Influence of the Lithium Cation Desolvation Process at the Electrolyte/Electrode Interface on the Performance of Lithium Batteries
Abstract
:1. Introduction
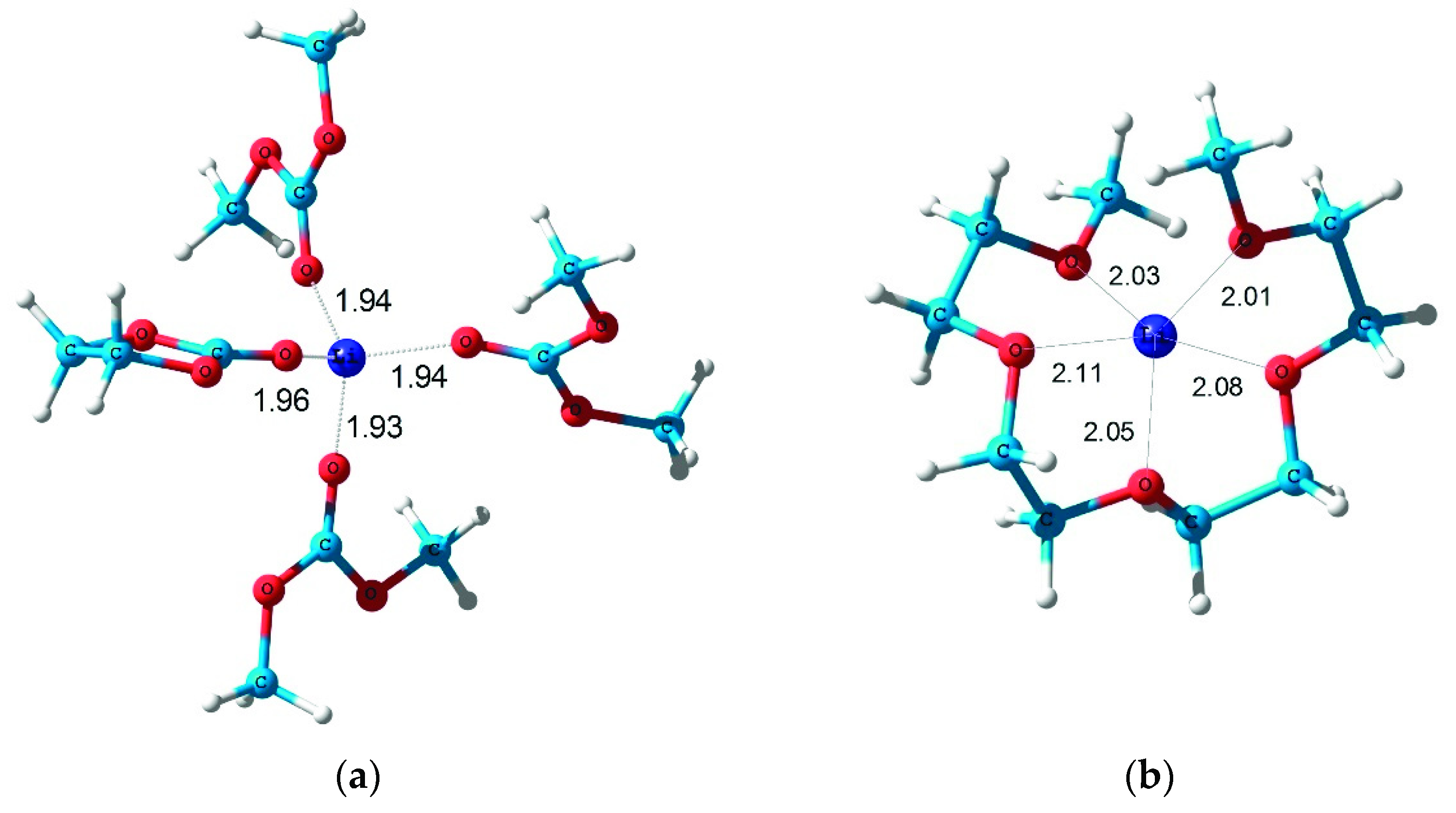
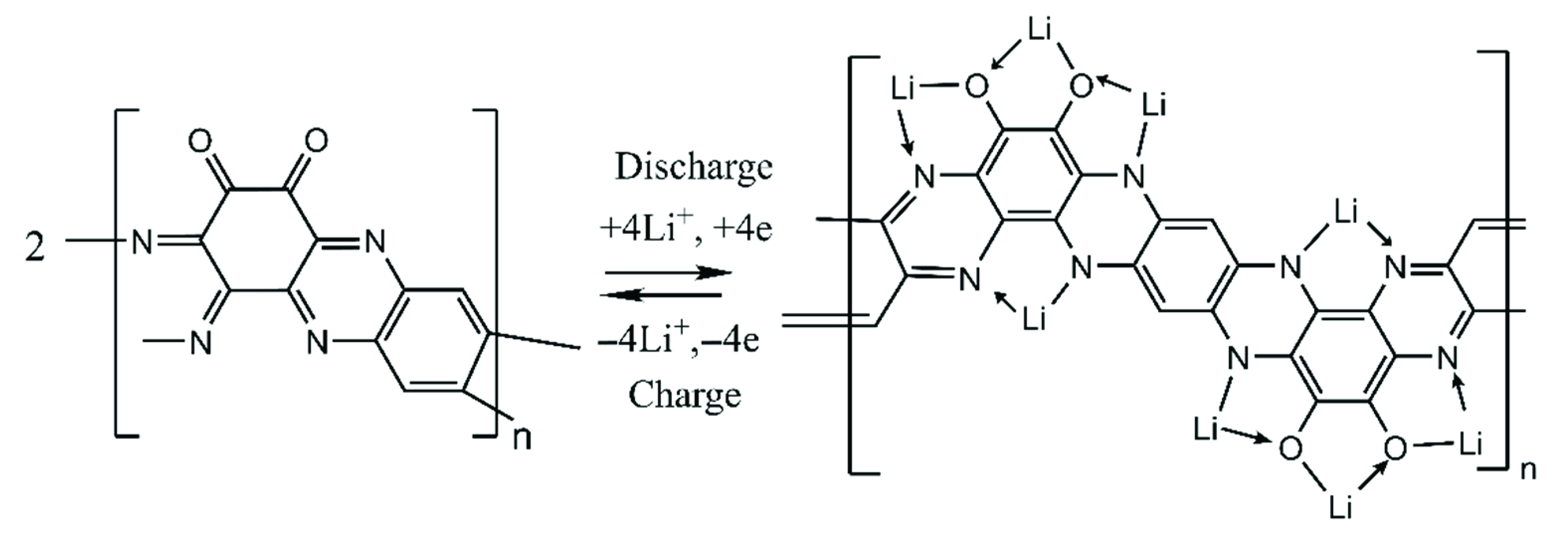
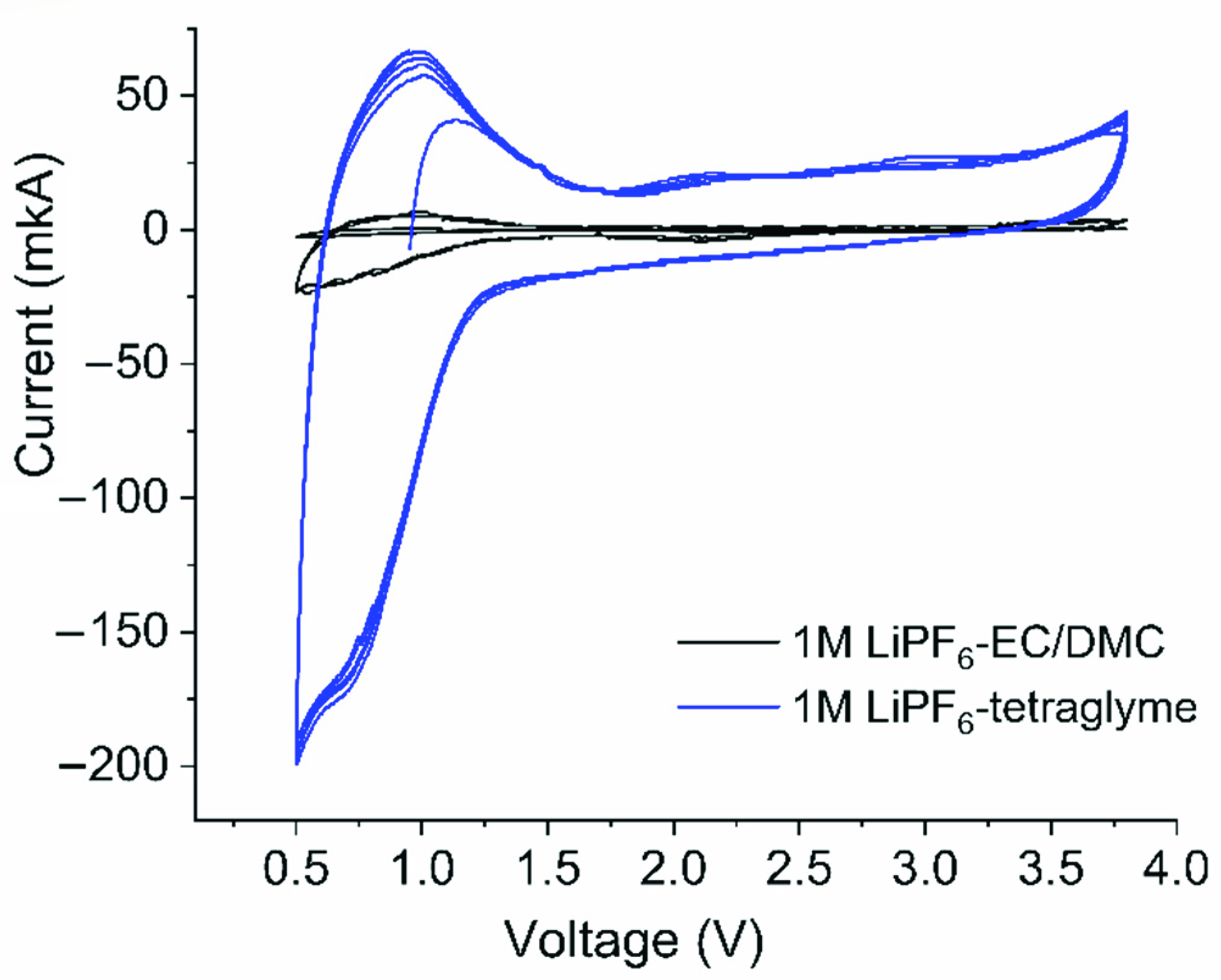
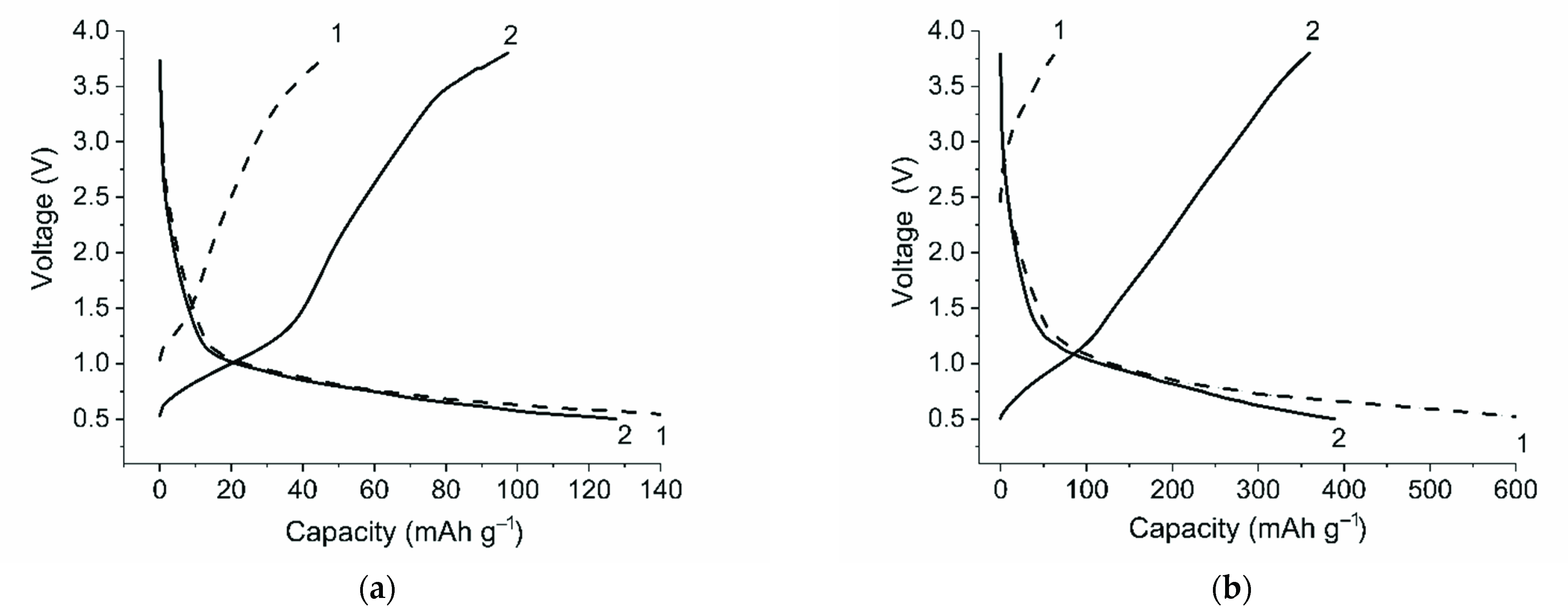
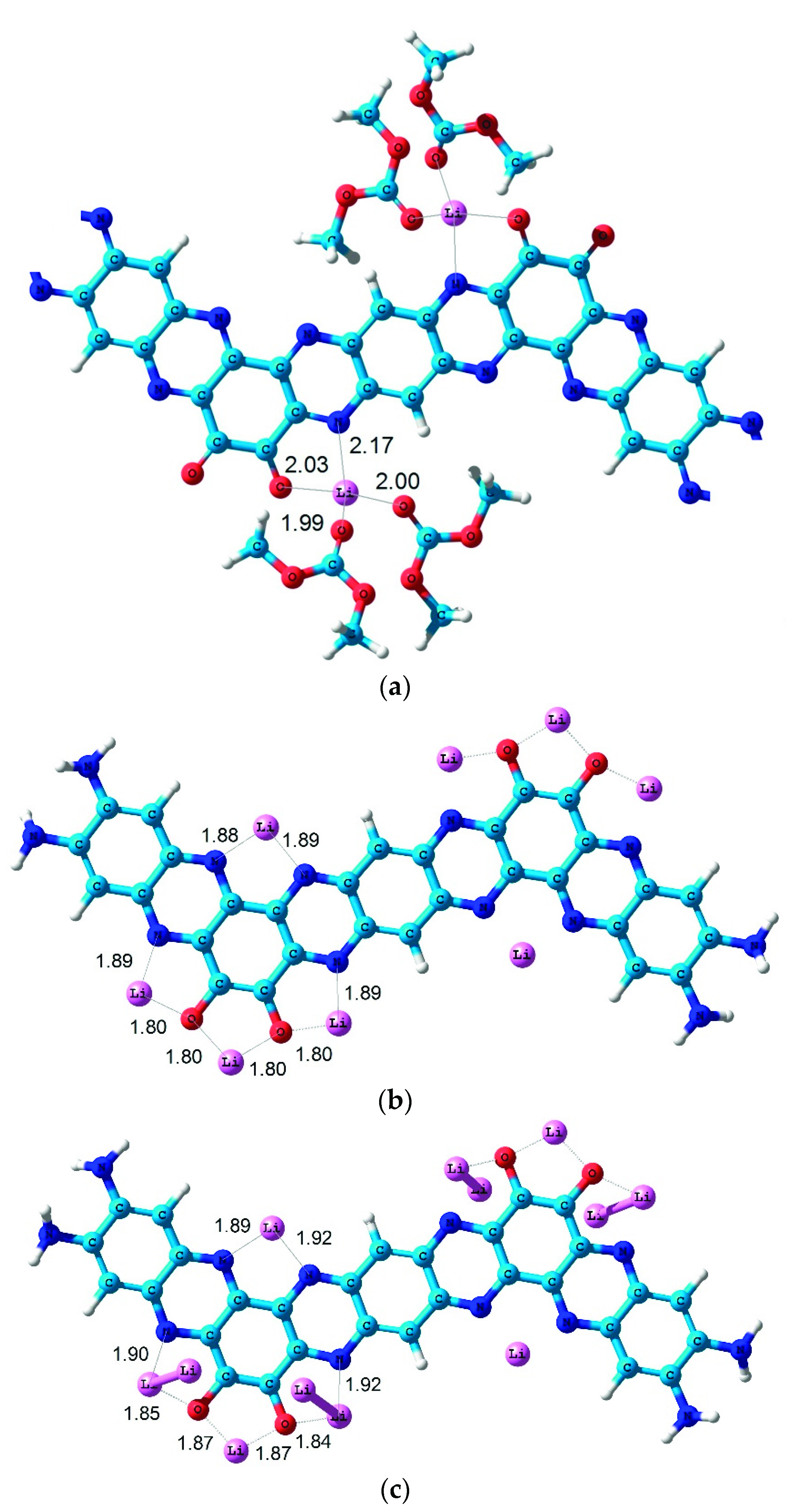
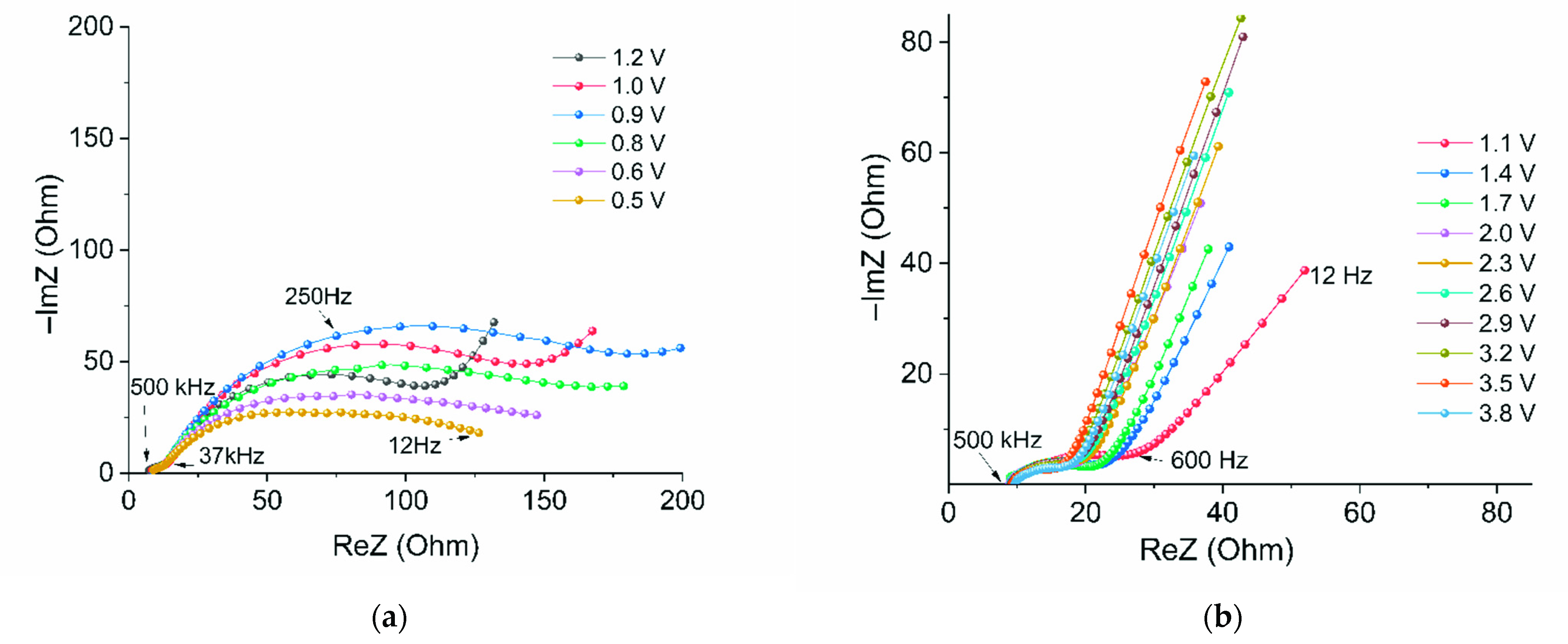
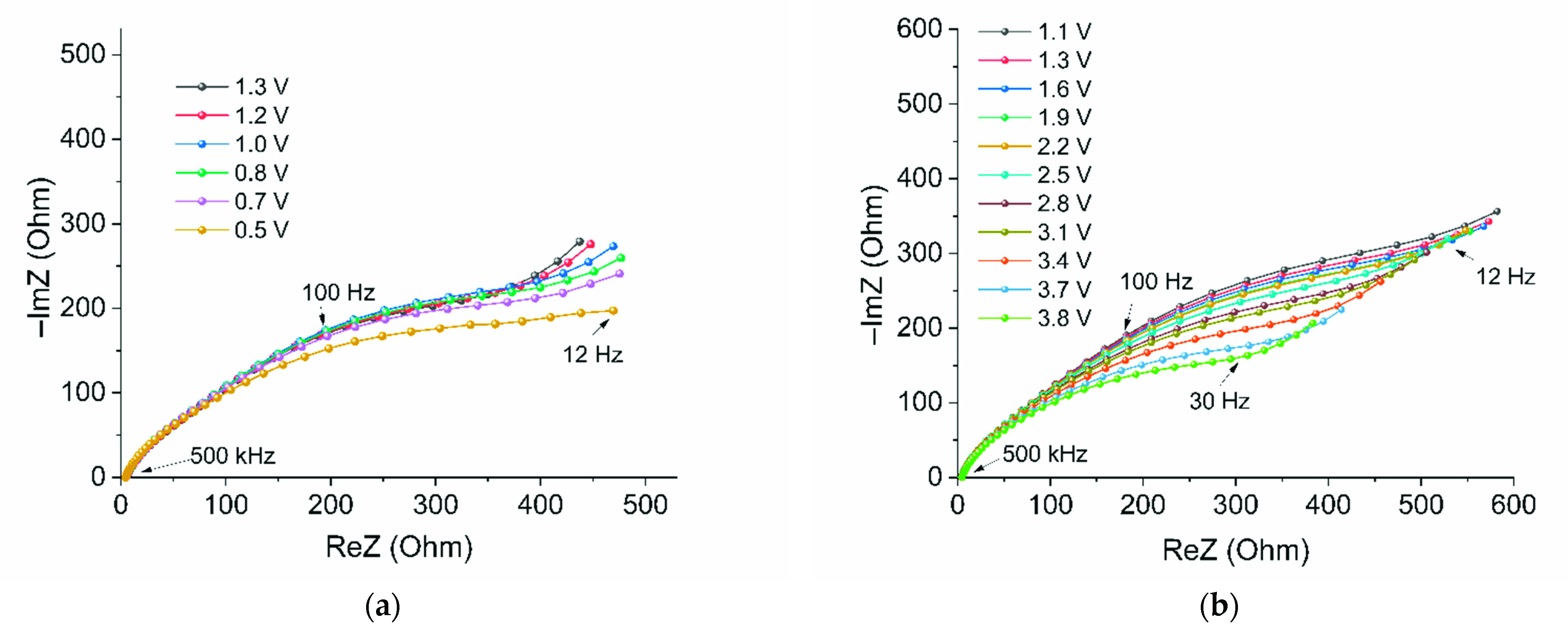
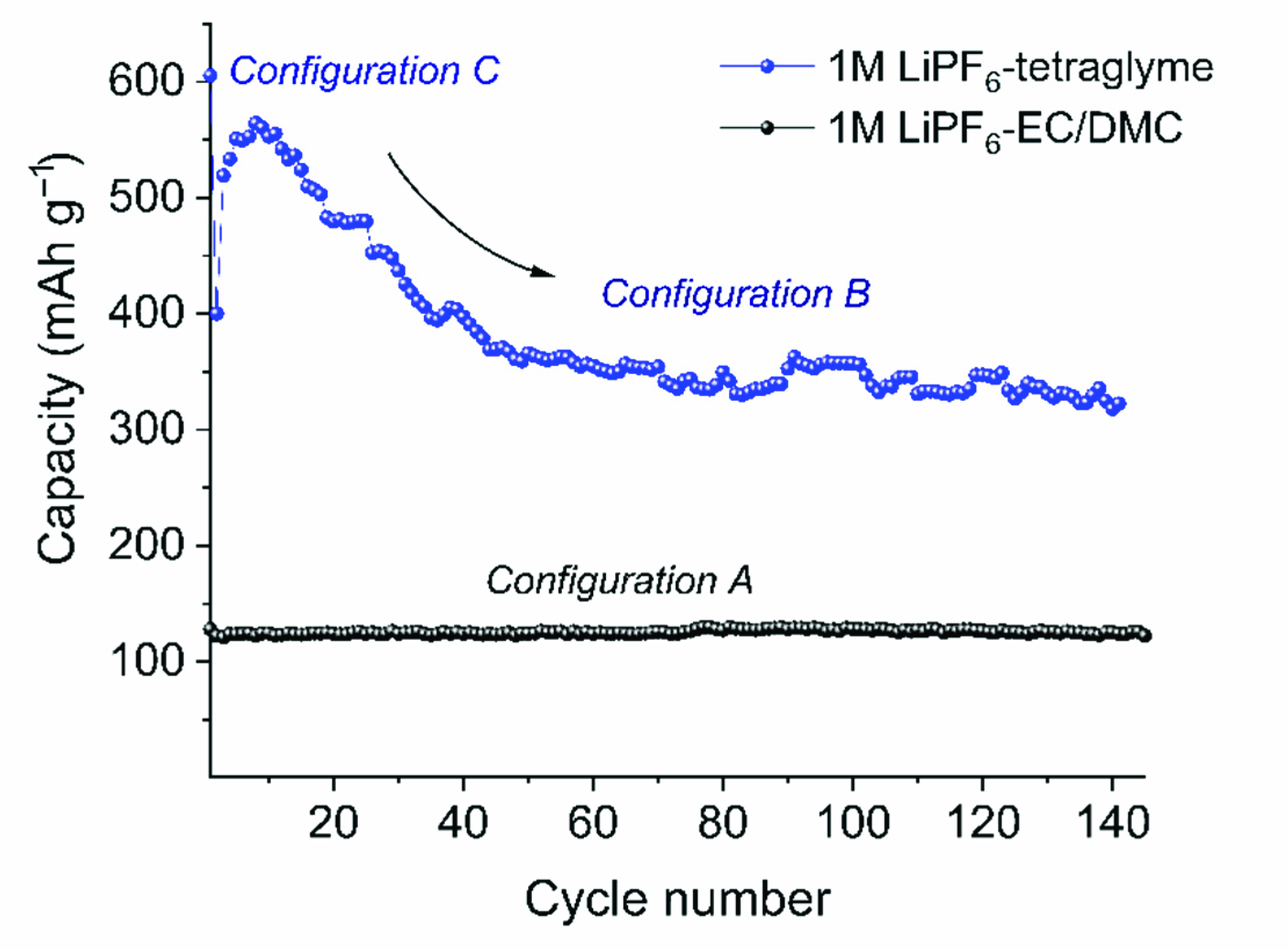
2. Results and Discussions
3. Conclusions
4. Materials and Methods
4.1. Components of Electrolytes and Electrodes
4.2. Li-Battery Fabrication
4.3. Li-Battery Testing
4.4. Quantum Chemical Modeling
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shea, J.J.; Luo, C. Organic Electrode Materials for Metal Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5361–5380. [Google Scholar] [CrossRef] [PubMed]
- Friebe, C.; Lex-Balducci, A.; Schubert, U.S. Sustainable Energy Storage: Recent Trends and Developments toward Fully Organic Batteries. ChemSusChem 2019, 12, 4093–4115. [Google Scholar] [CrossRef] [Green Version]
- Oubaha, H.; Gohy, J.; Melinte, S. Carbonyl-Based Π-Conjugated Materials: From Synthesis to Applications in Lithium-Ion Batteries. ChemPlusChem 2019, 84, 1179–1214. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, M.; Lei, Y. Organic Materials for Rechargeable Sodium-Ion Batteries. Mater. Today 2018, 21, 60–78. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, G.; Ku, K.; Yoon, K.; Jung, S.-K.; Lim, H.-D.; Kang, K. Recent Progress in Organic Electrodes for Li and Na Rechargeable Batteries. Adv. Mater. 2018, 30, 1704682. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J. Prospects of Organic Electrode Materials for Practical Lithium Batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Lu, B. Plum Pudding Model Inspired KVPO4F@3DC as High-Voltage and Hyperstable Cathode for Potassium Ion Batteries. Sci. Bull. 2020, 65, 1242–1251. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, H.; Zhang, Y.; Xiao, B.; Wang, L.; Li, M.; Wu, Y.; Sa, B.; Liao, H.; Zhang, L.; et al. Boosting the Potassium-Ion Storage Performance Enabled by Engineering of Hierarchical MoSSe Nanosheets Modified with Carbon on Porous Carbon Sphere. Sci. Bull. 2022, 67, 933–945. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Jia, X.; Li, W.; Zhang, Q.; Fan, L.; Ding, H.; Yang, H.; Yu, X.; Li, X.; et al. Graphene Armored with a Crystal Carbon Shell for Ultrahigh-Performance Potassium Ion Batteries and Aluminum Batteries. ACS Nano 2019, 13, 10631–10642. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Ding, H.; Chen, S.; Yu, X.; Lu, B. Carbon Nanoscrolls for Aluminum Battery. ACS Nano 2018, 12, 8456–8466. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in Electrolytes for Rechargeable Li-Based Batteries and Beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Xu, K. Li-Ion Battery Electrolytes. Nat. Energy 2021, 6, 763. [Google Scholar] [CrossRef]
- Flamme, B.; Rodriguez Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Guidelines to Design Organic Electrolytes for Lithium-Ion Batteries: Environmental Impact, Physicochemical and Electrochemical Properties. Green Chem. 2017, 19, 1828–1849. [Google Scholar] [CrossRef]
- Baymuratova, G.R.; Mumyatov, A.V.; Kapaev, R.R.; Troshin, P.A.; Yarmolenko, O.V. The Effect of Electrolyte Composition on the Parameters of Batteries of the Polyimide–Lithium System. Russ. J. Electrochem. 2021, 57, 725–732. [Google Scholar] [CrossRef]
- Aguilera, L.; Xiong, S.; Scheers, J.; Matic, A. A Structural Study of LiTFSI–Tetraglyme Mixtures: From Diluted Solutions to Solvated Ionic Liquids. J. Mol. Liq. 2015, 210, 238–242. [Google Scholar] [CrossRef]
- Tobishima, S.; Morimoto, H.; Aoki, M.; Saito, Y.; Inose, T.; Fukumoto, T.; Kuryu, T. Glyme-Based Nonaqueous Electrolytes for Rechargeable Lithium Cells. Electrochim. Acta 2004, 49, 979–987. [Google Scholar] [CrossRef]
- Henderson, W.A. Glyme−Lithium Salt Phase Behavior. J. Phys. Chem. B 2006, 110, 13177–13183. [Google Scholar] [CrossRef]
- Henderson, W.A.; Brooks, N.R.; Young, V.G. Tetraglyme−Li+ Cation Solvate Structures: Models for Amorphous Concentrated Liquid and Polymer Electrolytes (II). Chem. Mater. 2003, 15, 4685–4690. [Google Scholar] [CrossRef]
- Di Lecce, D.; Marangon, V.; Jung, H.-G.; Tominaga, Y.; Greenbaum, S.; Hassoun, J. Glyme-Based Electrolytes: Suitable Solutions for next-Generation Lithium Batteries. Green Chem. 2022, 24, 1021–1048. [Google Scholar] [CrossRef]
- Baymuratova, G.R.; Khatmullina, K.G.; Yakuschenko, I.K.; Tulibaeva, G.Z.; Savinykh, T.A.; Troshin, P.A.; Shestakov, A.F.; Yarmolenko, O.V. Synthesis and Investigation of a New Organic Electrode Material Based on Condensation Product of Triquinoyl with 1,2,4,5-Tetraaminobenzene. J. Electroanal. Chem. 2021, 889, 115234. [Google Scholar] [CrossRef]
- Ramezankhani, V.; Yakuschenko, I.K.; Vasilyev, S.; Savinykh, T.A.; Mumyatov, A.V.; Zhidkov, I.S.; Shchurik, E.V.; Kurmaev, E.Z.; Shestakov, A.F.; Troshin, P.A. High-Capacity Polymer Electrodes for Potassium Batteries. J. Mater. Chem. A 2022, 10, 3044–3050. [Google Scholar] [CrossRef]
- Baymuratova, G.R.; Khatmullina, K.G.; Tulibaeva, G.Z.; Yakushenko, I.K.; Troshin, P.A.; Yarmolenko, O.V. Gelled Tetraglyme-Based Electrolyte for Organic Electrode Materials. Russ. Chem. Bull. Int. Ed. 2022, in press. [Google Scholar]
- Wang, H.; He, J.; Liu, J.; Qi, S.; Wu, M.; Wen, J.; Chen, Y.; Feng, Y.; Ma, J. Electrolytes Enriched by Crown Ethers for Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2002578. [Google Scholar] [CrossRef]
- Ignatova, A.A.; Yarmolenko, O.V.; Tulibaeva, G.Z.; Shestakov, A.F.; Fateev, S.A. Influence of 15-Crown-5 Additive to a Liquid Electrolyte on the Performance of Li/CFx–Systems at Temperatures up to −50 °C. J. Power Sources 2016, 309, 116–121. [Google Scholar] [CrossRef]
- Oral, I.; Tamm, S.; Herrmann, C.; Abetz, V. Lithium Selectivity of Crown Ethers: The Effect of Heteroatoms and Cavity Size. Sep. Purif. Technol. 2022, 294, 121142. [Google Scholar] [CrossRef]
- Borodin, O.; Olguin, M.; Ganesh, P.; Kent, P.R.C.; Allen, J.L.; Henderson, W.A. Competitive Lithium Solvation of Linear and Cyclic Carbonates from Quantum Chemistry. Phys. Chem. Chem. Phys. 2016, 18, 164–175. [Google Scholar] [CrossRef]
- Sun, Y.; Hamada, I. Insight into the Solvation Structure of Tetraglyme-Based Electrolytes via First-Principles Molecular Dynamics Simulation. J. Phys. Chem. B 2018, 122, 10014–10022. [Google Scholar] [CrossRef]
- Morales, D.; Ruther, R.E.; Nanda, J.; Greenbaum, S. Ion Transport and Association Study of Glyme-Based Electrolytes with Lithium and Sodium Salts. Electrochim. Acta 2019, 304, 239–245. [Google Scholar] [CrossRef]
- Kartal, M.; Alp, A.; Akbulut, H. Electrical Conductivity, Viscosity and Thermal Properties of TEGDME-Based Composite Electrolytes for Lithium-Air Batteries. Acta Phys. Pol. A 2016, 129, 816–818. [Google Scholar] [CrossRef]
- Laoire, C.Ó.; Mukerjee, S.; Plichta, E.J.; Hendrickson, M.A.; Abraham, K.M. Rechargeable Lithium/TEGDME-LiPF6∕O2 Battery. J. Electrochem. Soc. 2011, 158, A302. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theoret. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Laikov, D.N. Fast Evaluation of Density Functional Exchange-Correlation Terms Using the Expansion of the Electron Density in Auxiliary Basis Sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarmolenko, O.V.; Baymuratova, G.R.; Khatmullina, K.G.; Tulibayeva, G.Z.; Yudina, A.V.; Savinykh, T.A.; Yakushchenko, I.K.; Troshin, P.A.; Shestakov, A.F. Influence of the Lithium Cation Desolvation Process at the Electrolyte/Electrode Interface on the Performance of Lithium Batteries. Inorganics 2022, 10, 176. https://doi.org/10.3390/inorganics10110176
Yarmolenko OV, Baymuratova GR, Khatmullina KG, Tulibayeva GZ, Yudina AV, Savinykh TA, Yakushchenko IK, Troshin PA, Shestakov AF. Influence of the Lithium Cation Desolvation Process at the Electrolyte/Electrode Interface on the Performance of Lithium Batteries. Inorganics. 2022; 10(11):176. https://doi.org/10.3390/inorganics10110176
Chicago/Turabian StyleYarmolenko, Olga V., Guzaliya R. Baymuratova, Kyunsylu G. Khatmullina, Galiya Z. Tulibayeva, Alena V. Yudina, Tatiana A. Savinykh, Igor K. Yakushchenko, Pavel A. Troshin, and Alexander F. Shestakov. 2022. "Influence of the Lithium Cation Desolvation Process at the Electrolyte/Electrode Interface on the Performance of Lithium Batteries" Inorganics 10, no. 11: 176. https://doi.org/10.3390/inorganics10110176






