New Aspects of Ruthenium-Mediated Polyhedral Contraction of Monocarbollides
Abstract
:1. Introduction
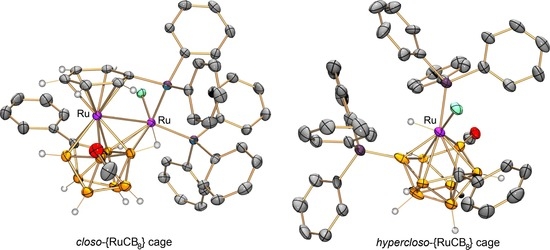
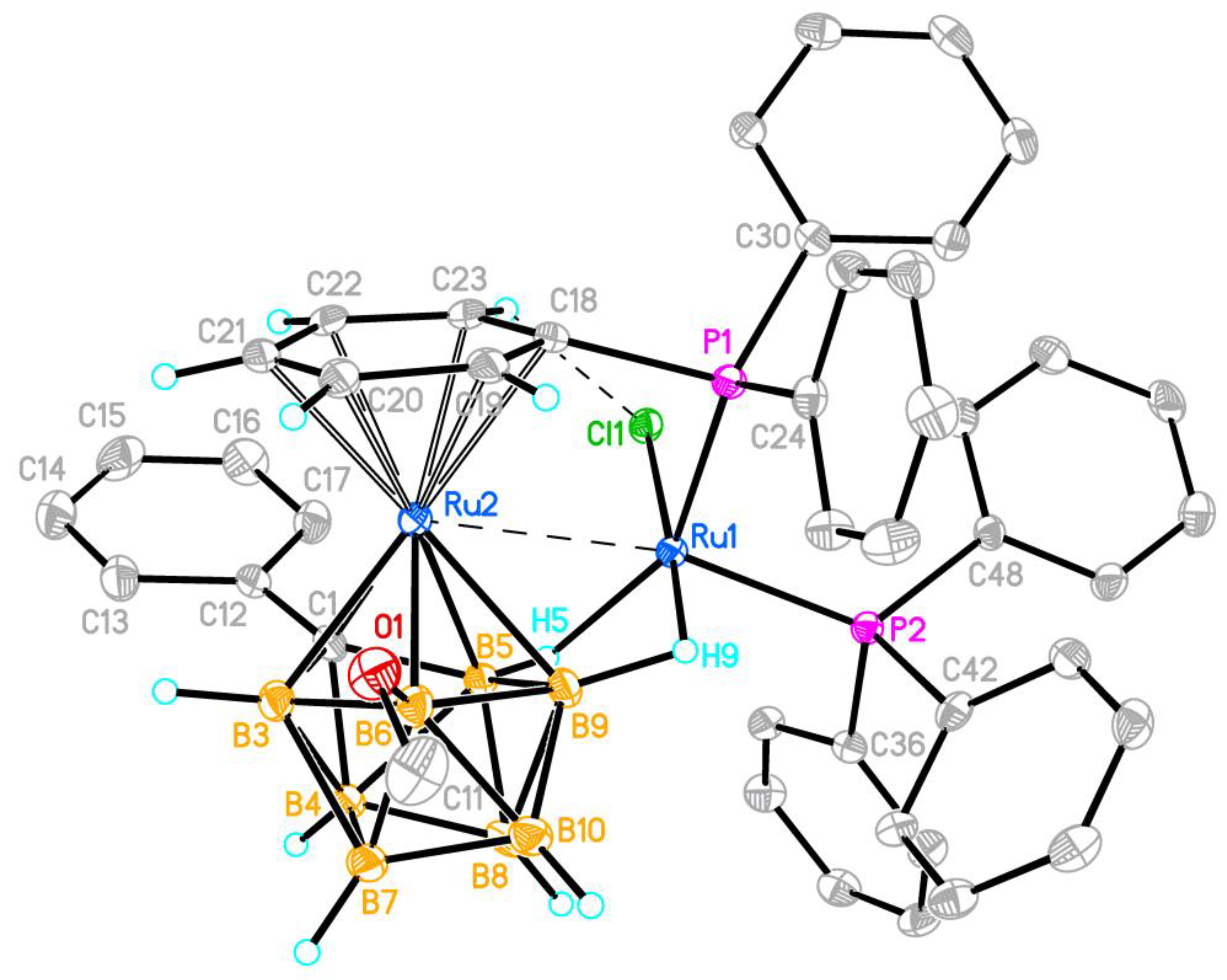
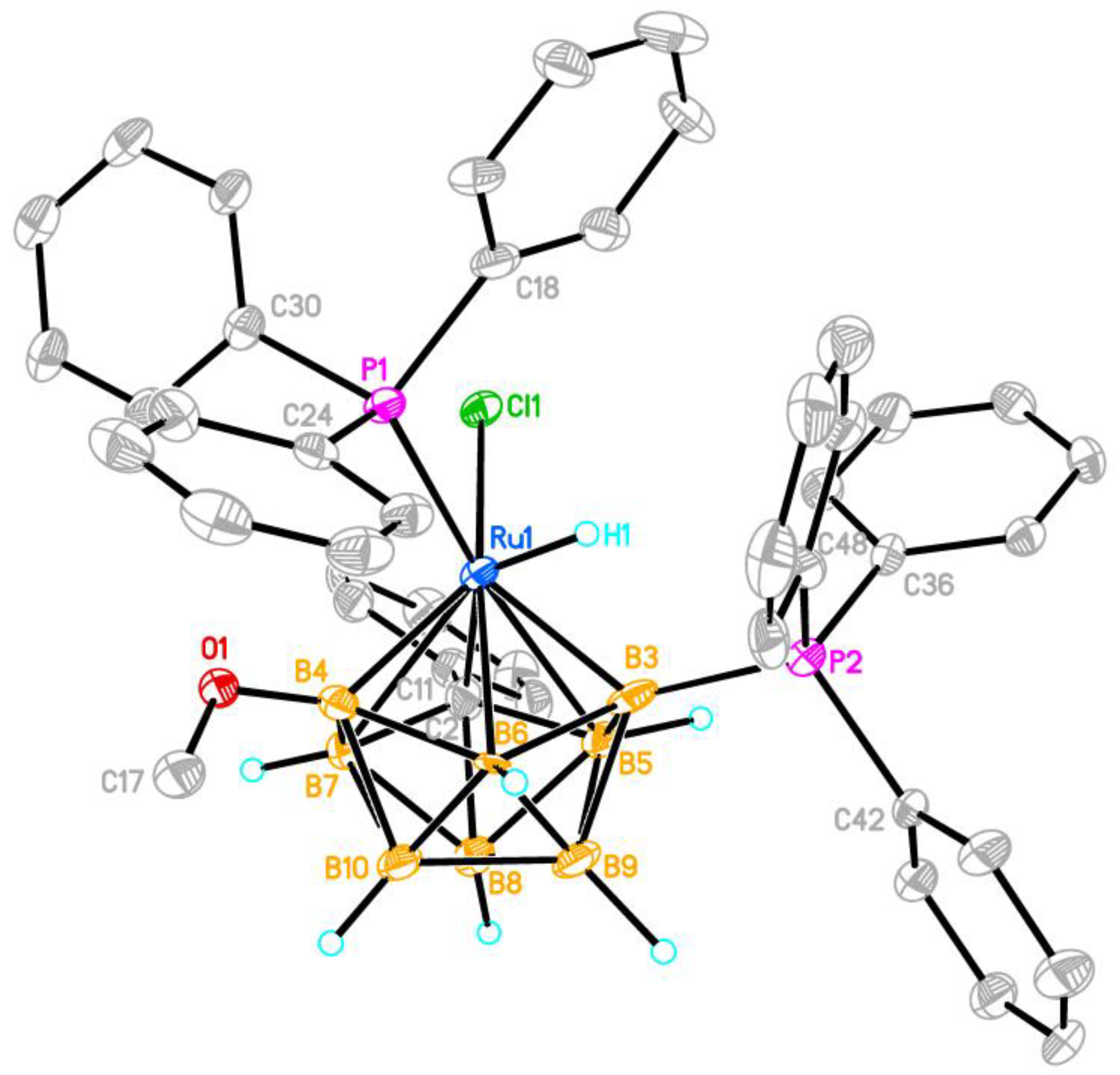
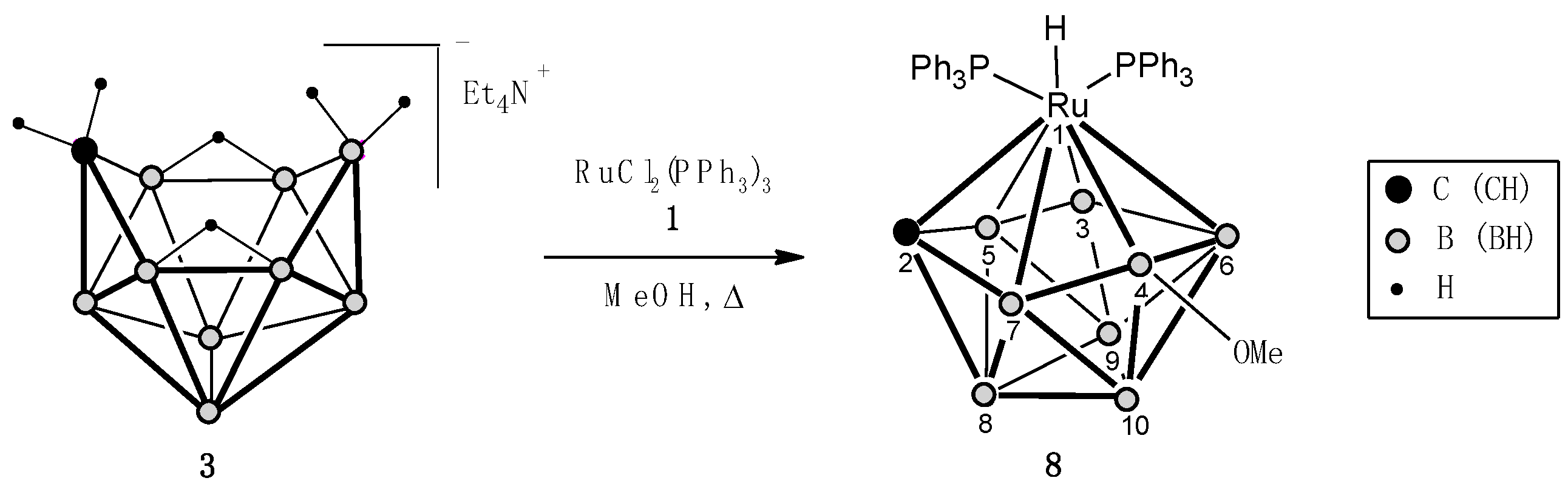
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Reaction of RuCl2(PPh3)3 (1) with Tetraethylammonium 6-Phenyl-Nido-6-Carbadecaborate (2)
3.3. Reaction of RuCl2(PPh3)3 (1) with Tetraethylammonium Arachno-6-Carbadecaborate (3)
3.4. X-ray Diffraction Study of 4a and 5a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosmane, N.S. (Ed.) Boron Science: New Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Kar, S.; Pradhan, A.N.; Ghosh, S. Comprehensive Organometallic Chemistry IV; Parkin, G., O’Hare, D., Meyer, K., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2022; Volume 9, pp. 263–369. [Google Scholar]
- Loginov, D.A.; Konoplev, V.E. Oxidative coupling of benzoic acids with alkynes: Catalyst design and selectivity. J. Organomet. Chem. 2018, 867, 14–24. [Google Scholar] [CrossRef]
- Vinogradov, M.M.; Loginov, D.A. Rhoda- and iridacarborane halide complexes: Synthesis, structure and application in homogeneous catalysis. J. Organomet. Chem. 2020, 910, 121135. [Google Scholar] [CrossRef]
- Molotkov, A.P.; Vinogradov, M.M.; Moskovets, A.P.; Chusova, O.; Timofeev, S.V.; Fastovskiy, V.A.; Nelyubina, Y.V.; Pavlov, A.A.; Chusov, D.A.; Loginov, D.A. Iridium halide complexes [1,1-X2-8-SMe2-1,2,8-IrC2B9H10]2 (X = Cl, Br, I): Synthesis, reactivity and catalytic activity. Eur. J. Inorg. Chem. 2017, 2017, 4635–4644. [Google Scholar] [CrossRef]
- Grishin, I.D.; Knyazeva, N.A.; Penkal, A.M. Novel ruthenium(II) and (III) carborane complexes with diphosphine ligands and their application in radical polymerization. Russ. Chem. Bull. 2020, 69, 1520–1529. [Google Scholar] [CrossRef]
- Kaltenberg, A.A.; Somov, N.V.; Malysheva, Y.B.; Knyazeva, N.A.; Piskunov, A.V.; Grishin, I.D. Novel carborane complexes of ruthenium with tridentate phosphine ligands: Synthesis and application in atom transfer radical polymerization. J. Organomet. Chem. 2020, 917, 121291. [Google Scholar] [CrossRef]
- Grishin, D.F.; Grishin, I.D. Modern trends in controlled synthesis of functional polymers: Fundamental aspects and practical applications. Russ. Chem. Rev. 2021, 90, 231–264. [Google Scholar] [CrossRef]
- Grishin, I.D.; Zimina, A.M.; Anufriev, S.A.; Knyazeva, N.A.; Piskunov, A.V.; Dolgushin, F.M.; Sivaev, I.B. Synthesis and Catalytic Properties of Novel Ruthenacarboranes Based on nido-[5-Me-7,8-C2B9H10]2− and nido-[5,6-Me2-7,8-C2B9H9]2− Dicarbollide Ligands. Catalysts 2021, 11, 1409. [Google Scholar] [CrossRef]
- Loginov, D.A.; Belova, A.O.; Vologzhanina, A.V.; Kudinov, A.R. Cationic iridacarboranes [3-(arene)-3,1,2-IrC2B9H11]+ and [3-(MeCN)3-3,1,2-IrC2B9H11]+: Synthesis, reactivity, and bonding. Catalysis of oxidative coupling of benzoic acid with alkynes. J. Organomet. Chem. 2015, 793, 232–240. [Google Scholar] [CrossRef]
- Loginov, D.A.; Muratov, D.V.; Nelyubina, Y.V.; Laskova, J.; Kudinov, A.R. µ-Borole triple-decker complexes as catalysts for oxidative couplingof benzoic acid with alkynes. Structure of a hybridrhodacyclopentadienyl/borole triple-decker complex. J. Mol. Catal. A Chem. 2017, 426, 393–397. [Google Scholar] [CrossRef]
- Molotkov, A.P.; Timofeev, S.V.; Loginov, D.A. Trimethylammonium-containing rhodacarborane [(9-NMe3-7,8-C2B9H10)RhCl2]2 as a catalyst for the annulation of arylcarboxylic acids with alkynes. Russ. Chem. Bull. 2021, 70, 1922–1926. [Google Scholar] [CrossRef]
- Kharitonov, V.B.; Muratov, D.V.; Nelyubina, Y.V.; Loginov, D.A. Formation of a Naphthalene Framework by Rhodium(III)-Catalyzed Double C–H Functionalization of Arenes with Alkynes: Impact of a Supporting Ligand and an Acid Additive. Synthesis 2022. [Google Scholar] [CrossRef]
- Wang, L.; Perveen, S.; Ouyang, Y.; Zhang, S.; Jiao, J.; He, G.; Nie, Y.; Li, P. Well-Defined, Versatile and Recyclable Half-Sandwich Nickelacarborane Catalyst for Selective Carbene-Transfer Reactions. Chem. Eur. J. 2021, 27, 5754–5760. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, L.S.; Lyubimov, S.E.; Dolgushin, F.M.; Novikov, V.V.; Davankov, V.A.; Chizhevsky, I.T. New Rhodacarborane−Phosphoramidite Catalyst System for Enantioselective Hydrogenation of Functionalized Olefins and Molecular Structure of the Chiral Catalyst Precursor [3,3-{(S)-PipPhos}2-3-H-1,2-(o-xylylene)-closo-3,1,2-RhC2B9H9]. Organometallics 2011, 30, 1942–1950. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, T.; Wu, S.; Guo, W.; Zhang, H. A stable ruthenium complex bearing a 1,2-dicarbadodecaborane(12)-1,2-dithiolate ligand and its activation for olefin metathesis. J. Organomet. Chem. 2018, 865, 95–99. [Google Scholar] [CrossRef]
- Chan, A.P.Y.; Parkinson, J.A.; Rosair, G.M.; Welch, A.J. Bis(phosphine)hydridorhodacarborane derivatives of 1,1′-bis(ortho-carborane) and their catalysis of alkene isomerization and the hydrosilylation of acetophenone. Inorg. Chem. 2020, 59, 2011–2023. [Google Scholar] [CrossRef]
- Guerrero, I.; Kelemen, Z.; Viñas, C.; Romero, I.; Teixidor, F. Metallacarboranes as Photoredox Catalysts in Water. Chem. Eur. J. 2020, 26, 5027–5036. [Google Scholar] [CrossRef]
- Guerrero, I.; Viñas, C.; Romero, I.; Teixidor, F. A stand-alone cobalt bis(dicarbollide) photoredox catalyst epoxidates alkenes in water at extremely low catalyst load. Green Chem. 2021, 23, 10123–10131. [Google Scholar] [CrossRef]
- Guerrero, I.; Viñas, C.; Fontrodona, X.; Romero, I.; Teixidor, F. Aqueous Persistent Noncovalent Ion-Pair Cooperative Coupling in a Ruthenium Cobaltabis(dicarbollide) System as a Highly Efficient Photoredox Oxidation Catalyst. Inorg. Chem. 2021, 60, 8898–8907. [Google Scholar] [CrossRef]
- Jones, C.J.; Francis, J.N.; Hawthorne, M.F. New 10- and 11-atom polyhedral metallocarboranes prepared by polyhedral contraction. J. Am. Chem. Soc. 1972, 94, 8391–8399. [Google Scholar] [CrossRef]
- Hanusa, T.P.; Huffman, J.C.; Curtis, T.L.; Todd, L.J. Synthesis of (π-arene)metallacarbaboranes containing ruthenium or osmium and a (π-cyclohexadienyl)cobaltacarbaborane. Crystal structure of 2,5,6-(η-C6H6)RuC2B7H11. Inorg. Chem. 1985, 24, 787–792. [Google Scholar] [CrossRef]
- Buckner, S.W.; Fischer, M.J.; Jelliss, P.A.; Luo, R.; Minteer, S.D.; Rath, N.P.; Siemarczuk, A. Dual Fluorescence from an Isonido ReIII Rhenacarborane Phosphine Complex, [7,10-μ-H-7-CO-7,7-(PPh3)2-isonido-7,8,9-ReC2B7H9]. Inorg. Chem. 2006, 45, 7339–7347. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, V.E.; Pisareva, I.V.; Vorontsov, E.V.; Dolgushin, F.M.; Franken, A.; Kennedy, J.D.; Chizhevsky, I.T. Ten-vertex osmamonocarbaboranes via arachno and nido {CB9} monocarbaboranes. Polyhedral contraction promoted by [OsCl2(PPh3)3] in MeOH and the crystal and molecular structure of [1-H-1,1-(PPh3)2-2-Ph-3-(OMe)-isocloso-1,2-OsCB8H7]. Inorg. Chem. Commun. 2003, 6, 1454–1458. [Google Scholar] [CrossRef]
- Konoplev, V.E.; Dolgushin, F.M.; Loginov, D.A.; Tachaev, M.V. Step-by-step polyhedral contraction of the osmacarborane framework {OsCB10} → {OsCB9} → {OsCB8}. J. Organomet. Chem. 2021, 949, 121948. [Google Scholar] [CrossRef]
- Du, S.; Kautz, J.A.; McGrath, T.D.; Stone, F.G.A. Synthesis and Structure of the Novel 11-Vertex Rhenacarborane Dianion [1,1,1-(CO)3-2-Ph-closo-1,2-ReCB9H9]2− and Its Reactivity toward Cationic Transition Metal Fragments. Organometallics 2003, 22, 2842–2850. [Google Scholar] [CrossRef]
- Kolomnikova, G.D.; Petrovskii, P.V.; Sorokin, P.V.; Dolgushin, F.M.; Yanovsky, A.I.; Chizhevsky, I.T. Synthesis, structure and isomerism of “three-bridge” exo-nido-osmacarborane clusters. Rus. Chem. Bull. 2001, 50, 706–715. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Grimes, R.N.; Sinn, E.; Pipal, J.R. Tetracarbon metallacarboranes. 9. New types of nido cage geometry. Crystal and molecular structures of [(C6H5)2PCH2]2Ni(CH3)4C4B8H8 (isomer 1) and (η5-C5H5)Co(CH3)4C4B7H7 (isomer 2). Inorg. Chem. 1980, 19, 2087–2095. [Google Scholar] [CrossRef]
- Ditzel, E.J.; Fontaine, X.L.R.; Greenwood, N.N.; Kennedy, J.D.; Sisan, Z.; Štíbr, B.; Thornton-Pett, M. An unusual direct conversion of metallaboranes to metallacarbaboranes and the isolation of a novel isoarachno twelve-vertex cluster compound. J. Chem. Soc. Chem. Commun. 1990, 1741–1743. [Google Scholar] [CrossRef]
- Pisareva, I.V.; Konoplev, V.E.; Petrovskii, P.V.; Vorontsov, E.V.; Dolgushin, F.M.; Yanovsky, A.I.; Chizhevsky, I.T. Synthesis and characterization of novel monocarbollide exo-closo-(π-arene)biruthenacarboranes [(PPh3)mClRu(η6-C6H5R)Ru′CB10H11-n(OMe)n] (where R = H, m = 2, n = 1; R = μ-PPh2, m = 1, n = 0, 1). Inorg. Chem. 2004, 43, 6228–6237. [Google Scholar] [CrossRef]
- Beckett, M.A.; Brellochs, B.; Chizhevsky, I.T.; Damhus, T.; Hellwich, K.-H.; Kennedy, J.D.; Laitinen, R.; Powella, W.H.; Rabinovich, D.; Viñas, C.; et al. Nomenclature for boranes and related species (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 355–381. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.D. NMR in Inorganic and Organometallic Chemistry. In Multinuclear NMR; Mason, J., Ed.; Plenum: London, UK; New York, NY, USA, 1987; p. 221. [Google Scholar]
- Chizhevsky, I.T.; Yanovsky, A.I.; Struchkov, Y.T. Semi-sandwich platinum metals metallacarboranes derived from nido-C2B9H12−: Chemistry and structural studies. J. Organomet. Chem. 1997, 51, 51–63. [Google Scholar] [CrossRef]
- Pisareva, I.V.; Dolgushin, F.M.; Yanovsky, A.I.; Balagurova, E.V.; Petrovskii, P.V.; Chizhevsky, I.T. The first 8-vertex monocarbon metallacarborane with an “isolated” boron-capped pentagonal bipyramidal cage. Synthesis and molecular structure of capped closo-2,2-(Ph3P)2-2-H-3,6,8-(MeO)3-RuCB6H4. Inorg.Chem. 2001, 40, 5318–5319. [Google Scholar] [CrossRef] [PubMed]
- Perekalin, D.S.; Kudinov, A.R. Cyclopentadienyl ruthenium complexes with naphthalene and other polycyclic aromatic ligands. Coord. Chem. Rev. 2014, 276, 153–173. [Google Scholar] [CrossRef]
- Stephenson, T.A.; Wilkinson, G.J. New complexes of ruthenium (II) and (III) with triphenylphosphine, triphenylarsine, trichlorostannate, pyridine and other ligands. J. Inorg. Nucl. Chem. 1966, 28, 945–956. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]

| NMR | 7a | 7b | (PPh3)2HRuCB6H4(OMe)3 [36] | |
|---|---|---|---|---|
| 1H | OMe | 4.24 s (3H); 2.96 s (3H) | 4.27 s (3H); 2.80 s (6H) | 4.17 s (3H); 3.05 s (6H) |
| HRu | −9.84 br t (1H), 2J(H,P) = 24 Hz | −10.43 br t (1H), 2J (H,P) = 24 Hz | −9.02 br t (1H), 2J(H,P) = 20.7 Hz | |
| 31P{1H} | PPh3 | 46.2 d (1P), 2J = 10 Hz; 46.0 d (1P), 2J = 10 Hz | 48.3 s (2P) | 49.4 s (2P) |
| 11B{1H} | B-8 | 67.5 s (1B) | 70.1 s (1B) | 68.9 s (1B) |
| B-3,6 | 33.7 s (1B); 24.1 s (1B) | 32.8 s (2B) | 33.7 s (2B) | |
| B-7 | 14.0 s (1B) | 14.1 s (1B) | 12.8 s (1B) | |
| B-4,5 | −26.3 (1B); −30.1 (1B) | −32.4 s (2B) | −31.8 s (2B) |
| Compound | 4a | 5a |
|---|---|---|
| CCDC No. | 2201207 | 2201208 |
| Empirical formula | C44H45B8ClOP2Ru2 | C44H45B8ClOP2Ru · 2(CH2Cl2) |
| Molecular weight | 975.81 | 1044.59 |
| Temperature (K) | 120(2) | 110(2) |
| Crystal system | triclinic | monoclinic |
| Space group | P21/c | |
| a (Å) | 10.560(2) | 18.467(11) |
| b (Å) | 20.205(4) | 15.515(9) |
| c (Å) | 22.352(4) | 18.611(11) |
| α (deg) | 114.814(4) | 90 |
| β (deg) | 101.570(4) | 100.170(11) |
| γ (deg) | 90.753(4) | 90 |
| V (Å3) | 4215.4(14) | 5249(5) |
| Z | 4 | 4 |
| Dcalc (g cm−3) | 1.538 | 1.322 |
| linear absorption μ (cm−1) | 8.92 | 6.47 |
| Tmin/Tmax | 0.659/0.862 | 0.560/0.928 |
| 2θmax (deg) | 58 | 52 |
| Reflections collected | 54553 | 45233 |
| Independent reflections (Rint) | 22374 (0.0329) | 10275 (0.1263) |
| Observed reflections (I > 2σ(I)) | 15232 | 5005 |
| Number of parameters | 1102 | 602 |
| R1 (on F for I > 2σ(I)) a | 0.0462 | 0.0987 |
| wR2 (on F2 for all data) b | 0.1000 | 0.2767 |
| GOOF | 1.075 | 1.009 |
| Largest diff. peak/hole (e Å−3) | 1.792/−0.643 | 2.931/−1.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loginov, D.A.; Dolgushin, F.M.; Konoplev, V.E.; Tachaev, M.V. New Aspects of Ruthenium-Mediated Polyhedral Contraction of Monocarbollides. Inorganics 2022, 10, 158. https://doi.org/10.3390/inorganics10100158
Loginov DA, Dolgushin FM, Konoplev VE, Tachaev MV. New Aspects of Ruthenium-Mediated Polyhedral Contraction of Monocarbollides. Inorganics. 2022; 10(10):158. https://doi.org/10.3390/inorganics10100158
Chicago/Turabian StyleLoginov, Dmitry A., Fedor M. Dolgushin, Vitalii E. Konoplev, and Maxim V. Tachaev. 2022. "New Aspects of Ruthenium-Mediated Polyhedral Contraction of Monocarbollides" Inorganics 10, no. 10: 158. https://doi.org/10.3390/inorganics10100158