Advances of Biowaste-Derived Porous Carbon and Carbon–Manganese Dioxide Composite in Supercapacitors: A Review
Abstract
:1. Introduction
2. Raw Materials
| Raw Material | SBET (m2/g) | Current Density (A/g) | Specific Capacitance (F/g) | Types of Test Cells | Reference |
|---|---|---|---|---|---|
| Petroleum coke | 2964 | 0.05 | 220 | Two-electrode cell with symmetrical electrodes. | [25] |
| Coal | 1032 | 0.5 | 108 | Three-electrode cell with Hg/HgO as the reference electrode, AC as the working | [26] |
| Pitch | 3145 | 0.05 | 272 | electrode and platinum plate as the counter electrode. Two-electrode cell with symmetrical electrodes. | [27] |
Non-Conventional Biowaste Raw Materials
3. Electrode Materials
3.1. Biowaste-Derived Activated Carbon
3.1.1. Physical Activation
| Raw Mterial | Agent | Temperature (°C) | BET (m2/g) | VMicro (m3/g) | Reference |
|---|---|---|---|---|---|
| Barley straw | CO2 | 800 | 789 | 0.3268 | [63] |
| Palm shell | CO2 | 850 | 1653 | 0.8900 | [64] |
| Coconut shell | steam | 900 | 1194 | 0.4460 | [65] |
| Artrocarpus waste | steam | 750 | 917 | 0.4800 | [66] |
| Acorn shell | CO2/steam | 600 | 1799 | 0.9270 | [67] |
3.1.2. Chemical Activation
| Raw Material | Agent | Activation Temperature (°C) | SBET (m2/g) | VMicro (cm3/g) | Reference |
|---|---|---|---|---|---|
| Alkaline | |||||
| Apricot shell | NaOH | 700 | 2335.00 | 0.797 | [84] |
| Rice husk | NaOH | 800 | 2682.00 | 1.011 | [85] |
| Rice straw | KOH | 800 | 1917.00 | 0.730 | [86] |
| Acidic | |||||
| Walnut shells | H3PO4 | 400 | 2095.00 | 0.520 | [87] |
| Sunflower oil cake | H2SO4 | 600 | 240.02 | 0.111 | [88] |
| Neutral | |||||
| Date pits | FeCl3 | 700 | 780.00 | 0.468 | [89] |
| Pine-fruit residue | ZnCl2 | 500 | 774.20 | 0.305 | [90] |
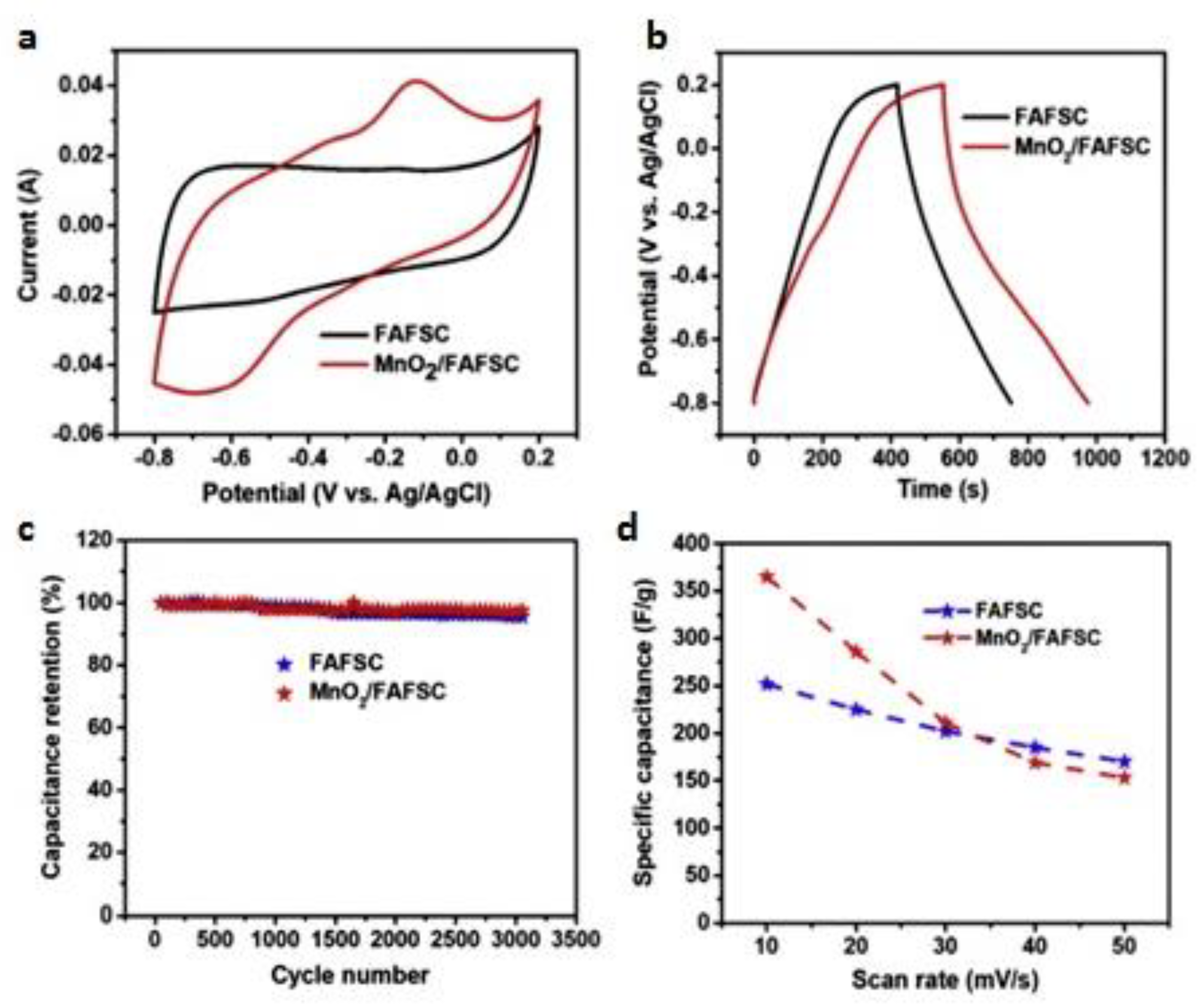
3.2. AC-MnO2 Composites
3.3. Methods of Obtaining AC-MnO2 Composites
4. The Performance of Supercapacitors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, K.; Zhang, Z.; Peng, H.; Zhao, G.; Ma, G.; Lei, Z. Hybrid symmetric supercapacitor assembled by renewable corn silks based porous carbon and redox-active electrolytes. Mater. Chem. Phys. 2018, 218, 229–238. [Google Scholar] [CrossRef]
- Chang, L.; Hang Hu, Y. Supercapacitors. Compr. Energy Syst. 2018, 2, 663–695. [Google Scholar]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Manyala, N.; Bello, A.; Barzegar, F.; Khaleed, A.A.; Momodu, D.Y.; Dangbegnon, J.K. Coniferous pine biomass: A novel insight into sustainable carbon materials for supercapacitors electrode. Mater. Chem. Phys. 2016, 182, 139–147. [Google Scholar] [CrossRef]
- Barik, R.; Ingole, P.P. ScienceDirect Electrochemistry Challenges and prospects of metal sulfide materials for supercapacitors. Curr. Opin. Electrochem. 2020, 21, 327–334. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, C.; Sheng, L. Heteroatoms-doped Pillared Porous Carbon Architectures with Ultrafast Electron and Ion Transport Capabilities under High Mass Loadings for High-rate Supercapacitors Heteroatoms-doped Pillared Porous Carbon Architectures with Ultrafast Electron and Ion Tra. ACS Sustain. Chem. Eng. 2020, 8, 8664–8674. [Google Scholar] [CrossRef]
- Sim, G.; De Oliveira, H.P.; Larsson, S.H.; Thyrel, M.; Lima, E.C. A Short Review on the Electrochemical Performance of Hierarchical and Nitrogen-Doped Activated Biocarbon-Based Electrodes for Supercapacitors. Nanomaterials 2021, 11, 424. [Google Scholar]
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Wu, D.; Xie, X.; Zhang, Y.; Zhang, D.; Du, W.; Zhang, X.; Wang, B. MnO2/Carbon Composites for Supercapacitor: Synthesis and Electrochemical Performance. Front. Mater. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Ali, S.; Saleh, L.; Hadi, R.; Farhoudian, S.; Nedaei, M. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2020, 857, 158281. [Google Scholar] [CrossRef]
- Wadekar, P.H.; Khose, R.V.; Pethsangave, D.A.; Some, S. Waste-Derived Heteroatom-Doped Activated Carbon/Manganese Dioxide Trio-Composite for Supercapacitor Applications. Energy Technol. 2020, 8, 1901402. [Google Scholar] [CrossRef]
- Guo, W.; Yu, C.; Li, S.; Wang, Z.; Yu, J.; Huang, H.; Qiu, J. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives. Nano Energy 2019, 57, 459–472. [Google Scholar] [CrossRef]
- Yang, D.; Ionescu, M.I. Metal Oxide–Carbon Hybrid Materials for Application in Supercapacitors; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128104644. [Google Scholar]
- Martin-Sanchez, N.; Sanchez-Montero, M.J.; Izquierdo, C.; Salvador, F. Highlighting the role of surface groups in the gasification of carbonized raw materials of different natures with steam and supercritical water at pressures ranging from 1 to 1000 bar. Carbon N. Y. 2016, 99, 502–513. [Google Scholar] [CrossRef]
- Volperts, A.; Dobele, G.; Zhurinsh, A.; Vervikishko, D.; Shkolnikov, E.; Ozolinsh, J. Wood-based activated carbons for supercapacitor electrodes with a sulfuric acid electrolyte. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 319–326. [Google Scholar] [CrossRef]
- Veksha, A.; Sasaoka, E.; Uddin, M.A. The influence of porosity and surface oxygen groups of peat-based activated carbons on benzene adsorption from dry and humid air. Carbon N. Y. 2009, 47, 2371–2378. [Google Scholar] [CrossRef]
- Xiao, N.; Wei, Y.; Li, H.; Wang, Y.; Bai, J.; Qiu, J. Boosting the sodium storage performance of coal-based carbon materials through structure modification by solvent extraction. Carbon N. Y. 2020, 162, 431–437. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Xiao, N.; Li, H.; Ji, Y.; Guo, Z.; Liu, C.; Qiu, J. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction. Carbon N. Y. 2019, 151, 46–52. [Google Scholar] [CrossRef]
- Li, G.Q.; Tian, F.H.; Zhang, Y.F.; Ding, J.L.; Fu, Y.L.; Wang, Y.; Zhang, G.J. Bamboo/lignite-based activated carbons produced by steam activation with and without ammonia for SO2 adsorption. Xinxing Tan Cailiao/New Carbon Mater. 2014, 29, 486–492. [Google Scholar] [CrossRef]
- Starck, J.; Burg, P.; Muller, S.; Bimer, J.; Furdin, G.; Fioux, P.; Vix-Guterl, C.; Begin, D.; Faure, P.; Azambre, B. The influence of demineralisation and ammoxidation on the adsorption properties of an activated carbon prepared from a Polish lignite. Carbon N. Y. 2006, 44, 2549–2557. [Google Scholar] [CrossRef]
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565. [Google Scholar] [CrossRef]
- Tabac, S.; Eisenberg, D. Pyrolyze this paper: Can biomass become a source for precise carbon electrodes? Curr. Opin. Electrochem. 2021, 25, 100638. [Google Scholar] [CrossRef]
- Vallero, D. Air Pollutant Emissions. In Fundamentals of Air Pollution; Elsevier: Amsterdam, The Netherlands, 2014; pp. 787–827. [Google Scholar]
- Tan, M.H.; Zheng, J.T.; Li, P.; Noritatsu, T.; Wu, M.B. Preparation and modification of high performance porous carbons from petroleum coke for use as supercapacitor electrodes. Xinxing Tan Cailiao/New Carbon Mater. 2016, 31, 343–351. [Google Scholar] [CrossRef]
- Lu, Q.; Xu, Y.Y.; Mu, S.J.; Li, W.C. The effect of nitrogen and/or boron doping on the electrochemical performance of non-caking coal-derived activated carbons for use as supercapacitor electrodes. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 442–450. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, H.F.; He, X.J.; Ma, H.; Dong, S.A.; Xie, X.Y. Synthesis of porous carbons from coal tar pitch for high-performance supercapacitors. Xinxing Tan Cailiao/New Carbon Mater. 2019, 34, 132–139. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Zhong, Y.; Shen, T.; Wang, X.; Xia, X.; Tu, J. Natural biomass-derived carbons for electrochemical energy storage. Mater. Res. Bull. 2017, 88, 234–241. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon N. Y. 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Y.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Liu, Q. Optimized preparation of activated carbon from coconut shell and municipal sludge. Mater. Chem. Phys. 2020, 241, 122327. [Google Scholar] [CrossRef]
- Martínez-Casillas, D.C.; Mascorro-Gutiérrez, I.; Arreola-Ramos, C.E.; Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Ramos-Sánchez, V.H.; Cuentas-Gallegos, A.K. A sustainable approach to produce activated carbons from pecan nutshell waste for environmentally friendly supercapacitors. Carbon N. Y. 2019, 148, 403–412. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, J.; Hao, L.; Xue, R.; Sun, G.; Yi, B. High rate performance activated carbons prepared from ginkgo shells for electrochemical supercapacitors. Carbon N. Y. 2013, 56, 146–154. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Mutuma, B.K.; Manyala, N. Onion-derived activated carbons with enhanced surface area for improved hydrogen storage and electrochemical energy application. RSC Adv. 2020, 10, 26928–26936. [Google Scholar] [CrossRef]
- Li, J.; Luo, F.; Lin, T.; Yang, J.; Yang, S.; He, D.; Xiao, D.; Liu, W. Pomelo peel-based N, O-codoped hierarchical porous carbon material for supercapacitor application. Chem. Phys. Lett. 2020, 753, 137597. [Google Scholar] [CrossRef]
- Ji, T.; Han, K.; Teng, Z.; Li, J.; Wang, M.; Zhang, J.; Cao, Y.; Qi, J. Synthesis of Activated Carbon Derived from Garlic Peel and Its Electrochemical Properties. Int. J. Electrochem. Sci. 2021, 16, 150653. [Google Scholar] [CrossRef]
- Ranaweera, C.K.; Kahol, P.K.; Ghimire, M.; Mishra, S.R.; Gupta, R.K. Orange-Peel-Derived Carbon: Designing Sustainable and High-Performance Supercapacitor Electrodes. J. Carbon Res. Artic. 2017, 3, 25. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B. High surface area and oxygen-enriched activated carbon synthesized from animal cellulose and evaluated in electric double-layer capacitors. RSC Adv. 2015, 5, 31375–31383. [Google Scholar] [CrossRef]
- Niu, L.; Shen, C.; Yan, L.; Zhang, J.; Lin, Y.; Gong, Y.; Li, C.; Sun, C.Q.; Xu, S. Waste bones derived nitrogen–doped carbon with high micropore ratio towards supercapacitor applications. J. Colloid Interface Sci. 2019, 547, 92–101. [Google Scholar] [CrossRef]
- Sirengo, K.; Jande, Y.A.C.; Kibona, T.E.; Hilonga, A.; Muiva, C.; King’ondu, C.K. Fish bladder-based activated carbon/Co3O4/TiO2 composite electrodes for supercapacitors. Mater. Chem. Phys. 2019, 232, 49–56. [Google Scholar] [CrossRef]
- Elanthamilan, E.; Sriram, B.; Rajkumar, S.; Dhaneshwaran, C.; Nagaraj, N.; Princy Merlin, J.; Vijayan, A.; Wang, S.F. Couroupita guianansis dead flower derived porous activated carbon as efficient supercapacitor electrode material. Mater. Res. Bull. 2019, 112, 390–398. [Google Scholar] [CrossRef]
- Chen, H.; Yu, F.; Wang, G.; Chen, L.; Dai, B.; Peng, S. Nitrogen and Sulfur Self-Doped Activated Carbon Directly Derived from Elm Flower for High-Performance Supercapacitors. ACS Omega 2018, 3, 4724–4732. [Google Scholar] [CrossRef]
- Zheng, L.H.; Chen, M.H.; Liang, S.X.; Lü, Q.F. Oxygen-rich hierarchical porous carbon derived from biomass waste-kapok flower for supercapacitor electrode. Diam. Relat. Mater. 2021, 113, 108267. [Google Scholar] [CrossRef]
- Sun, W.; Lipka, S.M.; Swartz, C.; Williams, D.; Yang, F. Hemp-derived activated carbons for supercapacitors. Carbon N. Y. 2016, 103, 181–192. [Google Scholar] [CrossRef]
- Roy, C.K.; Shah, S.S.; Reaz, A.H.; Sultana, S.; Chowdhury, A.N.; Firoz, S.H.; Zahir, M.H.; Ahmed Qasem, M.A.; Aziz, M.A. Preparation of Hierarchical Porous Activated Carbon from Banana Leaves for High-performance Supercapacitor: Effect of Type of Electrolytes on Performance. Chem.—Asian J. 2020, 16, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Cheng, Y.; Zhong, H.; Tian, H.; Pan, J.; Pareek, V.K.; Jiang, S.P.; Lamonier, J.F.; Jaroniec, M.; Liu, J. From waste Coca Cola® to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors. Carbon N. Y. 2017, 116, 490–499. [Google Scholar] [CrossRef]
- Boota, M.; Paranthaman, M.P.; Naskar, A.K.; Li, Y.; Akato, K.; Gogotsi, Y. Waste tire derived carbon-polymer composite paper as pseudocapacitive electrode with long cycle life. ChemSusChem 2015, 8, 3576–3581. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, Y.; Wang, F.; Wang, G.; Qi, P.; Guo, X.; Dai, B.; Yu, F. An activated carbon derived from tobacco waste for use as a supercapacitor electrode material. Carbon N. Y. 2018, 130, 848. [Google Scholar] [CrossRef]
- Utetiwabo, W.; Yang, L.; Tufail, M.K.; Zhou, L.; Chen, R.; Lian, Y.; Yang, W. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors. Chin. Chem. Lett. 2020, 31, 1474–1489. [Google Scholar] [CrossRef]
- Hu, X.; Lin, Z. Transforming waste polypropylene face masks into S-doped porous carbon as the cathode electrode for supercapacitors. Ionics 2021, 27, 2169–2179. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Sarkar, S.; Arya, A.; Gaur, U.K.; Gaur, A. Investigations on porous carbon derived from sugarcane bagasse as an electrode material for supercapacitors. Biomass Bioenergy 2020, 142, 105730. [Google Scholar] [CrossRef]
- Ma, F.; Ding, S.; Ren, H.; Liu, Y. Sakura-based activated carbon preparation and its performance in supercapacitor applications. RSC Adv. 2019, 9, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, H.D.; Rochmadi; Prasetyo, I.; Ariyanto, T. Mesoporous manganese oxide/lignin-derived carbon for high performance of supercapacitor electrodes. Molecules 2021, 26, 7104. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Nitta, N.; Yushin, G. Lithographically patterned thin activated carbon films as a new technology platform for on-chip devices. ACS Nano 2013, 7, 6498–6506. [Google Scholar] [CrossRef]
- Joanna Conder, K.F.; Ghimbeu, C.M. Supercapacitors (electrochemical capacitors). In Char and Carbon Materials Derived from Biomass Production, Characterization and Applications; Mejdi Jeguirim, L.L., Ed.; Elsevier Inc.: Mulhouse, France, 2019; pp. 383–427. ISBN 9780128148938. [Google Scholar]
- Xie, L.; Jin, Z.; Dai, Z.; Chang, Y.; Jiang, X.; Wang, H. Porous carbons synthesized by templating approach from fluid precursors and their applications in environment and energy storage: A review. Carbon N. Y. 2020, 170, 100–118. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Rodrfguez-reinoso, F.; Sepveda-escribano, A.; Inorgdnica, D.D.Q.; Alicante, U. De Chapter 9 Porous Carbons in Adsorption and Catalysis. In Handbook of Surfaces and Interfaces of Materials; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 5, pp. 309–355. [Google Scholar]
- Ayinla, R.T.; Dennis, J.O.; Zaid, H.M.; Sanusi, Y.K.; Usman, F.; Adebayo, L.L. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activation Processes (Thermal or Physical); Elsevier: Oxford, UK, 2006; Volume 2. [Google Scholar]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Arami-Niya, A.; Daud, W.M.A.W.; Mjalli, F.S. Comparative study of the textural characteristics of oil palm shell activated carbon produced by chemical and physical activation for methane adsorption. Chem. Eng. Res. Des. 2011, 89, 657–664. [Google Scholar] [CrossRef]
- Sun, K.; Leng, C.Y.; Jiang, J.C.; Bu, Q.; Lin, G.F.; Lu, X.C.; Zhu, G.Z. Microporous activated carbons from coconut shells produced by self-activation using the pyrolysis gases produced from them, that have an excellent electric double layer performance. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 451–459. [Google Scholar] [CrossRef]
- Kazmierczak-Razna, J.; Nowicki, P.; Wiśniewska, M.; Nosal-Wiercińska, A.; Pietrzak, R. Thermal and physicochemical properties of phosphorus-containing activated carbons obtained from biomass. J. Taiwan Inst. Chem. Eng. 2017, 80, 1006–1013. [Google Scholar] [CrossRef]
- Şahin, Ö.; Saka, C. Preparation and characterization of activated carbon from acorn shell by physical activation with H2O-CO2 in two-step pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y. Production and performance of activated carbon from rice husks for removal of natural organic matter from water: A review. Chem. Eng. Res. Des. 2018, 129, 271–296. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Dong, Q.; Zhan, F.; Zhang, X.; Li, S.; Qiao, H.; Qiu, J. Starch Derived Porous Carbon Nanosheets for High-performance Photovoltaic Capacitive Deionization. Environ. Sci. Technol. 2017, 51, 9244–9251. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Hao, F.; Qu, Z.; Gao, J.; Liu, M.; Wang, K. A green trace K2CO3 induced catalytic activation strategy for developing coal-converted activated carbon as advanced candidate for CO2 adsorption and supercapacitors. Chem. Eng. J. 2019, 383, 123205. [Google Scholar] [CrossRef]
- Kong, J.; Gu, R.; Yuan, J.; Liu, W.; Wu, J.; Fei, Z. Ecotoxicology and Environmental Safety Adsorption behavior of Ni (II) onto activated carbons from hide waste and high-pressure steaming hide waste. Ecotoxicol. Environ. Saf. 2018, 156, 294–300. [Google Scholar] [CrossRef]
- Santos, S.; Cristina, R.; Bonomo, F.; Costa, R.; Fontan, I. Adsorption of the textile dye Dianix® royal blue CC onto carbons obtained from yellow mombin fruit stones and activated with KOH and H3PO4: Kinetics, adsorption equilibrium and thermodynamic studies. Powder Technol. 2018, 339, 334–343. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S.; Kwon, E.E.; Song, H.; Poon, C.S. Phosphoric acid-activated wood biochar for catalytic conversion of starch-rich food waste into glucose and 5-hydroxymethylfurfural. Bioresour. Technol. 2018, 267, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Legrouri, K.; Khouya, E.; Ezzine, M.; Hannache, H.; Denoyel, R.; Pallier, R.; Naslain, R. Production of activated carbon from a new precursor molasses by activation with sulphuric acid. J. Hazard. Mater. 2005, 118, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Yang, S.; Zhang, J.; Zhang, C.; Wu, H. Physicochemical characteristics and sorption capacities of heavy metal ions of activated carbons derived by activation with different alkyl phosphate triesters. Appl. Surf. Sci. 2014, 316, 443–450. [Google Scholar] [CrossRef]
- Sayğili, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. A green and scalable route to yield porous carbon sheets from biomass for supercapacitors with high capacity. J. Mater. Chem. A 2018, 6, 1244–1254. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, F.; Bian, Y.; Ji, R.; Song, Y.; Jiang, X. Co- and self-activated synthesis of tailored multimodal porous carbons for solid-phase microextraction of chlorobenzenes and polychlorinated biphenyls. J. Chromatogr. A 2019, 1585, 1–9. [Google Scholar] [CrossRef]
- Wu, X.; Ding, B.; Zhang, C.; Li, B.; Fan, Z. Self-activation of potassium gluconate derived nitrogen and sulfur dual-doping hierarchical porous carbons for asymmetric supercapacitors with high energy densities. Carbon N. Y. 2019, 153, 225–233. [Google Scholar] [CrossRef]
- De Yuso, A.M.; Rubio, B.; Izquierdo, M.T. Influence of activation atmosphere used in the chemical activation of almond shell on the characteristics and adsorption performance of activated carbons. Fuel Process. Technol. 2014, 119, 74–80. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis Strategies of Porous Carbon for Supercapacitor Applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Y.; Wei, G.; Cao, G.; Zhang, H.; Yang, Y. Activated carbon with high capacitance prepared by NaOH activation for supercapacitors. Mater. Chem. Phys. 2010, 124, 504–509. [Google Scholar] [CrossRef]
- Le Van, K.; Luong Thi, T.T. Activated carbon derived from rice husk by NaOH activation and its application in supercapacitor. Prog. Nat. Sci. Mater. Int. 2014, 24, 191–198. [Google Scholar] [CrossRef]
- Basta, A.H.; Fierro, V.; El-Saied, H.; Celzard, A. 2-Steps KOH activation of rice straw: An efficient method for preparing high-performance activated carbons. Bioresour. Technol. 2009, 100, 3941–3947. [Google Scholar] [CrossRef]
- Pavlenko, V.V.; Abbas, Q.; Przygocki, P.; Kon’kova, T.; Supiyeva, Z.; Abeykoon, N.; Prikhodko, N.; Bijsenbayev, M.; Kurbatov, A.; Lesbayev, B.T.; et al. Temperature dependent characteristics of activated carbons from walnut shells for improved supercapacitor performance. Eurasian Chem. J. 2018, 20, 99–105. [Google Scholar] [CrossRef]
- Karagöz, S.; Tay, T.; Ucar, S.; Erdem, M. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 2008, 99, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Theydan, S.K. Adsorptive removal of p-nitrophenol on microporous activated carbon by FeCl3 activation: Equilibrium and kinetics studies. Desalin. Water Treat. 2015, 55, 522–531. [Google Scholar] [CrossRef]
- Madankar, C.S.; Bhagwat, S.S.; Meshram, P.D. Cd2+ removal from synthetic waters by ZnCl2-activated carbon. Mater. Today Proc. 2021, 45, 4684–4688. [Google Scholar] [CrossRef]
- Li, X.R.; Jiang, Y.H.; Wang, P.Z.; Mo, Y.; Lai, W.D.; Li, Z.J.; Yu, R.J.; Du, Y.T.; Zhang, X.R.; Chen, Y. Effect of the oxygen functional groups of activated carbon on its electrochemical performance for supercapacitors. Xinxing Tan Cailiao/New Carbon Mater. 2020, 35, 232–243. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Activated carbons from KOH-activation of argan (Argania spinosa) seed shells as supercapacitor electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Park, M.; Park, S.J. Current progress on the surface chemical modification of carbonaceous materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef]
- Ding, Z.; Trouillet, V.; Dsoke, S. Are Functional Groups Beneficial or Harmful on the Electrochemical Performance of Activated Carbon Electrodes? J. Electrochem. Soc. 2019, 166, A1004–A1014. [Google Scholar] [CrossRef]
- Tran, V.M.; Ha, A.T.; Loan, M.; Le, P. Capacitance behavior of nanostructured ε-MnO2/C composite electrode using different carbons matrix. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 025005. [Google Scholar] [CrossRef]
- Adorna, J.; Borines, M.; Dang, V.D.; Doong, R.A. Coconut shell derived activated biochar–manganese dioxide nanocomposites for high performance capacitive deionization. Desalination 2020, 492, 114602. [Google Scholar] [CrossRef]
- Dujearic-stephane, K.; Gupta, M.; Kumar, A.; Sharma, V.; Pandit, S. The Effect of Modifications of Activated Carbon Materials on the Capacitive Performance: Surface, Microstructure, and Wettability. J. Compos. Sci. 2021, 5, 66. [Google Scholar] [CrossRef]
- Wang, J.G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.; Li, X.; Wang, X.; Jia, Y. Simultaneous removal and oxidation of arsenic from water by δ-MnO2 modifie d activate d carbon. J. Environ. Sci. 2020, 94, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Fashedemi, O.O.; Barzegar, F.; Madito, M.J.; Momodu, D.Y.; Masikhwa, T.M.; Dangbegnon, J.K.; Manyala, N. Microwave synthesis: Characterization and electrochemical properties of amorphous activated carbon-MnO2 nanocomposite electrodes. J. Alloys Compd. 2016, 681, 293–300. [Google Scholar] [CrossRef]
- Song, X.; Duan, H.; Zhang, Y.; Wang, H.; Cao, H. Facile synthesis of -MnO2/rice husk-based-activated carbon and its electrochemical properties. Funct. Mater. Lett. 2017, 10, 9–11. [Google Scholar] [CrossRef]
- Liu, Y.H.; Hsi, H.C.; Li, K.C.; Hou, C.H. Electrodeposited manganese dioxide/activated carbon composite as a high-performance electrode material for capacitive deionization. ACS Sustain. Chem. Eng. 2016, 4, 4762–4770. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Huang, T.; Qiu, Z.; Wu, D.; Hu, Z. Bamboo-based activated carbon @ MnO2 nanocomposites for flexible high-performance supercapacitor electrode materials. Int. J. Electrochem. Sci. 2015, 10, 6312–6323. [Google Scholar]
- Mohammed, A.A.; Chen, C.; Zhu, Z. Green and high performance all-solid-state supercapacitors based on MnO2/Faidherbia albida fruit shell derived carbon sphere electrodes. J. Power Sources 2019, 417, 1–13. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, Y.; Song, X.; Liu, Y.; Wang, H.; Duan, H.; Kong, X. Facile hydrothermal synthesis of actiniaria-shaped α-MnO2/activated carbon and its electrochemical performances of supercapacitor. J. Alloys Compd. 2019, 770, 926–933. [Google Scholar] [CrossRef]
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Synthesis of hierarchically porous MnO2/rice husks derived carbon composite as high-performance electrode material for supercapacitors. Appl. Energy 2016, 178, 260–268. [Google Scholar] [CrossRef]
- Kong, S.; Jin, B.; Quan, X.; Zhang, G.; Guo, X.; Zhu, Q.; Yang, F.; Cheng, K.; Wang, G.; Cao, D. MnO2 nanosheets decorated porous active carbon derived from wheat bran for high-performance asymmetric supercapacitor. J. Electroanal. Chem. 2019, 850, 113412. [Google Scholar] [CrossRef]
- Performance, E.; Li, X.; Xu, M.; Yang, Y.; Huang, Q.; Wang, X.; Ren, J.; Wang, X. MnO 2 @ Corncob Carbon Composite Electrode and All-Solid-State Supercapacitor with Improved. Materials 2019, 15, 2379. [Google Scholar]
- Wang, Y.; Liu, H.; Sun, X.; Zhitomirsky, I. Manganese dioxide-carbon nanotube nanocomposites for electrodes of electrochemical supercapacitors. Scr. Mater. 2009, 61, 1079–1082. [Google Scholar] [CrossRef]
- Şahin, M.E.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Li, L.; Wu, Z.; Yuan, S.; Zhang, X.B. Advances and challenges for flexible energy storage and conversion devices and systems. Energy Environ. Sci. 2014, 7, 2101–2122. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]



| Raw Material | SBET (m2/g) | Current Density (A/g) | Specific Capacitance (F/g) | Types of Test Cells | Reference |
|---|---|---|---|---|---|
| Plant waste | |||||
| Sugarcane bagasse | 725 | 0.2 | 265 | Three-electrode cell (working (AC), reference (saturated calomel electrode), a counter (Pt wire). | [52] |
| Elm flower | 2048.6 | 20 | 216 | Two-electrode cell with symmetrical electrodes. | [41] |
| Kapok flower | 1904.1 | 1 | 286.8 | Three-electrode cell (counter (Pt wire), reference electrode (Ag/AgCl), working (AC) cell. | [42] |
| Sakura petals | 1433.8 | 0.2 | 265.8 | Three-electrode working (AC), counter (Pt sheet), reference (a saturated calomel electrode). | [53] |
| Lignin | 1425 | 10 mV/s | 140.9 | Three-electrode cell (working (AC), counter (Pt), and references (Ag/AgCl)). | [54] |
| Food waste | |||||
| Garlic peel | 3325.2 | 1 | 424.42 | Two-electrode cell with symmetrical electrodes. | [35] |
| Onion peel | 3150 | 0.5 | 169 | Three-electrode cell (working (AC), counter (glassy carbon), and references (Ag/AgCl)). | [33] |
| Pomelo peel | 1582 | 0.5 | 180 | Three-electrode cell (reference (Hg/HgO), a counter (graphite sheet), working (porous carbon)). | [34] |
| Orange peel | 1391 | 0.5 | 407 | Three-electrode cell (working (porous carbon), counter (Pt strip), and reference (saturated calomel electrode). | [36] |
| Animal waste | |||||
| Crab shell | 3442 | 0.2 | 280.6 | Two-electrode cell with symmetrical electrodes. | [37] |
| Pork bone | 1260 | 0.5 | 263 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
| Blackfish bone | 1202 | 0.5 | 302 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
| Eel bone | 1163 | 0.5 | 264 | Three-electrode cell (working (AC), counter (Pt foil), reference (saturated calomel electrode)). | [38] |
| The Raw Material of AC | Current Density | Specific Capacitance | Types of Test Cells | Reference |
|---|---|---|---|---|
| Banana peel | 10 A/g | 70 F/g | Three-electrode cell (counter (Pt wire), reference (a saturated calomel), working (composite)). | [103] |
| Bamboo | 1 A/g | 221.45 F/g | Three-electrode cell (counter (Pt plate), reference (a saturated calomel), working (composite). | [104] |
| Faidherbia albida shell | 1 A/g | 426.66 F/g | Three-electrode cell (counter (Pt wire), reference (Ag/AgCl), working (composite). | [105] |
| Rice husk | 0.2 A/g | 977.4 C/g | Two-electrode cell with symmetrical electrode). | [106] |
| Rice husk | 0.5 A/g | 210.3 F/g | Three-electrode cell (counter (Pt plate), reference (saturated calomel), working (composite). | [107] |
| Wheat bran | 1 A/g | 258 F/g | Three-electrode cell (counter (Pt foil), reference (Ag/AgCl), working (composite). | [108] |
| Lignin | 10 mV/s | 345.3 F/g | Three-electrode cell (working (composite), counter (Pt), and references (Ag/AgCl)). | [54] |
| Corncob | - | 1281.3 mF/cm2 | Three-electrode cell (working (composite), counter (Pt plate), and references (Ag/AgCl)). | [109] |
| Material | Capacitance Retention, % | Reference |
|---|---|---|
| Rice husk-derived carbon | 98.5 (after 5000 charge–discharge cycles) | [107] |
| Rice husk-derived carbon/MnO2 | 80.2 (after 5000 charge–discharge cycles) | [107] |
| Bamboo-based activated carbon | 99.98 (after 1000 charge–discharge cycles) | [104] |
| Bamboo-based activated carbon/MnO2 | 89.29 (after 1000 charge–discharge cycles) | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zekenova, A.; Nazhipkyzy, M.; Li, W.; Kalybayeva, A.; Zhumanova, G.; Zubova, O. Advances of Biowaste-Derived Porous Carbon and Carbon–Manganese Dioxide Composite in Supercapacitors: A Review. Inorganics 2022, 10, 160. https://doi.org/10.3390/inorganics10100160
Zekenova A, Nazhipkyzy M, Li W, Kalybayeva A, Zhumanova G, Zubova O. Advances of Biowaste-Derived Porous Carbon and Carbon–Manganese Dioxide Composite in Supercapacitors: A Review. Inorganics. 2022; 10(10):160. https://doi.org/10.3390/inorganics10100160
Chicago/Turabian StyleZekenova, Akzhibek, Meruyert Nazhipkyzy, Wanlu Li, Akmaral Kalybayeva, Guldarikha Zhumanova, and Olga Zubova. 2022. "Advances of Biowaste-Derived Porous Carbon and Carbon–Manganese Dioxide Composite in Supercapacitors: A Review" Inorganics 10, no. 10: 160. https://doi.org/10.3390/inorganics10100160





