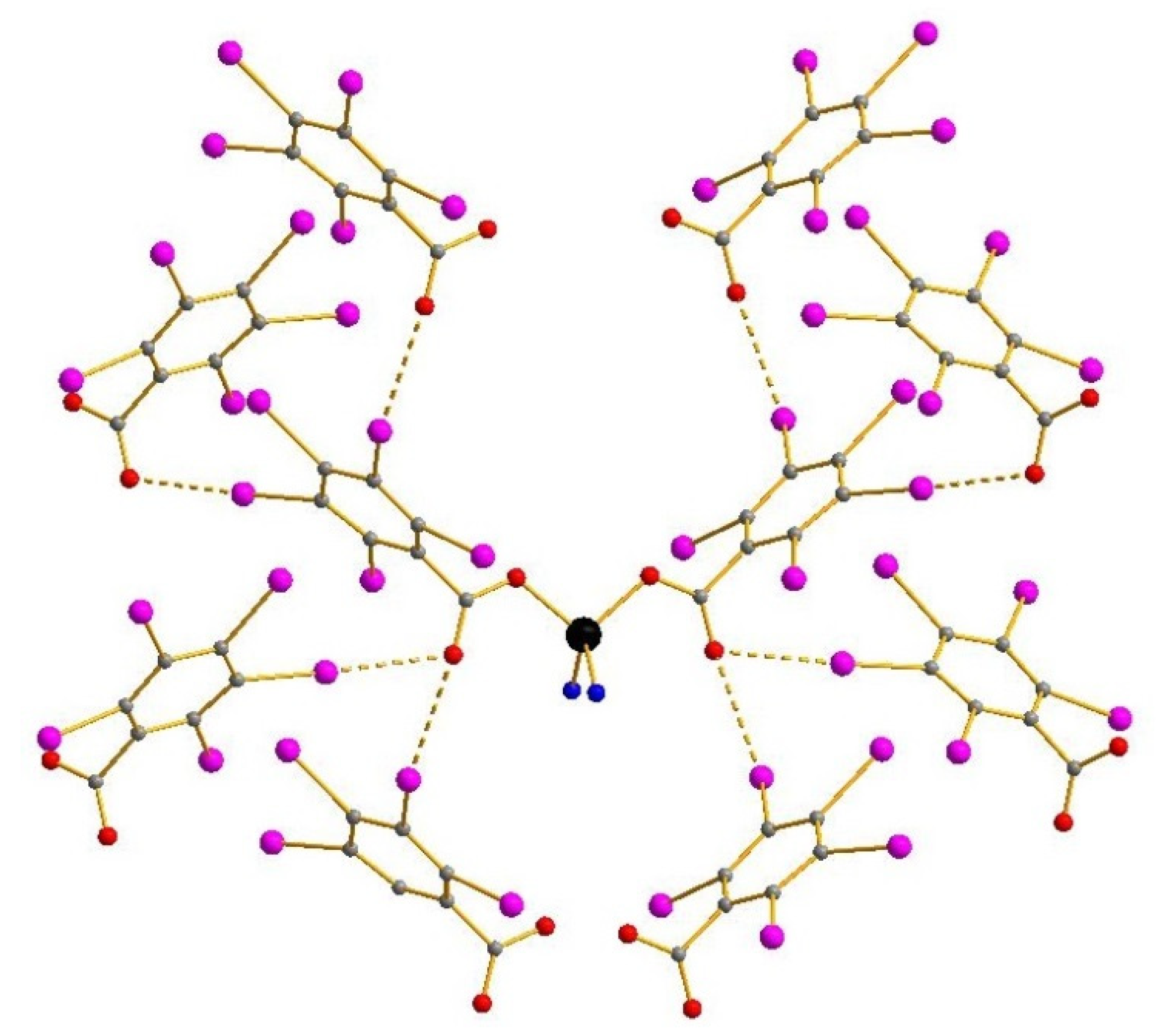
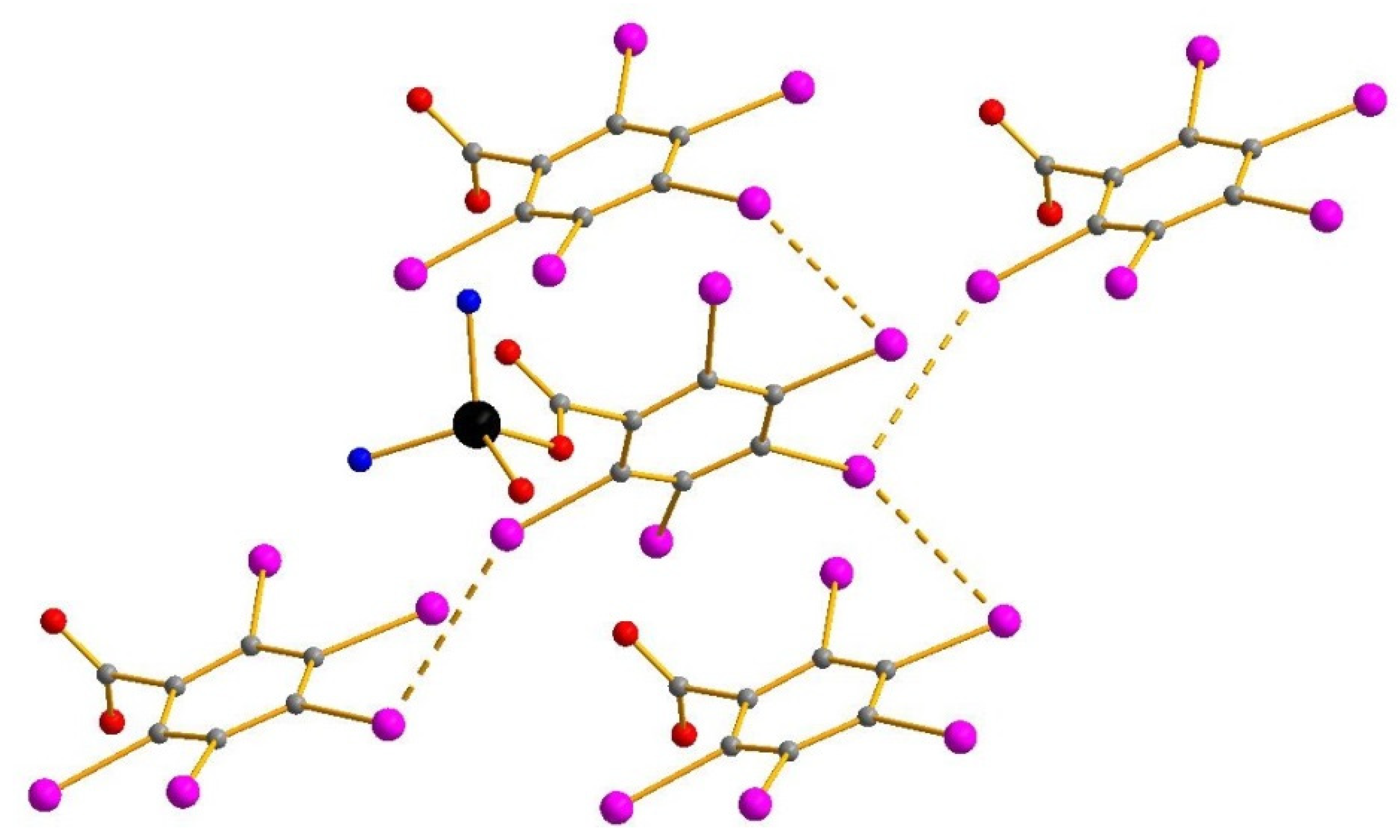
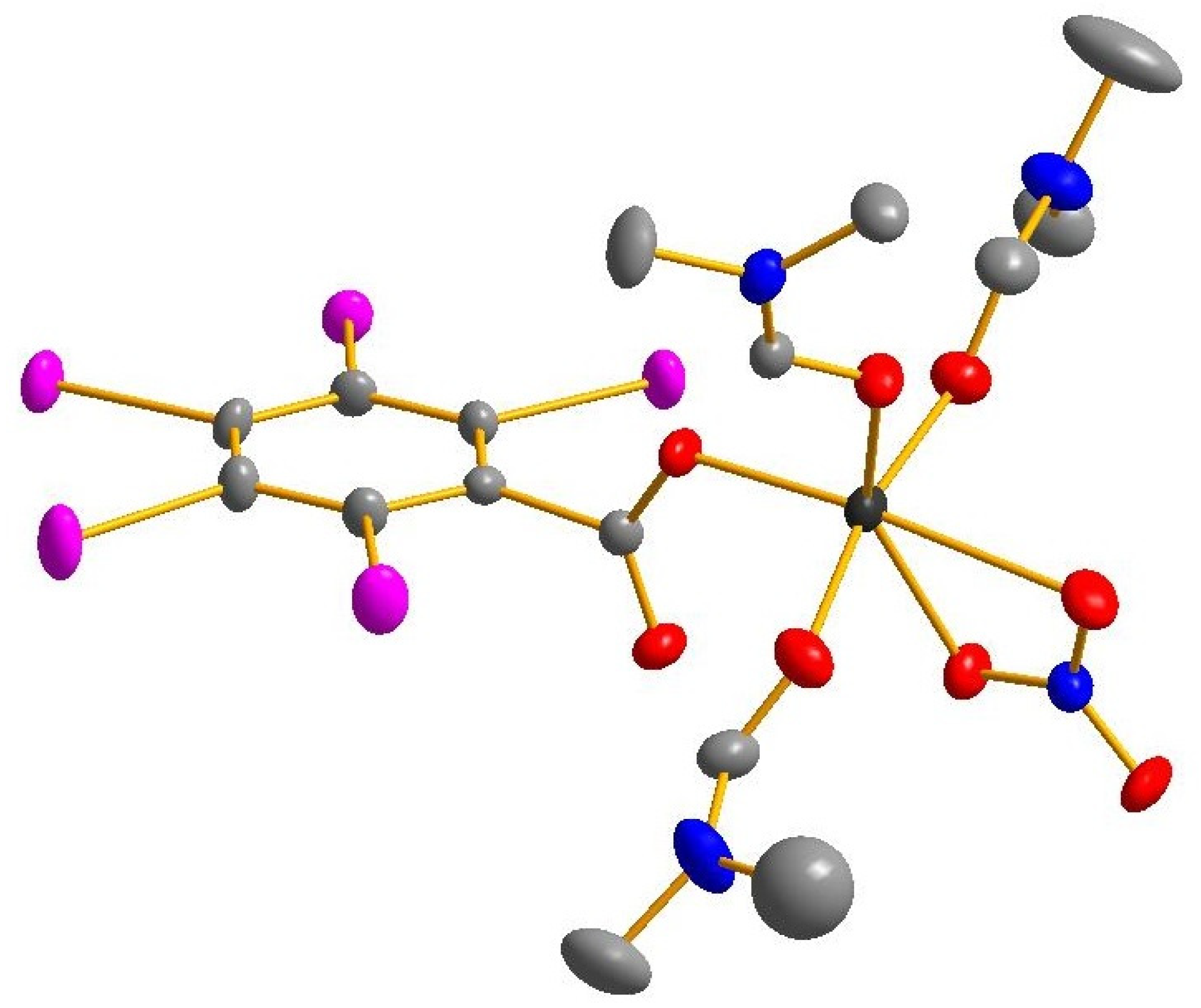
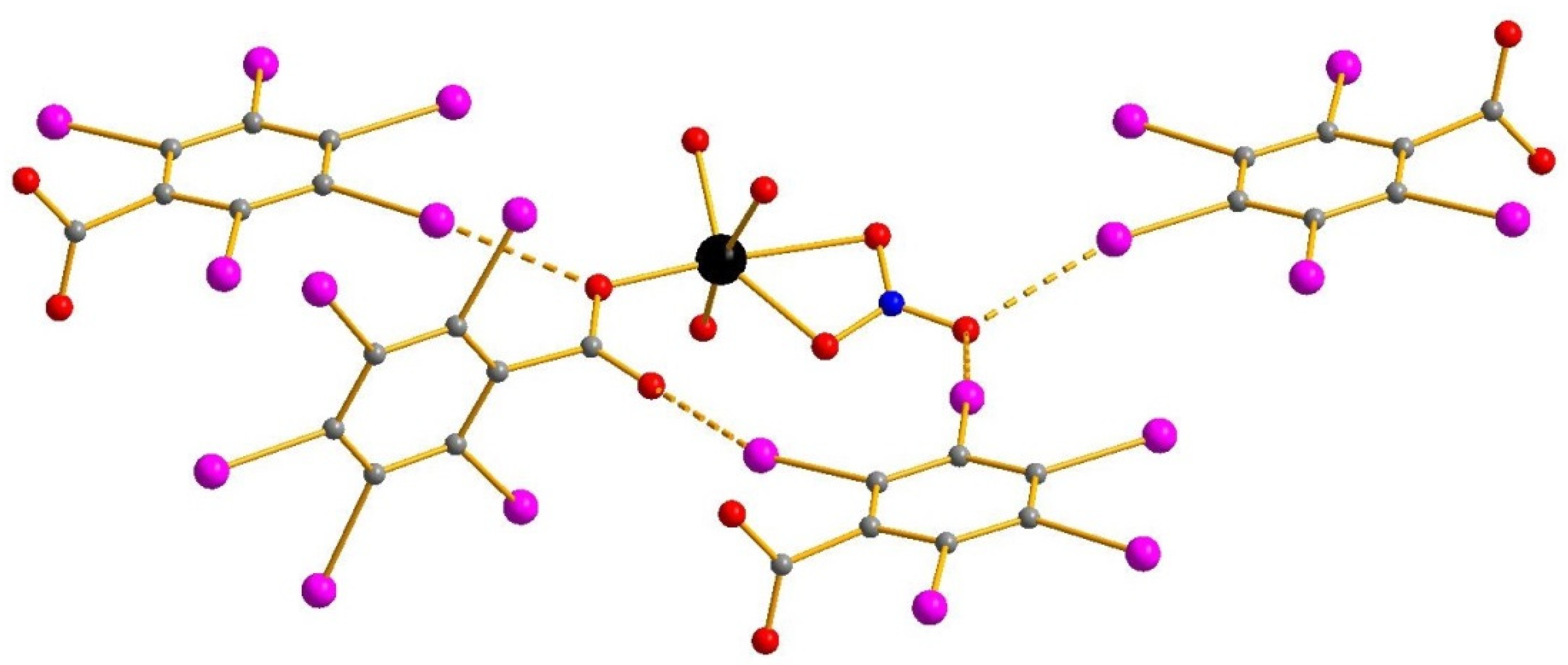
3. Results and Discussion
| Contact | Length | ρ (r) | ∇2ρ (r) | −λ2 | Hb | −V (r) | G (r) | Eint * |
|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||
| I–I | 3.908 | 0.007 | 0.029 | 0.007 | 0.002 | 0.004 | 0.006 | 1.7 |
| I–I | 3.829 | 0.009 | 0.033 | 0.009 | 0.001 | 0.006 | 0.007 | 2.6 |
| I–O | 3.321 | 0.008 | 0.035 | 0.008 | 0.002 | 0.005 | 0.007 | 2.1 |
| I–O | 3.045 | 0.015 | 0.054 | 0.015 | 0.001 | 0.011 | 0.012 | 4.7 |
| 2 | ||||||||
| I–O | 3.181 | 0.011 | 0.047 | 0.011 | 0.002 | 0.008 | 0.010 | 3.4 |
| I–O | 3.129 | 0.012 | 0.047 | 0.012 | 0.001 | 0.009 | 0.010 | 3.8 |
| I–O | 3.079 | 0.014 | 0.053 | 0.014 | 0.002 | 0.010 | 0.012 | 4.3 |
| I–O | 2.997 | 0.017 | 0.060 | 0.017 | 0.002 | 0.012 | 0.014 | 5.1 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yashkova, K.A.; Mel’nikov, S.N.; Nikolaevskii, S.A.; Shmelev, M.A.; Sidorov, A.A.; Kiskin, M.A.; Eremenko, I.L. Synthesis and structure of an nontrivial coordination polymer {[Na2Co(pfb)i2 (H2O)8](pfb)2}n with pentafluorobenzoate anions. J. Struct. Chem. 2021, 62, 1378–1384. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kuznetsova, G.N.; Dolgushin, F.M.; Voronina, Y.K.; Gogoleva, N.V.; Kiskin, M.A.; Ivanov, V.K.; Sidorov, A.A.; Eremenko, I.L. Influence of the Fluorinated Aromatic Fragments on the Structures of the Cadmium and Zinc Carboxylate Complexes Using Pentafluorobenzoates and 2,3,4,5-Tetrafluorobenzoates as Examples. Russ. J. Coord. Chem. 2021, 47, 127–143. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Kuznetsova, G.N.; Kiskin, M.A.; Voronina, Y.K.; Yakushev, I.A.; Ivanova, T.M.; Nelyubina, Y.V.; Sidorov, A.A.; Eremenko, I.L. Cd(II) and Cd(II)–Eu(III) Complexes with Pentafluorobenzoic Acid Anions and N-Donor Ligands: Synthesis and Structures. Russ. J. Coord. Chem. 2020, 46, 557–572. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kiskin, M.A.; Voronina, J.K.; Babeshkin, K.A.; Efimov, N.N.; Varaksina, E.A.; Korshunov, V.M.; Taydakov, I.V.; Gogoleva, N.V.; Sidorov, A.A.; et al. Molecular and polymer ln2m2 (Ln = eu, gd, tb, dy; m = zn, cd) complexes with pentafluorobenzoate anions: The role of temperature and stacking effects in the structure; magnetic and luminescent properties. Materials 2020, 13, 5689. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Grishko, A.; Vashchenko, A.; Goloveshkin, A.; Averin, A.; Kuzmina, N. Lanthanide Tetrafluoroterephthalates for Luminescent Ink-Jet Printing. Eur. J. Inorg. Chem. 2017, 2017, 5635–5639. [Google Scholar] [CrossRef]
- Kalyakina, A.S.; Utochnikova, V.V.; Bushmarinov, I.S.; Le-Deygen, I.M.; Volz, D.; Weis, P.; Schepers, U.; Kuzmina, N.P.; Bräse, S. Lanthanide Fluorobenzoates as Bio-Probes: A Quest for the Optimal Ligand Fluorination Degree. Chem. A Eur. J. 2017, 23, 14944–14953. [Google Scholar] [CrossRef]
- Mironova, A.D.; Mikhaylov, M.A.; Maksimov, A.M.; Brylev, K.A.; Gushchin, A.L.; Stass, D.V.; Novikov, A.S.; Eltsov, I.V.; Abramov, P.A.; Sokolov, M.N. Phosphorescent Complexes of {Mo6I8}4+ and {W6I8}4+ with Perfluorinated Aryl Thiolates featuring Unusual Molecular Structures. Eur. J. Inorg. Chem. 2022, 2022, e202100890. [Google Scholar] [CrossRef]
- Rogozhin, A.F.; Silantyeva, L.I.; Yablonskiy, A.N.; Andreev, B.A.; Grishin, I.D.; Ilichev, V.A. Near infrared luminescence of Nd, Er and Yb complexes with perfluorinated 2-mercaptobenzothiazolate and phosphine oxide ligands. Opt. Mater. 2021, 118, 111241. [Google Scholar] [CrossRef]
- Silantyeva, L.I.; Ilichev, V.A.; Shavyrin, A.S.; Yablonskiy, A.N.; Rumyantcev, R.V.; Fukin, G.K.; Bochkarev, M.N. Unexpected findings in a simple metathesis reaction of europium and ytterbium diiodides with perfluorinated mercaptobenzothiazolates of alkali metals. Organometallics 2020, 39, 2972–2983. [Google Scholar] [CrossRef]
- Bhat, S.A.; Iftikhar, K. Synthesis and NIR photoluminescence studies of novel Yb(III) complexes of asymmetric perfluoryl β-diketone. J. Lumin. 2019, 208, 334–341. [Google Scholar] [CrossRef]
- Balashova, T.V.; Burin, M.E.; Ilichev, V.A.; Starikova, A.A.; Marugin, A.V.; Rumyantcev, R.V.; Fukin, G.K.; Yablonskiy, A.N.; Andreev, B.A.; Bochkarev, M.N. Features of the molecular structure and luminescence of rare-earth metal complexes with perfluorinated (Benzothiazolyl)phenolate Ligands. Molecules 2019, 24, 2376. [Google Scholar] [CrossRef]
- Abramov, P.A.; Brylev, K.A.; Vorob’ev, A.Y.; Gatilov, Y.V.; Borodkin, G.I.; Kitamura, N.; Sokolov, M.N. Emission tuning in Re(I) complexes: Expanding heterocyclic ligands and/or introduction of perfluorinated ligands. Polyhedron 2017, 137, 231–237. [Google Scholar] [CrossRef]
- Kalyakina, A.S.; Utochnikova, V.V.; Bushmarinov, I.S.; Ananyev, I.V.; Eremenko, I.L.; Volz, D.; Rönicke, F.; Schepers, U.; Van Deun, R.; Trigub, A.L.; et al. Highly Luminescent, Water-Soluble Lanthanide Fluorobenzoates: Syntheses, Structures and Photophysics, Part I: Lanthanide Pentafluorobenzoates. Chem. A Eur. J. 2015, 21, 17921–17932. [Google Scholar] [CrossRef]
- Claus, A.; Bücher, A.W. Zur Kenntniss der Chlorbenzoësäuren. Ber. Dtsch. Chem. Ges. 1887, 20, 1621–1627. [Google Scholar] [CrossRef]
- Ballester, M.; Castañer, J.; Riera, J.; Tabernero, I. Synthesis and Chemical Behavior of Perchlorophenylacetylene. J. Org. Chem. 1986, 51, 1413–1419. [Google Scholar] [CrossRef]
- Ozaki, K.; Okuno, T. Crystal polymorphs and ab initio calculation of 2,3,4,5,6-pentachlorobenzoic acid. J. Mol. Struct. 2018, 1173, 959–963. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K. Synthesis and structure of triphenylbismuth bis(pentachlorobenzoate). Russ. J. Inorg. Chem. 2014, 59, 558–560. [Google Scholar] [CrossRef]
- Mattern, D.L. Direct aromatic periodination. J. Org. Chem. 1984, 49, 3051–3053. [Google Scholar] [CrossRef]
- deKrafft, K.E.; Xie, Z.; Cao, G.; Tran, S.; Ma, L.; Zhou, O.; Lin, W. Iodinated Nanoscale Coordination Polymers as Potential Contrast Agents for Computed Tomography. Angew. Chem. Int. Ed. 2009, 48, 9901–9904. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen bonding in the structures of pentaiodobenzoic acid and its salts. CrystEngComm 2019, 21, 6666–6670. [Google Scholar] [CrossRef]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Bondarenko, M.A.; Abramov, P.A.; Novikov, A.S.; Sokolov, M.N.; Adonin, S.A. Cu(II) pentaiodobenzoate complexes: “super heavy carboxylates” featuring strong halogen bonding. Polyhedron 2022, 214, 115644. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef]
- Takezawa, H.; Murase, T.; Resnati, G.; Metrangolo, P.; Fujita, M. Halogen-Bond-Assisted Guest Inclusion in a Synthetic Cavity. Angew. Chem. Int. Ed. 2015, 54, 8411–8414. [Google Scholar] [CrossRef]
- Decato, D.A.; Riel, A.M.S.; Berryman, O.B. Anion influence on the packing of 1,3-bis(4-ethynyl-3-iodopyridinium)-benzene halogen bond receptors. Crystals 2019, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fusco, N.; La Rocca, M.V.; Linati, L.; Lo Presti, E.; Mella, M.; Metrangolo, P.; Miljkovic, A. Novel hydrogen- and halogen-bonding anion receptors based on 3-iodopyridinium units. RSC Adv. 2016, 6, 67540–67549. [Google Scholar] [CrossRef]
- Chandran, S.K.; Thakuria, R.; Nangia, A. Silver(I) complexes of N-4-halophenyl-N′-4-pyridyl ureas. Isostructurality, urea⋯nitrate hydrogen bonding, and Ag⋯halogen interaction. CrystEngComm 2008, 10, 1891–1898. [Google Scholar] [CrossRef]
- Zisti, F.; Tehrani, A.A.; Alizadeh, R.; Abbasi, H.; Morsali, A.; Eichhorn, S.H. Synthesis and structural characterization of three nano-structured Ag(I) coordination polymers; Syntheses, characterization and X-ray crystal structural analysis. J. Solid State Chem. 2019, 271, 29–39. [Google Scholar] [CrossRef]
- Powell, J.; Horvath, M.J.; Lough, A. Silver-iodocarbon complexes: Crystal structures of eight compounds obtained from the reactions of AgPF6 or AgNO3 with CH2I2, I(CH2)3I and simple aryl iodides. J. Chem. Soc. Dalt. Trans. 1996, 1669–1677. [Google Scholar] [CrossRef]
- Melekhova, A.A.; Novikov, A.S.; Dubovtsev, A.Y.; Zolotarev, A.A.; Bokach, N.A. Tris(3,5-dimethylpyrazolyl)methane copper(I) complexes featuring one disubstituted cyanamide ligand. Inorg. Chim. Acta 2019, 484, 69–74. [Google Scholar] [CrossRef]
- Bulatova, M.; Melekhova, A.A.; Novikov, A.S.; Ivanov, D.M.; Bokach, N.A. Redox reactive (RNC)CuII species stabilized in the solid state via halogen bond with I2. Z. Krist. Cryst. Mater. 2018, 233, 371–377. [Google Scholar] [CrossRef]
- Bokach, N.A.; Suslonov, V.V.; Eliseeva, A.A.; Novikov, A.S.; Ivanov, D.M.; Dubovtsev, A.Y.; Kukushkin, V.Y.; Kukushkin, V.Y. Tetrachloroplatinate(ii) anion as a square-planar tecton for crystal engineering involving halogen bonding. CrystEngComm 2020, 22, 4180–4189. [Google Scholar]
- Eliseeva, A.A.; Ivanov, D.M.; Novikov, A.S.; Kukushkin, V.Y. Recognition of the π-hole donor ability of iodopentafluorobenzene-a conventional σ-hole donor for crystal engineering involving halogen bonding. CrystEngComm 2019, 21, 616–628. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Ananyev, I.V.; Romanova, A.A.; Boyarskiy, V.P.; Haukka, M.; Kukushkin, V.Y. H2 C(X)–X⋯X− (X = Cl, Br) Halogen Bonding of Dihalomethanes. Cryst. Growth Des. 2017, 17, 1353–1362. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Bartashevich, E.V.; Tsirelson, V.G. Interplay between non-covalent interactions in complexes and crystals with halogen bonds. Russ. Chem. Rev. 2014, 83, 1181–1203. [Google Scholar] [CrossRef]
- Goldberg, A.E.; Kiskin, M.A.; Nikolaevskii, S.A.; Zorina-Tikhonova, E.N.; Aleksandrov, G.G.; Sidorov, A.A.; Eremenko, I.L. Structural influence of the substituent in carboxylate anion on example of α- And β-naphthoate complexes of Co(II), Ni(II), Cu(II), and Zn(II). Russ. J. Coord. Chem. 2015, 41, 182–188. [Google Scholar] [CrossRef]
- Nikolaevskii, S.A.; Kiskin, M.A.; Starikova, A.A.; Efimov, N.N.; Sidorov, A.A.; Novotortsev, V.M.; Eremenko, I.L. Binuclear nickel(II) complexes with 3,5-di-tert-butylbenzoate and 3,5-di-tert-butyl-4-hydroxybenzoate anions and 2,3-lutidine: The synthesis, structure, and magnetic properties. Russ. Chem. Bull. 2016, 65, 2812–2819. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Lutsenko, I.A.; Kiskin, M.A.; Shmelev, M.A.; Bekker, O.B.; Efimov, N.N.; Ugolkova, E.A.; Minin, V.V.; Sidorov, A.A.; et al. Copper(II) Trimethylacetate Complex with Caffeine: Synthesis, Structure, and Biological Activity. Russ. J. Coord. Chem. 2020, 46, 772–778. [Google Scholar] [CrossRef]
- Yin, W.-D.; Li, G.-L.; Liu, M.-N.; Du, G.-J.; Shao, Y.; Liu, G.-Z. Syntheses, Structures, and Magnetic Properties of Two Cu(II) Coordination Polymers Based on 4-Nitrophthalic Acid. Russ. J. Inorg. Chem. 2021, 66, 2077–2083. [Google Scholar] [CrossRef]
- Nikolaevskii, S.A.; Yambulatov, D.S.; Starikova, A.A.; Sidorov, A.A.; Kiskin, M.A.; Eremenko, I.L. Molecular Structure and Photoluminescence Behavior of the Zn(II) Carboxylate Complex with Pyrazino [2,3-f][1,10]phenanthroline. Russ. J. Coord. Chem. 2020, 46, 260–267. [Google Scholar] [CrossRef]
- Dorofeeva, V.N.; Pavlishchuk, A.V.; Kiskin, M.A.; Efimov, N.N.; Minin, V.V.; Gavrilenko, K.S.; Kolotilov, S.V.; Pavlishchuk, V.V.; Eremenko, I.L. Generation of Long-Lived Phenoxyl Radical in the Binuclear Copper(II) Pivalate Complex with 2,6-Di-tert-butyl-4-(3,5-bis(4-pyridyl)pyridyl)phenol. Russ. J. Coord. Chem. 2022, 48, 422–429. [Google Scholar] [CrossRef]

| (1) | (2) | |
|---|---|---|
| Chemical formula | C28H18I10N2O4Zn | C16H21I5N4O8Zn |
| Mr | 1780.81 | 1097.24 |
| Crystal system, space group | Orthorhombic, Fdd2 | Monoclinic, P21/n |
| α, β, γ (°) | 90, 90, 90 | 90, 94.011 (1), 90 |
| V (Å3) | 8048.2 (3) | 2922.66 (10) |
| Z | 8 | 4 |
| µ (mm−1) | 8.32 | 6.17 |
| Tmin, Tmax | 0.642, 0.746 | 0.616, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 49,070, 6155, 6088 | 33,369, 5530, 5174 |
| Rint | 0.029 | 0.030 |
| θ values (°) | θmax = 30.5, θmin = 2.4 | θmax = 25.7, θmin = 2.3 |
| (sin θ/λ)max (Å−1) | 0.715 | 0.610 |
| Range of h, k, l | h = −72→72, k = −14→14, l = −21→21 | h = −13→13, k = −11→11, l = −32→32 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.014, 0.036, 0.99 | 0.025, 0.058, 1.07 |
| No. of reflections, parameters and restraints | 6155, 204, 1 | 5530, 307, 0 |
| Δρmax, Δρmin (e Å−3) | 0.74, −0.84 | 1.97, −1.20 |
| Absolute structure | Flack x determined using 2891 quotients [(I+)−(I-)]/[(I+)+(I-)] | |
| Absolute structure parameter | 0.000 (7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondarenko, M.A.; Novikov, A.S.; Sokolov, M.N.; Adonin, S.A. Heteroleptic Zn(II)–Pentaiodobenzoate Complexes: Structures and Features of Halogen–Halogen Non-Covalent Interactions in Solid State. Inorganics 2022, 10, 151. https://doi.org/10.3390/inorganics10100151
Bondarenko MA, Novikov AS, Sokolov MN, Adonin SA. Heteroleptic Zn(II)–Pentaiodobenzoate Complexes: Structures and Features of Halogen–Halogen Non-Covalent Interactions in Solid State. Inorganics. 2022; 10(10):151. https://doi.org/10.3390/inorganics10100151
Chicago/Turabian StyleBondarenko, Mikhail A., Alexander S. Novikov, Maxim N. Sokolov, and Sergey A. Adonin. 2022. "Heteroleptic Zn(II)–Pentaiodobenzoate Complexes: Structures and Features of Halogen–Halogen Non-Covalent Interactions in Solid State" Inorganics 10, no. 10: 151. https://doi.org/10.3390/inorganics10100151






