Synthesis of 3D Cadmium(II)-Carboxylate Framework Having Potential for Co-Catalyst Free CO2 Fixation to Cyclic Carbonates
Abstract
:1. Introduction
2. Experimental
2.1. Characterization Methods
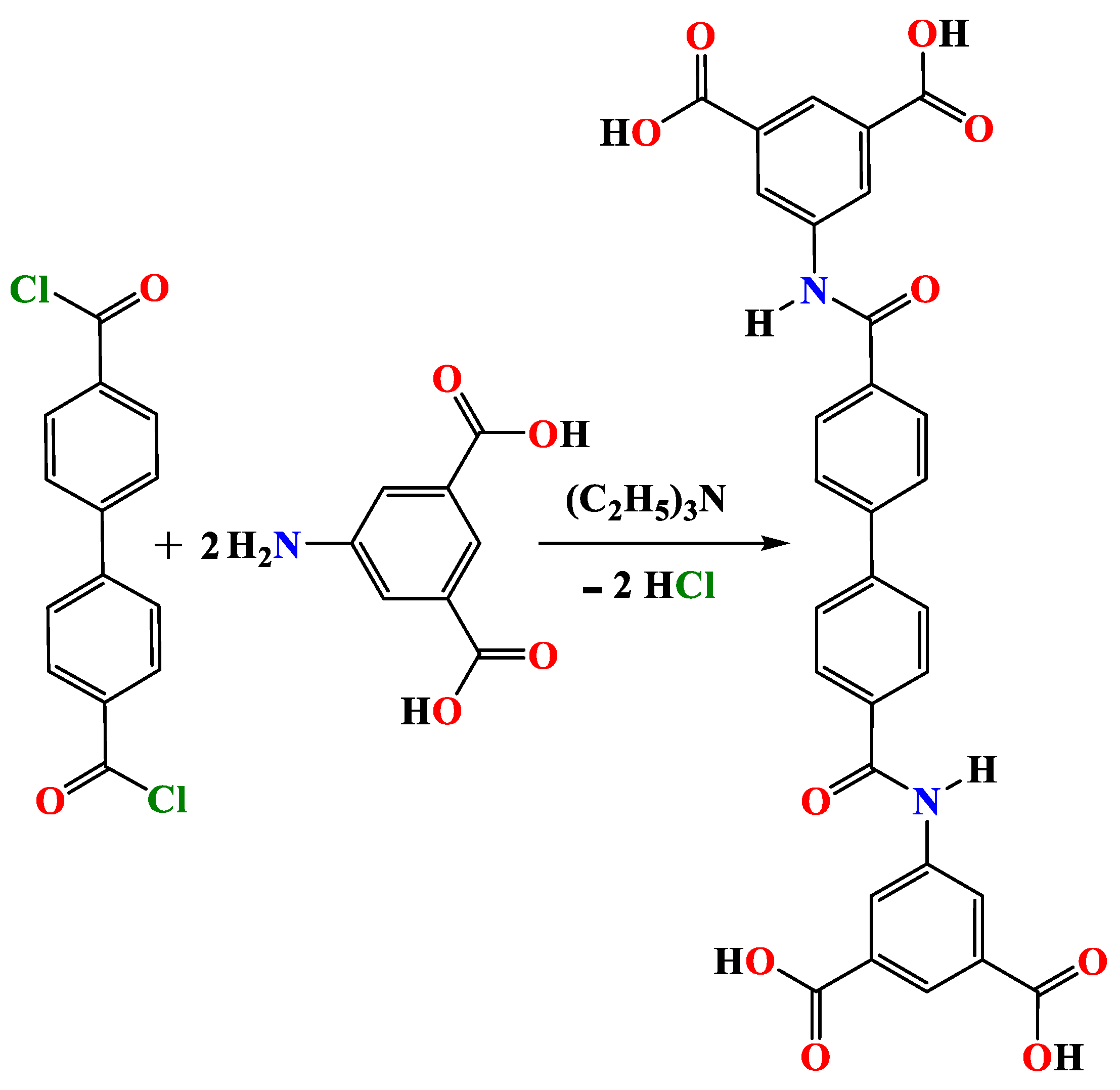
2.2. Synthesis of Ligand Acid (H4L)
2.3. Synthesis of CdMOF ([Cd2(L)(DMF)(H2O)2]n)
2.4. Catalytic Reactions
3. Result and Discussion
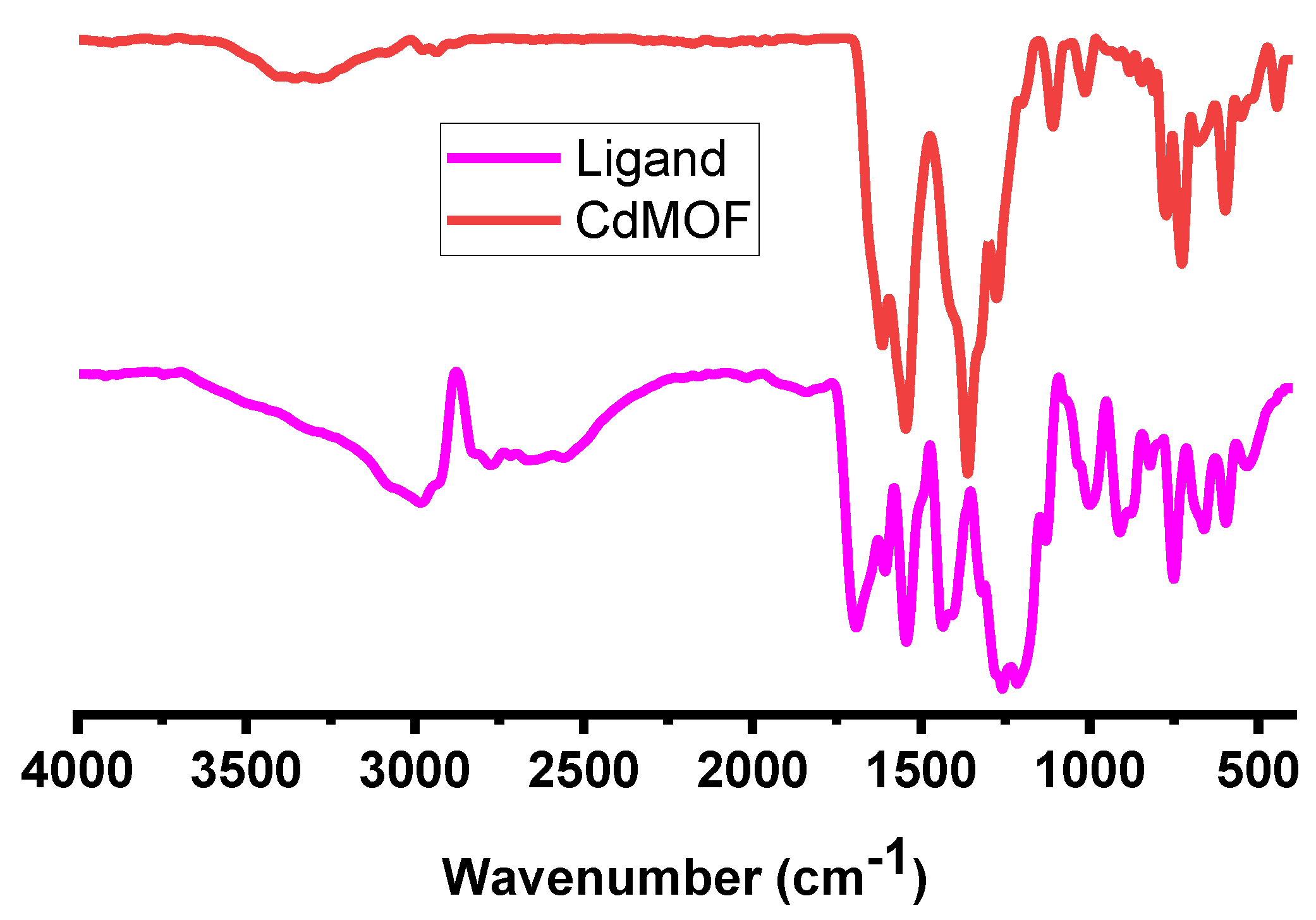
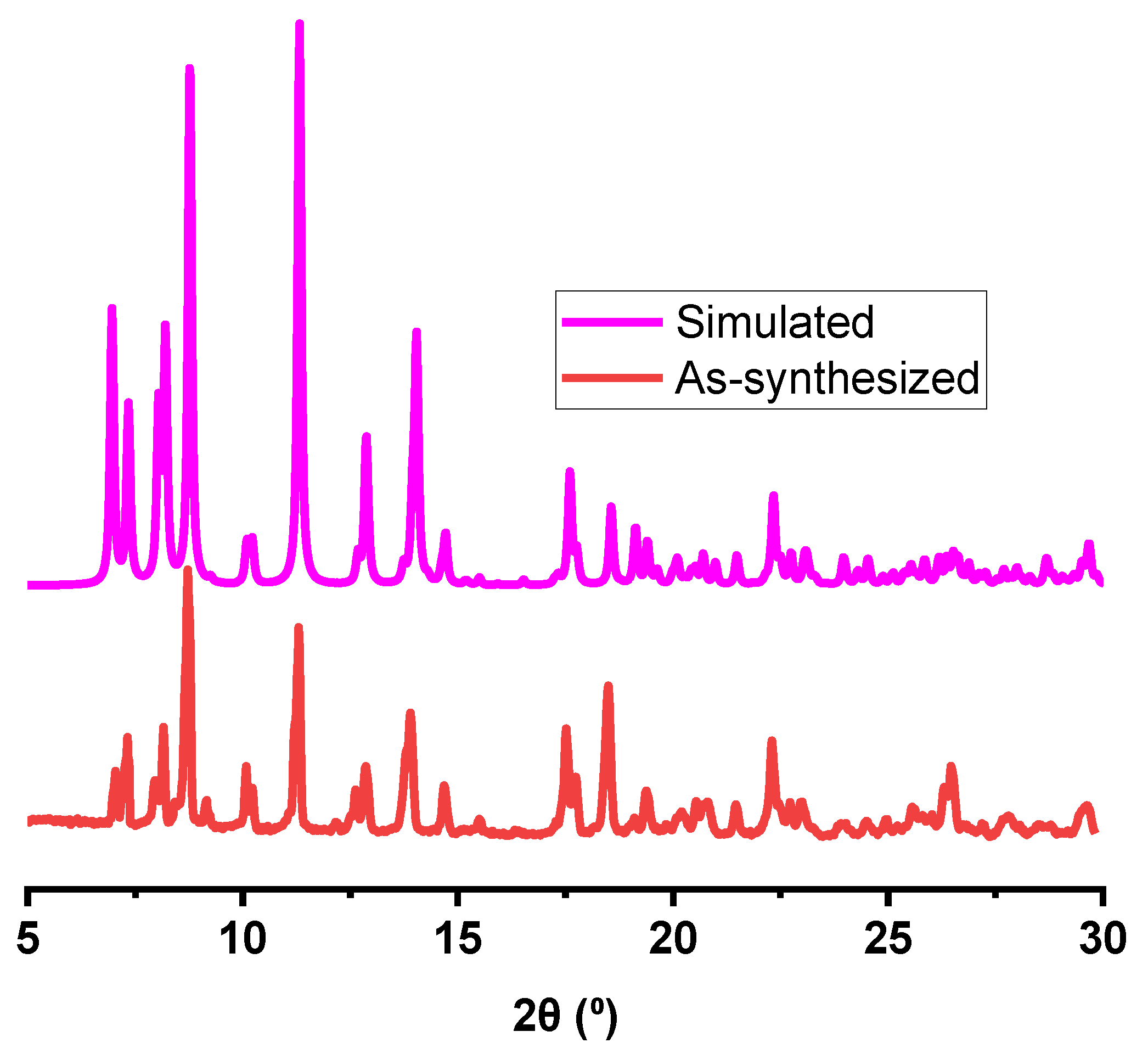
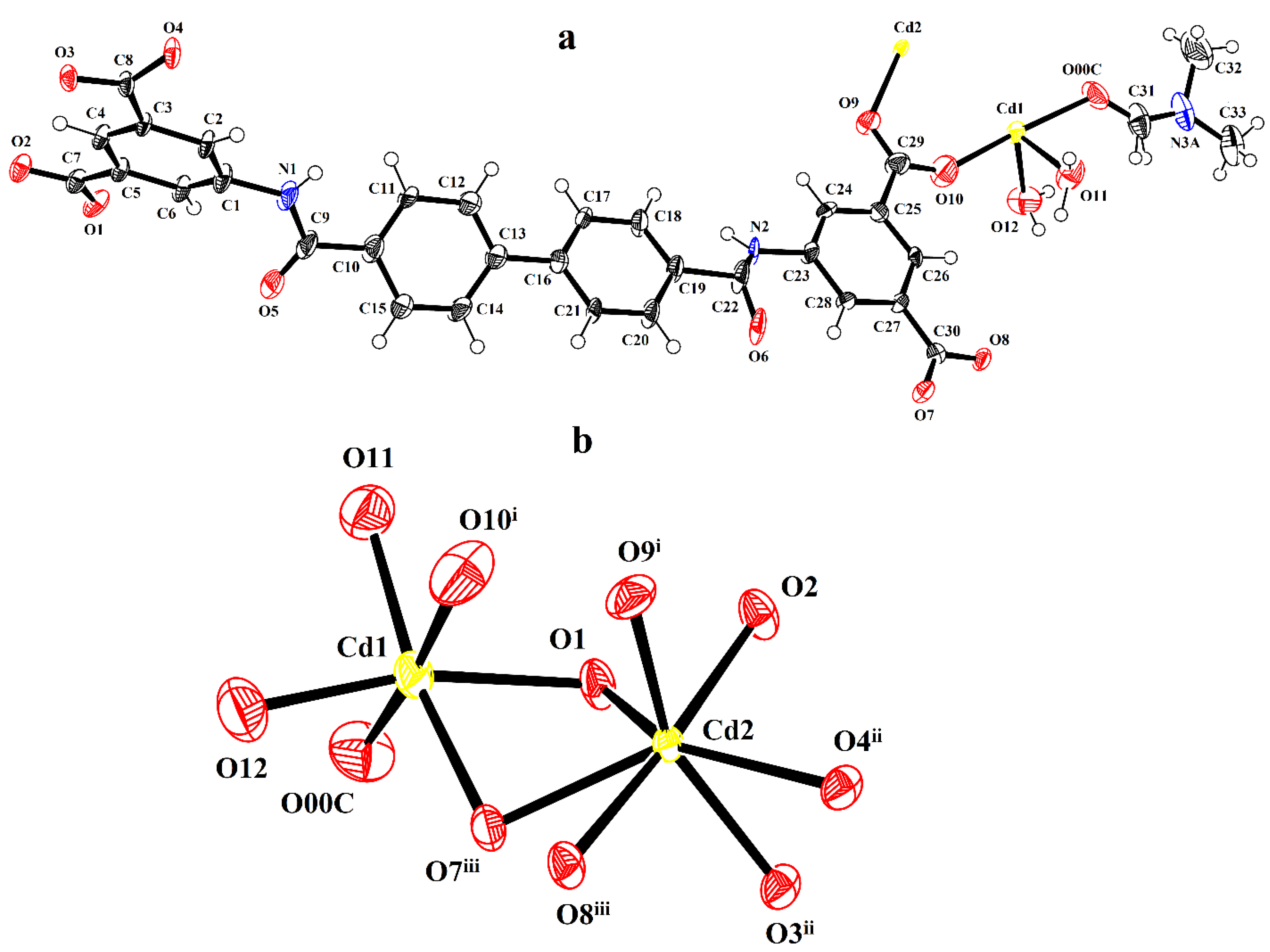
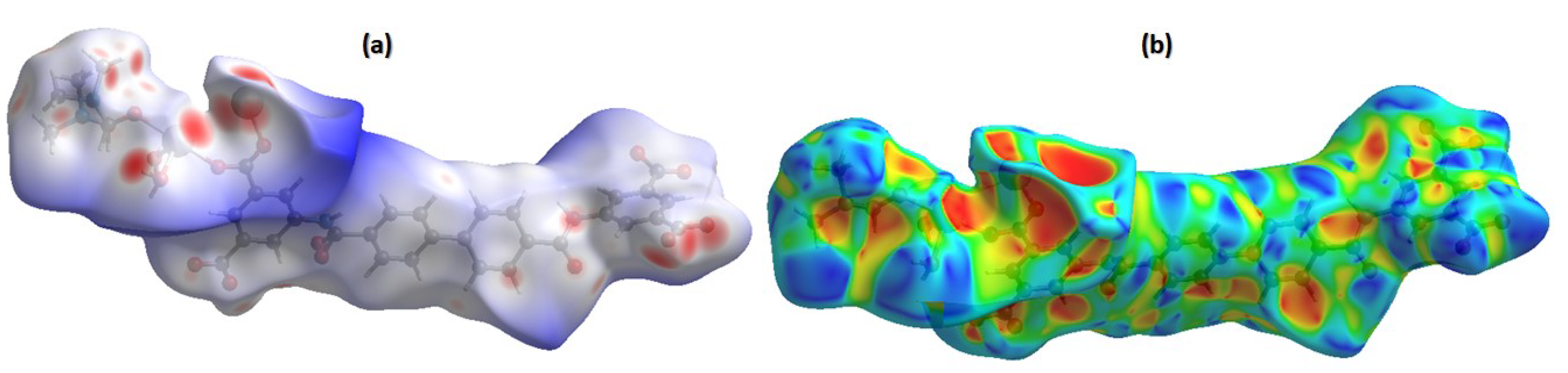
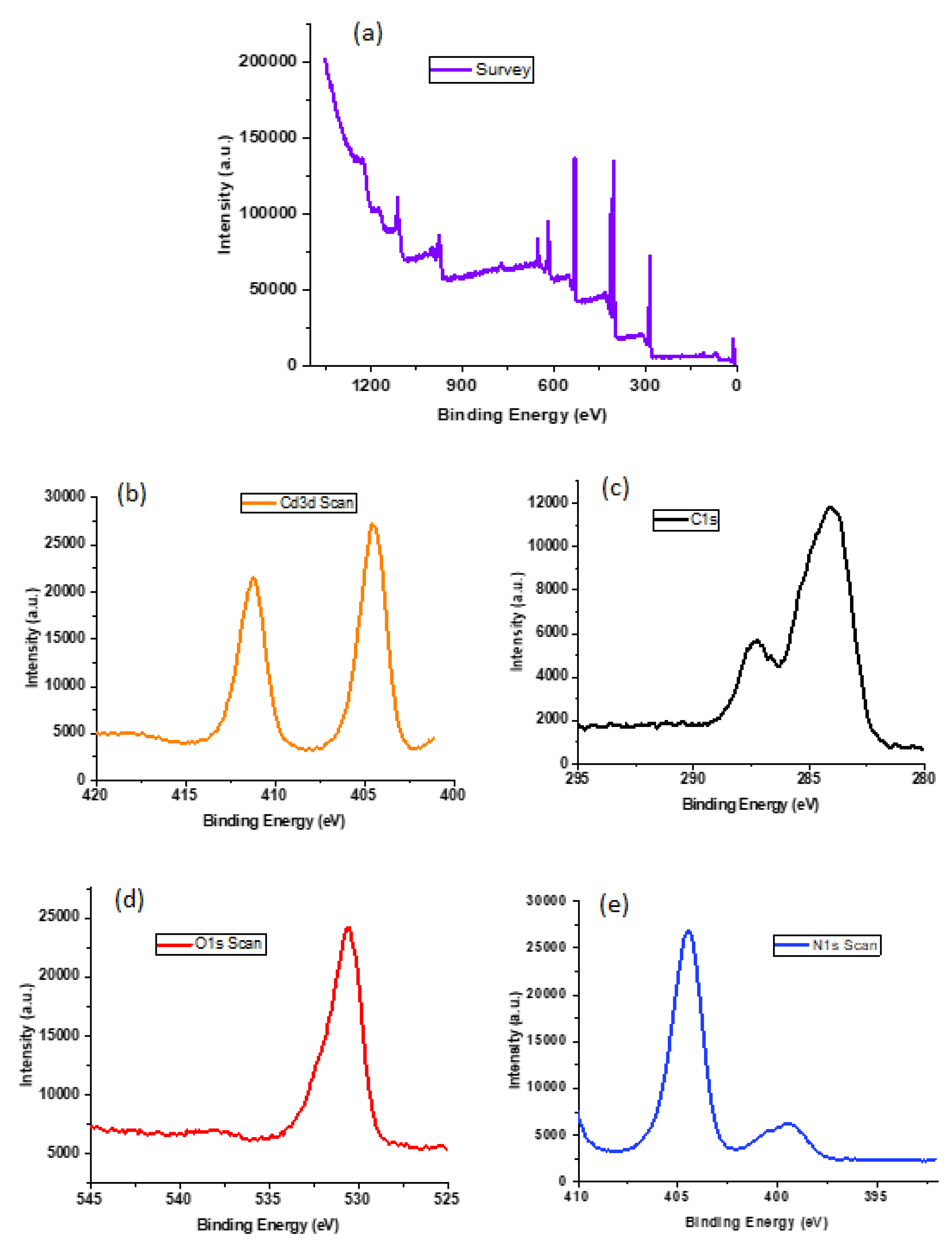
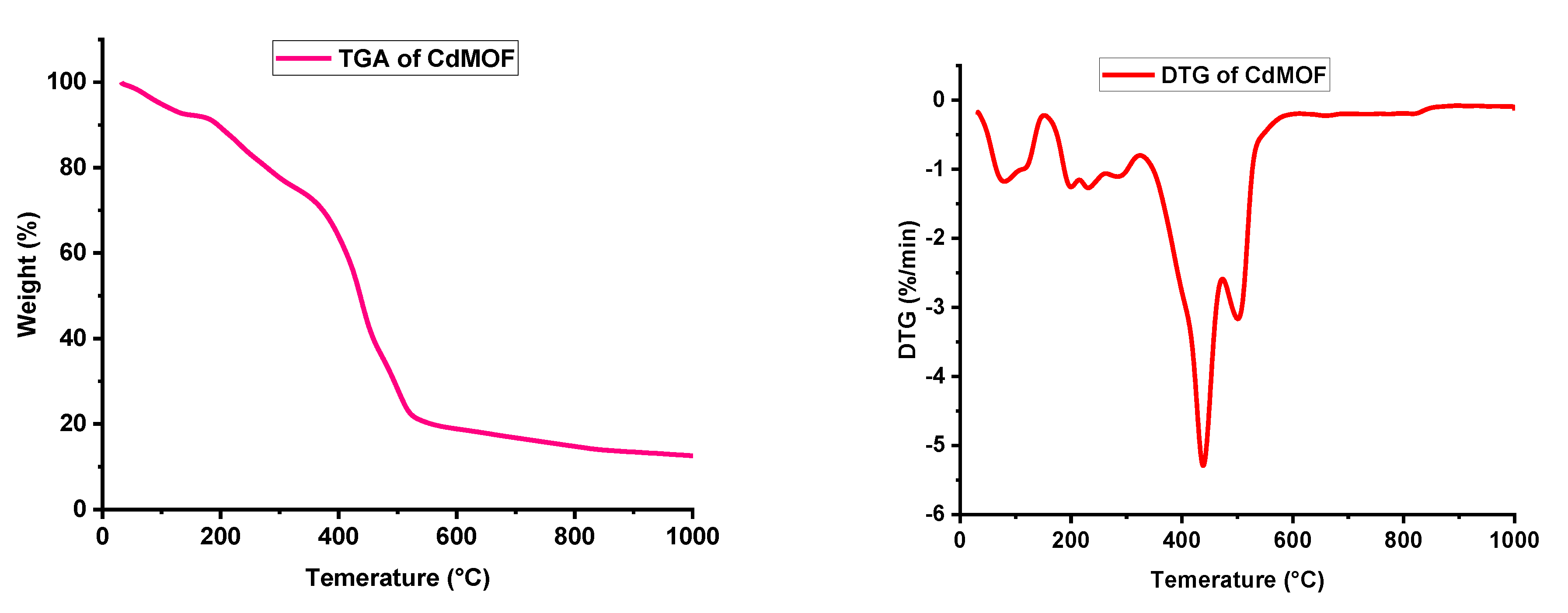
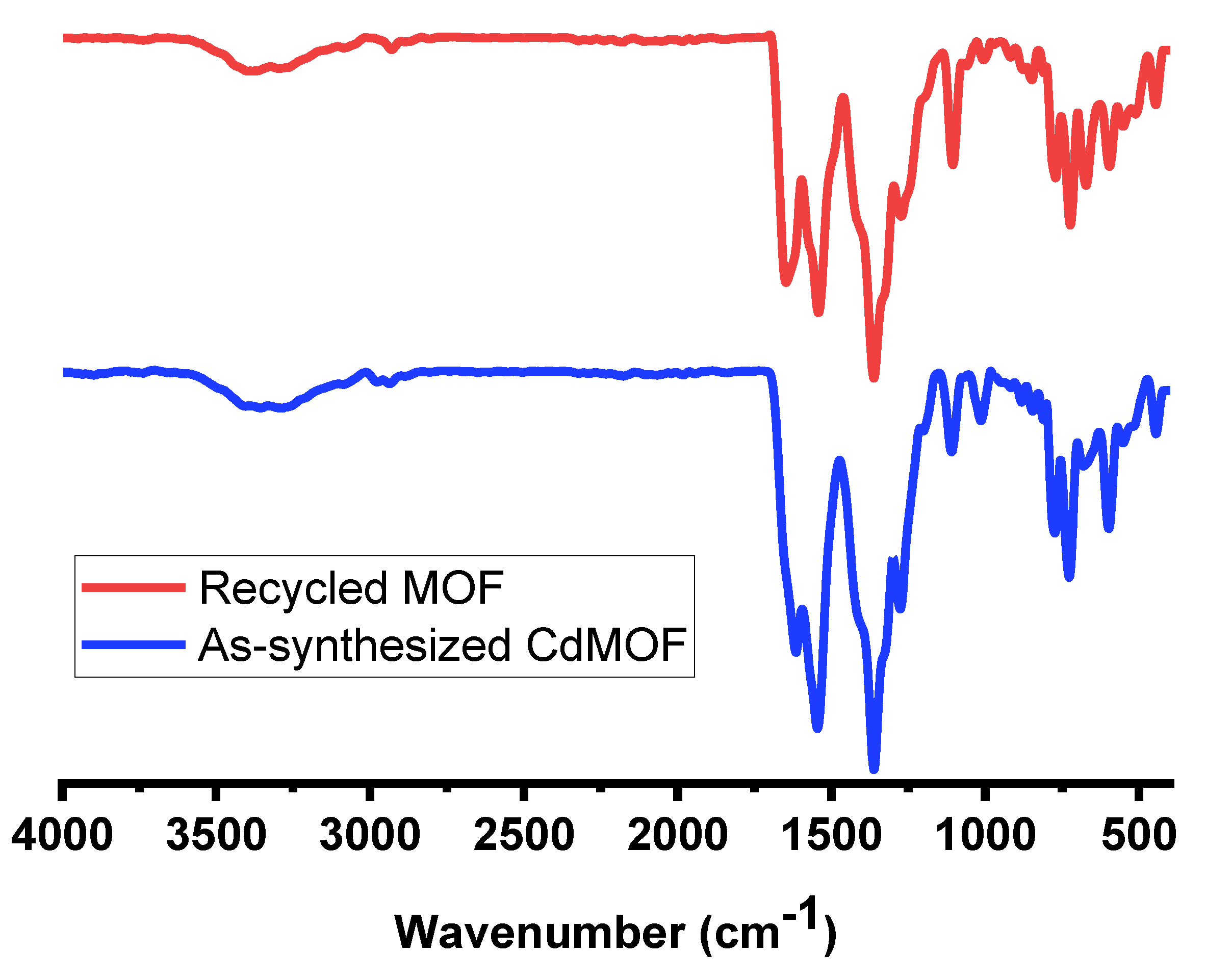
3.1. Characterizations of CdMOF
3.2. Catalytic Activity of CdMOF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [Green Version]
- Pal, T.K.; De, D.; Bharadwaj, P.K. Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coord. Chem. Rev. 2020, 408, 213173. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Sabouni, R.; Husseini, G.A. Biomedical applications of metal-organic frameworks for disease diagnosis and drug delivery: A review. Nanomaterials 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Basu, O.; Nasani, R.; Das, S.K. Evolution of metal organic frameworks as electrocatalysts for water oxidation. Chem. Commun. 2020, 56, 11735–11748. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Gaoke, Z.; Van Hecke, K.; Verpoort, F. Direct synthesis of the 2D copper(II) 5-prop-2-ynoxyisophthalate MOF: Comment on “surface functionalization of porous coordination nanocages via click chemistry and their application in drug delivery”. Adv. Mater. 2019, 31, 1801399. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Hoskins, B.F.; Robson, R. Two interpenetrating 3D networks which generate spacious sealed-off compartments enclosing of the order of 20 solvent molecules in the structures of Zn(CN)(NO3)(tpt)2/3.Solv (tpt = 2,4,6-tri(4-pyridyl)-1,3,5-triazine, solv = ~3/4C2H2CL4.3/4CH3OH or ~3/2CHCl3.1/3CH3OH). J. Am. Chem. Soc. 1995, 117, 5385–5386. [Google Scholar]
- Ezugwu, C.I.; Mousavi, B.; Asrafa, M.A.; Mehta, A.; Vardhan, H.; Verpoort, F. An n-heterocyclic carbene based MOF catalyst for Sonogashira cross-coupling reaction. Catal. Sci. Technol. 2016, 6, 2050–2054. [Google Scholar] [CrossRef]
- Ahmad, N.; Chughtai, A.H.; Younus, H.A.; Verpoort, F. Discrete metal-carboxylate self-assembled cages: Design, synthesis and applications. Coord. Chem. Rev. 2014, 280, 1–27. [Google Scholar] [CrossRef]
- Ezugwu, C.I.; Kabir, N.A.; Yusubov, M.; Verpoort, F. Metal–organic frameworks containing n-heterocyclic carbenes and their precursors. Coord. Chem. Rev. 2016, 307, 188–210. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Go, Y.B.; Ko, N.; Park, Y.K.; Uribe-Romo, F.J.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Isoreticular expansion of metal-organic frameworks with triangular and square building units and the lowest calculated density for porous crystals. Inorg. Chem. 2011, 50, 9147–9152. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.B.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yaghi, O.M. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Yang, A.-F.; Cao, C.-S.; Zhao, B. Applications of MOFs: Recent advances in photocatalytic hydrogen production from water. Coord. Chem. Rev. 2019, 390, 50–75. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Pang, H. A review of mofs and their composites-based photocatalysts: Synthesis and applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Manna, K.; Zhang, T.; Lin, W. Postsynthetic metalation of bipyridyl-containing metal–organic frameworks for highly efficient catalytic organic transformations. J. Am. Chem. Soc. 2014, 136, 6566–6569. [Google Scholar] [CrossRef] [PubMed]
- Forster, P.M.; Cheetham, A.K. Hybrid inorganic–organic solids: An emerging class of nanoporous catalysts. Top. Catal. 2003, 24, 79–86. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Platero Prats, A.E.; de la Peña-O'Shea, V.A.; Iglesias, M.; Snejko, N.; Monge, Á.; Gutiérrez-Puebla, E. Heterogeneous catalysis with alkaline-earth metal-based mofs: A green calcium catalyst. ChemCatChem 2010, 2, 147–149. [Google Scholar] [CrossRef]
- Liao, T.B.; Ling, Y.; Chen, Z.X.; Zhou, Y.M.; Weng, L.H. A rutile-type porous zinc(II)-phosphonocarboxylate framework: Local proton transfer and size-selected catalysis. Chem. Commun. 2010, 46, 1100–1102. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276. [Google Scholar] [CrossRef] [Green Version]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Singha, D.K.; Majee, P.; Mondal, S.K.; Mahata, P. A luminescent cadmium based MOF as selective and sensitive iodide sensor in aqueous medium. J. Photochem. Photobiol. A Chem. 2018, 356, 389–396. [Google Scholar] [CrossRef]
- Xu, X.-D.; Liang, Y.; Mensah, A.; Li, J.-F.; Zhou, L.; Chen, L.-Z.; Wang, F.-M. Synthesis, structures and fluorescence properties of two novel cadmium MOFs based on a tetraphenylethene(TPE)-core ligand. ChemistrySelect 2019, 4, 12380–12385. [Google Scholar] [CrossRef]
- Parmar, B.; Patel, P.; Kureshy, R.I.; Khan, N.H.; Suresh, E. Sustainable heterogeneous catalysts for CO2 utilization by using dual ligand Zn(II) /Cd(II) metal-organic frameworks. Chemistry 2018, 24, 15831–15839. [Google Scholar] [CrossRef] [PubMed]
- Rachuri, Y.; Kurisingal, J.F.; Chitumalla, R.K.; Vuppala, S.; Gu, Y.; Jang, J.; Choe, Y.; Suresh, E.; Park, D.-W. Adenine-based Zn(II)/Cd(II) metal–organic frameworks as efficient heterogeneous catalysts for facile co2 fixation into cyclic carbonates: A DFT-supported study of the reaction mechanism. Inorg. Chem. 2019, 58, 11389–11403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.L.; Li, J.; Ren, Y.; Jiang, H. A ni(salen)-based metal–organic framework: Synthesis, structure, and catalytic performance for CO2 cycloaddition with epoxides. Eur. J. Inorg. Chem. 2017, 2017, 4982–4989. [Google Scholar] [CrossRef]
- Zou, Y.; Park, M.; Hong, S.; Lah, M.S. A designed metal-organic framework based on a metal-organic polyhedron. Chem. Commun. 2008, 2340–2342. [Google Scholar] [CrossRef]
- Zou, Y.; Yu, C.; Li, Y.; Lah, M.S. A 3-dimensional coordination polymer with a rare lonsdaleite topology constructed from a tetrahedral ligand. CrystEngComm 2012, 14, 7174–7177. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Van Hecke, K.; Khattak, Z.A.K.; Gaoke, Z.; Danish, M.; Verpoort, F. Synthesis of 2D MOF having potential for efficient dye adsorption and catalytic applications. Catal. Sci. Technol. 2018, 8, 4010–4017. [Google Scholar] [CrossRef]
- Wang, G.-Y.; Song, C.; Kong, D.-M.; Ruan, W.-J.; Chang, Z.; Li, Y. Two luminescent metal-organic frameworks for the sensing of nitroaromatic explosives and DNA strands. J. Mater. Chem. A 2014, 2, 2213–2220. [Google Scholar] [CrossRef]
- Yang, J.-X.; Qin, Y.-Y.; Zhang, X.; Yao, Y.-G. Construction of three new metal-organic frameworks with distinct SBUs: Trinuclear {Cd3(COO)6} clusters, inorganic -Cd-O-Cd- chains, and heterometallic trinuclear {Cd2ba(COO)4} clusters. J. Coord. Chem. 2016, 69, 1568–1576. [Google Scholar] [CrossRef]
- Fu, H.-R.; Wang, F.; Zhang, J. The photoluminescence and gas sorption properties of three Cd(II) mofs based on 1,3,5-benzenetribenzoate with –NH2 or –OH groups. Dalton Trans. 2014, 43, 4668–4673. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wu, T.; Bu, X.; Feng, P. A twelve-connected porous framework built from rare linear cadmium tricarboxylate pentamer. Dalton Trans. 2012, 41, 3620–3622. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Z.-X.; Chen, W.-B.; Yang, M.; Dong, W. Syntheses, structures and photochromic properties of two tetrazolylazo-based K+ and Cd2+ complexes. J. Coord. Chem. 2014, 67, 3243–3251. [Google Scholar] [CrossRef]
- Zhu, X.-H.; Jiang, D.-Y.; Cheng, X.-C.; Li, D.-H.; Du, W.-G. A Mn(II) complex with an amide-containing ligand: Synthesis, structural characterization, and magnetic properties. Z. Für Nat. B 2019, 74, 409–414. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. Crystalexplorer: A program for hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Ashfaq, M.; Tahir, M.N.; Kuznetsov, A.E.; Mirza, S.H.; Khalid, M.; Ali, A. DFT and single crystal analysis of the pyrimethamine-based novel co-crystal salt: 2,4-diamino-5-(4-chloro-phenyl)-6-ethylpyrimidin-1-ium:4-hydroxybenzoate:Methanol:Hydrate (1:1:1:1) (DEHMH). J. Mol. Struct. 2020, 1199, 127041. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Ashfaq, M.; Bogdanov, G.; Glebov, V.; Ali, A.; Tahir, M.N.; Abdullah, S. Single crystal investigation, hirshfeld surface analysis and DFT exploration of the pyrimethamine-based novel organic salt: 2, 4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidin-1-ium 3-carboxybenzoate hydrate (1:1:1). J. Mol. Struct. 2021, 1224, 129309. [Google Scholar] [CrossRef]
- Ashfaq, M.; Ali, A.; Kuznetsov, A.E.; Tahir, M.N.; Khalid, M. DFT and single-crystal investigation of the pyrimethamine-based novel co-crystal salt: 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidin-1-ium-4-methylbenzoate hydrate (1:1:1) (DEMH). J. Mol. Struct. 2020, 1228, 129445. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Torabi, V.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Sonication-assisted synthesis of new schiff bases derived from 3-ethoxysalicylaldehyde: Crystal structure determination, hirshfeld surface analysis, theoretical calculations and spectroscopic studies. J. Mol. Struct. 2021, 1243, 130782. [Google Scholar] [CrossRef]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Fallah-Mehrjardi, M.; Torabi, V.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Ultrasound-based synthesis, SC-XRD, NMR, DFT, HSA of new Schiff bases derived from 2-aminopyridine: Experimental and theoretical studies. J. Mol. Struct. 2021, 1233, 130105. [Google Scholar] [CrossRef]
- Ashfaq, M.; Bogdanov, G.; Ali, A.; Tahir, M.N.; Abdullah, S. Pyrimethamine-based novel co-crystal salt: Synthesis, single-crystal investigation, hirshfeld surface analysis and DFT inspection of the 2,4-diamino-5-(4-chlorophenyl)-6-ethylpyrimidin-1-ium 2,4-dichlorobenzoate (1:1) (DECB). J. Mol. Struct. 2021, 1235, 130215. [Google Scholar] [CrossRef]
- Kargar, H.; Bazrafshan, M.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.A.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Synthesis, characterization, crystal structures, Hirshfeld surface analysis, DFT computational studies and catalytic activity of novel oxovanadium and dioxomolybdenum complexes with ono tridentate schiff base ligand. Polyhedron 2021, 202, 115194. [Google Scholar] [CrossRef]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Munawar, K.S.; Ashfaq, M.; Tahir, M.N. Titanium(IV) complex containing ono-tridentate schiff base ligand: Synthesis, crystal structure determination, hirshfeld surface analysis, spectral characterization, theoretical and computational studies. J. Mol. Struct. 2021, 1241, 130653. [Google Scholar] [CrossRef]
- Raza, H.; Yildiz, I.; Yasmeen, F.; Munawar, K.S.; Ashfaq, M.; Abbas, M.; Ahmed, M.; Younus, H.A.; Zhang, S.; Ahmad, N. Synthesis of a 2D copper(II)-carboxylate framework having ultrafast adsorption of organic dyes. J. Colloid Interface Sci. 2021, 602, 43–54. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Visualisation and characterisation of voids in crystalline materials. CrystEngComm 2011, 13, 1804–1813. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Tahir, M.N.; Imran, M.; Ashfaq, M.; Hussain, R.; Assiri, M.A.; Khan, I. Synthesis of diaminopyrimidine sulfonate derivatives and exploration of their structural and quantum chemical insights via SC-XRD and the DFT approach. ACS Omega 2021, 6, 7047–7057. [Google Scholar] [CrossRef]
- Amor, S.B.; Jacquet, M.; Fioux, P.; Nardin, M. XPS characterisation of plasma treated and zinc oxide coated pet. Appl. Surf. Sci. 2009, 255, 5052–5061. [Google Scholar] [CrossRef]
- Miralda, C.M.; Macias, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate. ACS Catal. 2012, 2, 180–183. [Google Scholar] [CrossRef]
- Mousavi, B.; Chaemchuen, S.; Moosavi, B.; Luo, Z.; Gholampour, N.; Verpoort, F. Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates. New J. Chem. 2016, 40, 5170–5176. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal−organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.-B.; Cao, R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates. Coord. Chem. Rev. 2019, 378, 32–65. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Jose, T.; Babu, R.; Hwang, G.-Y.; Kathalikkattil, A.C.; Kim, D.-W.; Park, D.-W. A room temperature synthesizable and environmental friendly heterogeneous ZIF-67 catalyst for the solvent less and co-catalyst free synthesis of cyclic carbonates. Appl. Catal. B Environ. 2016, 182, 562–569. [Google Scholar] [CrossRef]
- Macias, E.E.; Ratnasamy, P.; Carreon, M.A. Catalytic activity of metal organic framework Cu3(BTC)2 in the cycloaddition of CO2 to epichlorohydrin reaction. Catal. Today 2012, 198, 215–218. [Google Scholar] [CrossRef]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.M.; Hwang, G.-Y.; Jeong, G.S.; Kim, M.-I.; Kim, D.-W.; Park, D.-W. A solid solution zeolitic imidazolate framework as a room temperature efficient catalyst for the chemical fixation of CO2. Green Chem. 2016, 18, 6349–6356. [Google Scholar] [CrossRef]
- Ji, H.; Naveen, K.; Lee, W.; Kim, T.S.; Kim, D.; Cho, D.-H. Pyridinium-functionalized ionic metal–organic frameworks designed as bifunctional catalysts for CO2 fixation into cyclic carbonates. ACS Appl. Mater. Interfaces 2020, 12, 24868–24876. [Google Scholar] [CrossRef]
- Wan, Y.-L.; Wang, L.; Wen, L. Amide-functionalized organic cationic polymers toward enhanced catalytic performance for conversion of co2 into cyclic carbonates. J. CO2 Util. 2022, 64, 102174. [Google Scholar] [CrossRef]
- Hao, Y.; Yan, X.; Chang, T.; Liu, X.; Kang, L.; Zhu, Z.; Panchal, B.; Qin, S. Hydroxyl-anchored covalent organic crown-based polymers for co2 fixation into cyclic carbonates under mild conditions. Sustain. Energy Fuels 2022, 6, 121–127. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Yuan, J.; Xie, H.; Gao, C.; Zhao, T.; Zheng, Q. Agile construction of porous organic frameworks pending carboxylic acids and imidazolium-based ionic liquids for the efficient fixation of CO2 to cyclic carbonates. ACS Sustain. Chem. Eng. 2022, 10, 7990–8001. [Google Scholar] [CrossRef]
- Yuan, G.; Lei, Y.; Meng, X.; Ge, B.; Ye, Y.; Song, X.; Liang, Z. Metal-assisted synthesis of salen-based porous organic polymer for highly efficient fixation of CO2 into cyclic carbonates. Inorg. Chem. Front. 2022, 9, 1208–1216. [Google Scholar] [CrossRef]







| Catalyst/Co-Catalyst | Catalyst Loading (mol%) | T (°C) | P (bar) | t (h) | Conversion (%) | Selectivity (%) | Reference |
|---|---|---|---|---|---|---|---|
| Control (without catalyst) | - | 120 | 8 | 12 | 0 | 0 | This work |
| 3D CdMOF | 0.67 | 120 | 8 | 12 | 83 | 100 | This work |
| 3D CdMOF a | 0.67 | 120 | 8 | 12 | 82 | 100 | This work |
| Cd(NO3)2·4H2O | 0.60 | 80 | 4 | 8 | 21 | 98 | [25] |
| 3D PNU-22 (a CdMOF) b | 0.60 | 80 | 4 | 8 | 32 | >99 | [25] |
| 3D PNU-22/TBAB c | 0.60 f | 80 | 4 | 8 | 85 | >99 | [25] |
| 2D {[Cd(CHDC)(L)]·H2O}n d/ TBAB | 1.8 f | 80 | 10 | 18 | 89 | - | [24] |
| 3D [Cd2(Ni(salen))(DMF)3]·4DMF·7H2O e/ TBAB | 0.50 f | 80 | 10 | 12 | 99 | - | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattak, Z.A.K.; Ahmad, N.; Younus, H.A.; Ullah, H.; Yu, B.; Munawar, K.S.; Ashfaq, M.; Ali, S.; Shahadat, H.M.; Verpoort, F. Synthesis of 3D Cadmium(II)-Carboxylate Framework Having Potential for Co-Catalyst Free CO2 Fixation to Cyclic Carbonates. Inorganics 2022, 10, 162. https://doi.org/10.3390/inorganics10100162
Khattak ZAK, Ahmad N, Younus HA, Ullah H, Yu B, Munawar KS, Ashfaq M, Ali S, Shahadat HM, Verpoort F. Synthesis of 3D Cadmium(II)-Carboxylate Framework Having Potential for Co-Catalyst Free CO2 Fixation to Cyclic Carbonates. Inorganics. 2022; 10(10):162. https://doi.org/10.3390/inorganics10100162
Chicago/Turabian StyleKhattak, Zafar A. K., Nazir Ahmad, Hussein A. Younus, Habib Ullah, Baoyi Yu, Khurram S. Munawar, Muhammad Ashfaq, Sher Ali, Hossain M. Shahadat, and Francis Verpoort. 2022. "Synthesis of 3D Cadmium(II)-Carboxylate Framework Having Potential for Co-Catalyst Free CO2 Fixation to Cyclic Carbonates" Inorganics 10, no. 10: 162. https://doi.org/10.3390/inorganics10100162









