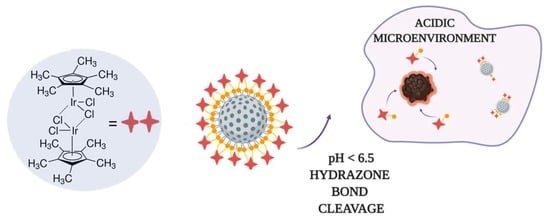
Mesoporous Silica Nanoparticles for pH-Responsive Delivery of Iridium Metallotherapeutics and Treatment of Glioblastoma Multiforme
Abstract
:1. Introduction
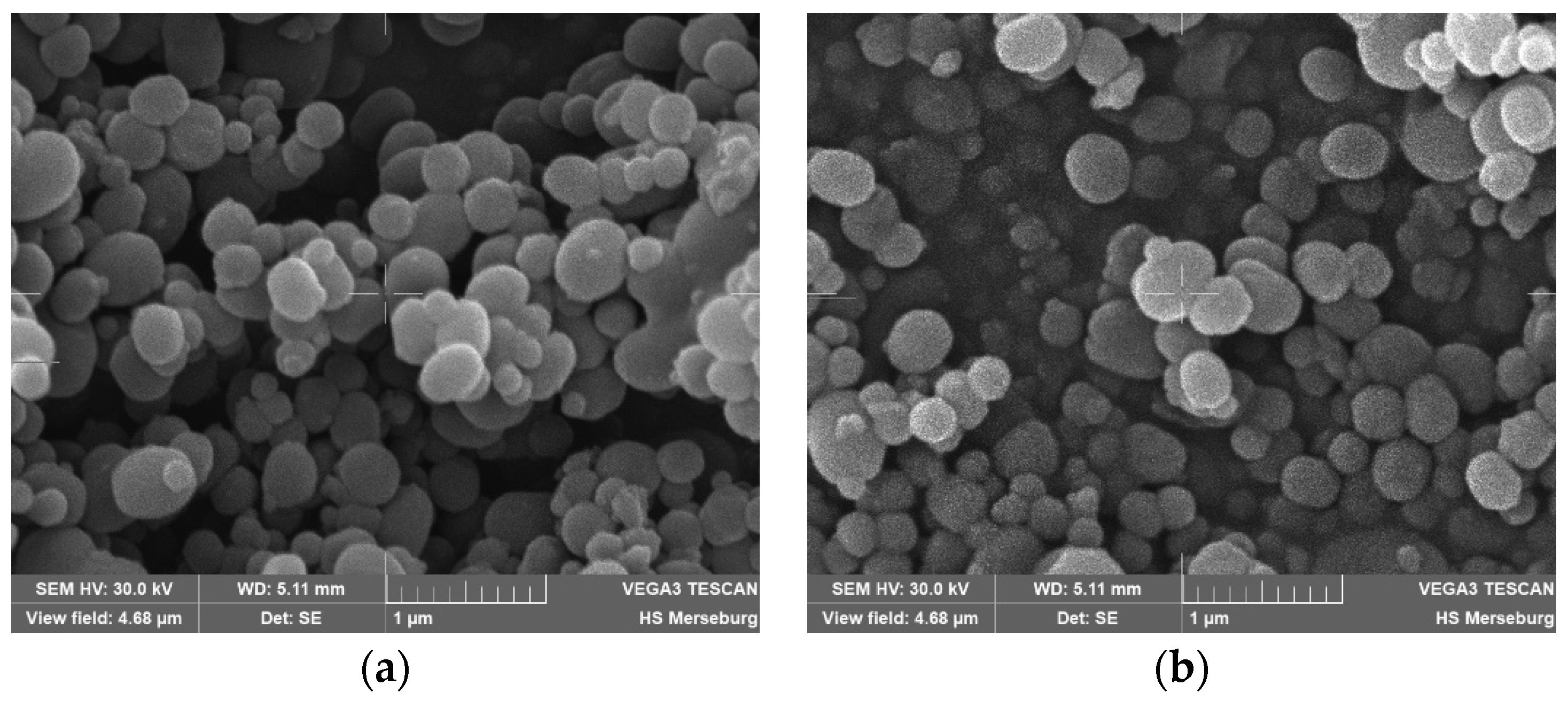
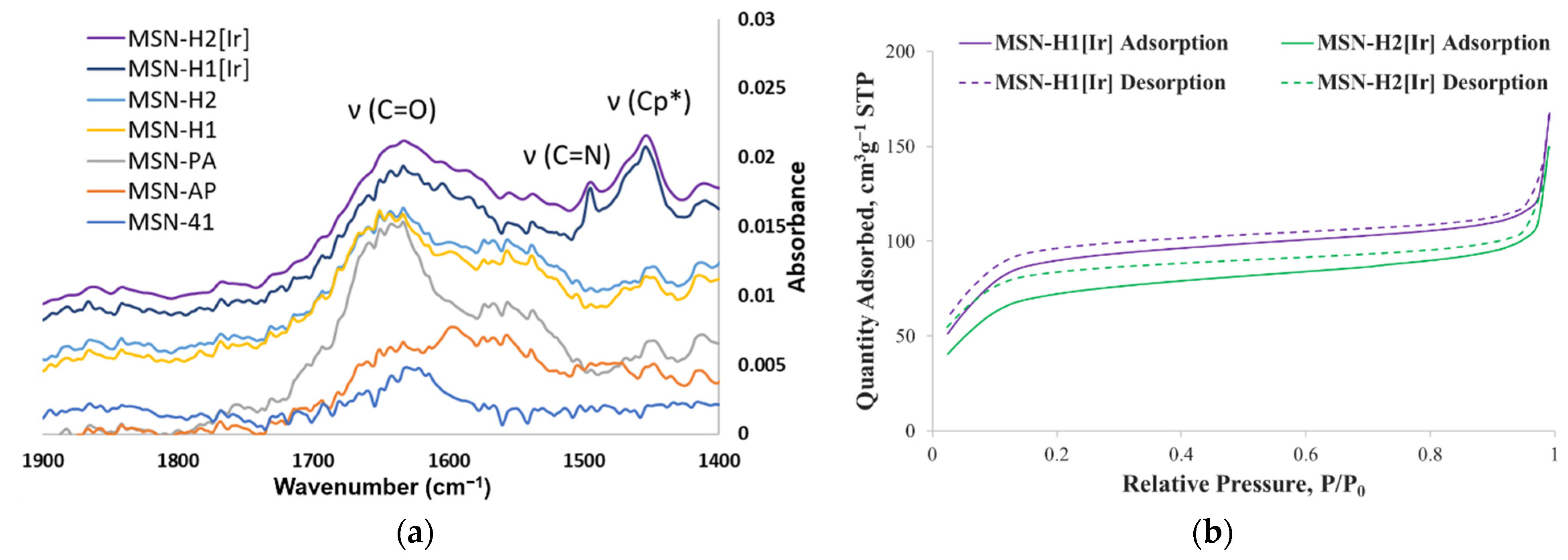
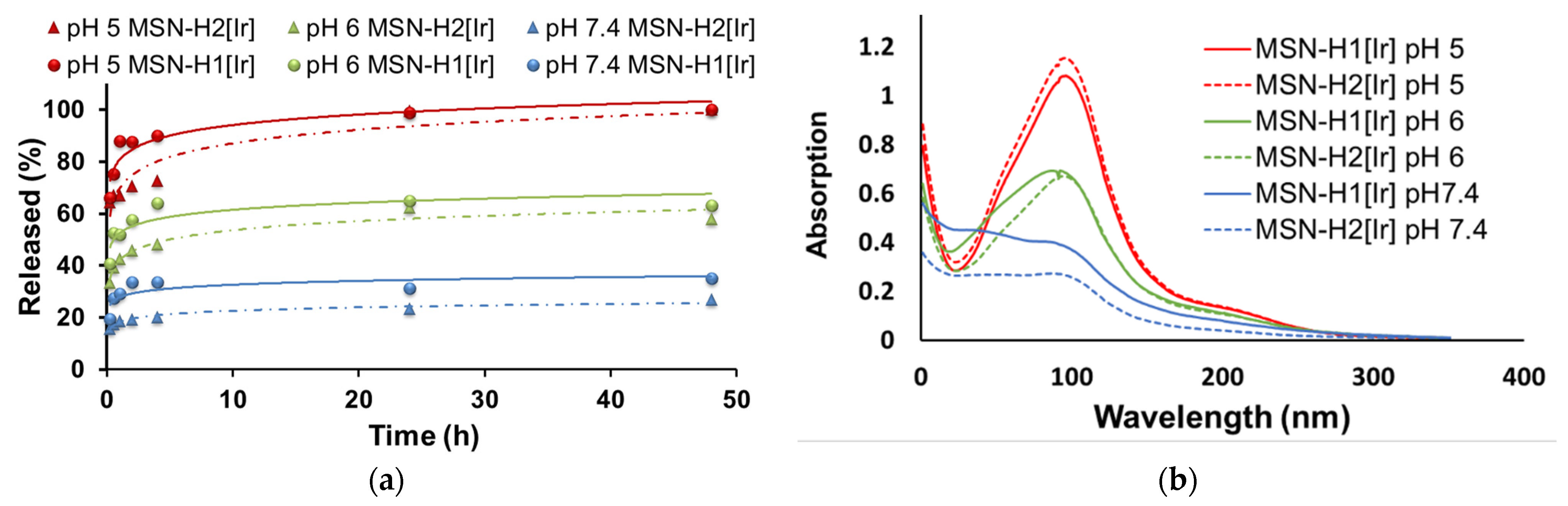
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis and Functionalization of MSN
3.2. Functionalization with Ir(III) Complex
3.3. Characterization
3.4. Drug Release Measurements
3.5. Cell Viability Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Z.; Zhou, Y.; Fan, T.; Lin, Y.; Zhang, H.; Mei, L. Inorganic nano-carriers based smart drug delivery systems for tumor therapy. Smart Mater. Med. 2020, 1, 32–47. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. J. Drug Target. 2007, 15, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Theranostic Antitumoral Nanomedicines. Pharmaceutics 2020, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Saroj, S.; Rajput, S.J. Composite smart mesoporous silica nanoparticles as promising therapeutic and diagnostic candidates: Recent trends and applications. J. Drug Deliv. Sci. Technol. 2018, 44, 349–365. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Durand, J.-O. Targeted Treatment of Cancer with Nanotherapeutics Based on Mesoporous Silica Nanoparticles. ChemPlusChem 2015, 80, 26–36. [Google Scholar] [CrossRef]
- Yan, Y.; Fu, J.; Wang, T.; Lu, X. Controlled release of silyl ether camptothecin from thiol-ene click chemistry-functionalized mesoporous silica nanoparticles. Acta Biomater. 2017, 51, 471–478. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Chen, Y.; Wang, L.; Huang, P.; Li, Y.; Liu, Z.; Yao, H.; Shi, J. Core-shell hierarchical mesostructured silica nanoparticles for gene/chemo-synergetic stepwise therapy of multidrug-resistant cancer. Biomaterials 2017, 133, 219–228. [Google Scholar] [CrossRef]
- Yang, K.; Luo, H.; Zeng, M.; Jiang, Y.; Li, J.; Fu, X. Intracellular pH-Triggered, Targeted Drug Delivery to Cancer Cells by Multifunctional Envelope-Type Mesoporous Silica Nanocontainers. ACS Appl. Mater. Interfaces. 2015, 7, 17399–17407. [Google Scholar] [CrossRef]
- Živojević, K.; Mladenović, M.; Djisalov, M.; Mundzic, M.; Ruiz-Hernandez, E.; Gadjanski, I.; Knežević, N.Ž. Advanced mesoporous silica nanocarriers in cancer theranostics and gene editing applications. J. Control. Release 2021, 337, 193–211. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Kaluđerović, G.N. Silicon-based nanotheranostics. Nanoscale 2017, 9, 12821–12829. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, D.; Lu, M.-M.; Zhao, Y.-W.; Zhang, F.; Tan, Y.-F.; Zheng, X.; Pan, Y.; Xiao, X.-A.; Wang, Z.; Dong, W.-F.; et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017, 49, 531–540. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo Biodistribution and Urinary Excretion of Mesoporous Silica Nanoparticles: Effects of Particle Size and PEGylation. Small 2011, 7, 271–280. [Google Scholar] [CrossRef]
- Lai, W.-F.; Tang, R.; Wong, W.-T. Ionically Crosslinked Complex Gels Loaded with Oleic Acid-Containing Vesicles for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 725. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, X.; Qiu, X.; Gao, T.; Yu, W.; Zhang, M.; Song, L.; Liu, D.; Dong, J.; Jiang, Z.; et al. Bioactive composite Janus nanofibrous membranes loading Ciprofloxacin and Astaxanthin for enhanced healing of full-thickness skin defect wounds. Appl. Surf. Sci. 2023, 610, 155290. [Google Scholar] [CrossRef]
- Sargazi, S.; Laraib, U.; Barani, M.; Rahdar, A.; Fatima, I.; Bilal, M.; Pandey, S.; Sharma, R.K.; Kyzas, G.Z. Recent trends in mesoporous silica nanoparticles of rode-like morphology for cancer theranostics: A review. J. Mol. Struct. 2022, 1261, 132922. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Yang, L.; Wang, Z.; Wang, Z.; Feng, J.; Wen, X.; Cheng, L.; Zhu, R. MgFe-LDH Nanoparticles: A Promising Leukemia Inhibitory Factor Replacement for Self-Renewal and Pluripotency Maintenance in Cultured Mouse Embryonic Stem Cells. Adv. Sci. 2021, 8, 2003535. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Sazanovich, I.V.; Baggaley, E.; Bonneau, M.; Guerchais, V.; Williams, J.A.G.; Weinstein, J.A.; Bryant, H.E. Metal Complexes for Two-Photon Photodynamic Therapy: A Cyclometallated Iridium Complex Induces Two-Photon Photosensitization of Cancer Cells under Near-IR Light. Chem.-A Eur. J. 2017, 23, 234–238. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Tian, Z.; Gong, Y.; Zheng, H.; Zhang, S.; Xu, Z.; Ge, X.; Liu, Z. Novel and Versatile Imine-N-Heterocyclic Carbene Half-Sandwich Iridium(III) Complexes as Lysosome-Targeted Anticancer Agents. Inorg. Chem. 2018, 57, 11087–11098. [Google Scholar] [CrossRef]
- Ludwig, G.; Ranđelović, I.; Maksimović-Ivanić, D.; Mijatović, S.; Bulatović, M.Z.; Miljković, D.; Korb, M.; Lang, H.; Steinborn, D.; Kaluđerović, G.N. Anticancer Potential of (Pentamethylcyclopentadienyl)chloridoiridium(III) Complexes Bearing κP and κP,κS-Coordinated Ph2PCH2CH2CH2S(O)xPh (x = 0–2) Ligands. ChemMedChem 2014, 9, 1586–1593. [Google Scholar] [CrossRef]
- Ludwig, G.; Steinborn, D.; Kaluđerović, G. Biological Potential of Halfsandwich Ruthenium(II) and Iridium(III) Complexes. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2016, 16, 1455–1460. [Google Scholar]
- Ludwig, G.; Mijatović, S.; Ranđelović, I.; Bulatović, M.; Miljković, D.; Maksimović-Ivanić, D.; Korb, M.; Lang, H.; Steinborn, D.; Kaluđerović, G.N. Biological activity of neutral and cationic iridium(III) complexes with κP and κP,κS coordinated Ph2PCH2S(O)xPh (x = 0–2) ligands. Eur. J. Med. Chem. 2013, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, A.C.; Rodríguez-Fanjul, V.; Habtemariam, A.; Pizarro, A.M. Structurally Strained Half-Sandwich Iridium(III) Complexes As Highly Potent Anticancer Agents. J. Med. Chem. 2020, 63, 4005–4021. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Wei, Y.; Xie, Y.; Cong, F. Reduction- and pH-Sensitive Hyaluronan Nanoparticles for Delivery of Iridium(III) Anticancer Drugs. Biomacromolecules 2017, 18, 2102–2117. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zheng, Y.; Zhang, H.; Sun, J.H.; Tan, C.P.; He, L.; Zhang, W.; Ji, L.N.; Mao, Z.W. Delivery of Phosphorescent Anticancer Iridium(III) Complexes by Polydopamine Nanoparticles for Targeted Combined Photothermal-Chemotherapy and Thermal/Photoacoustic/Lifetime Imaging. Adv. Sci. 2018, 5, 1800581. [Google Scholar] [CrossRef] [Green Version]
- Gou, Y.; Huang, G.; Li, J.; Yang, F.; Liang, H. Versatile delivery systems for non-platinum metal-based anticancer therapeutic agents. Coord. Chem. Rev. 2021, 441, 213975. [Google Scholar] [CrossRef]
- Estevão, B.M.; Vilela, R.R.C.; Geremias, I.P.; Zanoni, K.P.S.; de Camargo, A.S.S.; Zucolotto, V. Mesoporous silica nanoparticles incorporated with Ir(III) complexes: From photophysics to photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 40, 103052. [Google Scholar] [CrossRef] [PubMed]
- Mladenović, M.; Morgan, I.; Ilić, N.; Saoud, M.; Pergal, M.V.; Kaluđerović, G.N.; Knežević, N.Ž. pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers. Pharmaceutics 2021, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Ilić, N.; Kaluđerović, G.N. Functionalized Mesoporous Silica Nanoparticles for Drug Delivery to Glioblastoma Multiforme. In Proceedings of the 2022 IEEE 22nd International Conference on Nanotechnology (NANO), Palma de Mallorca, Spain, 4–8 July 2022; pp. 321–324. [Google Scholar]


| Material | Hydrodynamic Diameter/nm | Predominant Intensity-Weighted Peak/nm | Zeta Potential/mV |
|---|---|---|---|
| MSN-H1[Ir] | 539 ± 7 | 456 ± 40 | +61.7 |
| MSN-H2[Ir] | 366 ± 25 | 289 ± 14 | +55.9 |
| Material | U251 μg/mL (μM) | MRC-5 μg/mL (μM) | ||
|---|---|---|---|---|
| pH 5 | pH 7.4 | pH 5 | pH 7.4 | |
| MSN * | 1220 | >2000 | 1265 | >2000 |
| MSN-H1 * | 1082 | 628 | 902 | 1425 |
| MSN-H2 * | 1030 | 957 | 1045 | 561 |
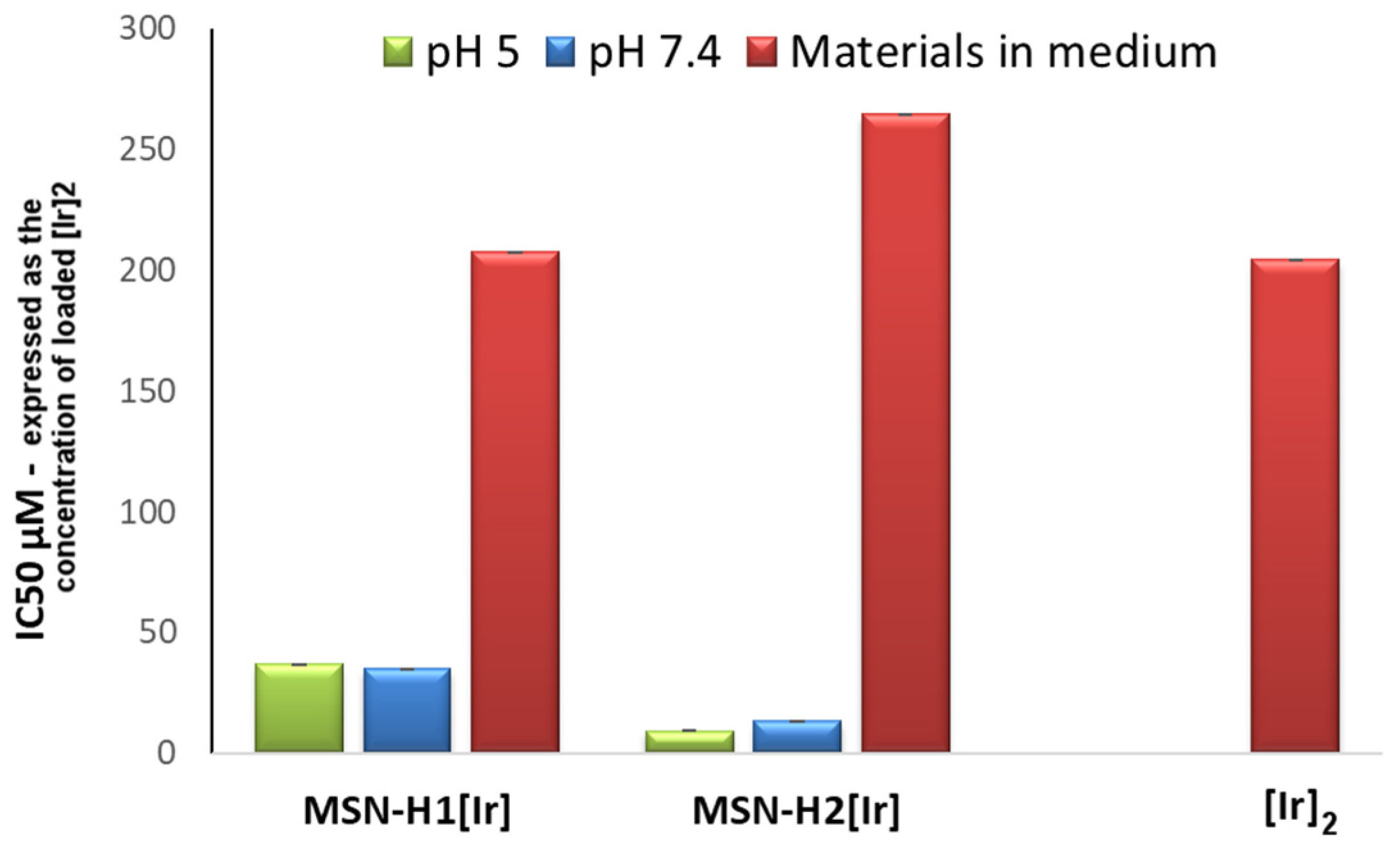
| MSN-H1[Ir] | 110 (36.8) ** | 104 (35.1) ** | 1512 (508.5) ** | >2000 (>672.7) ** |
| MSN-H2[Ir] | 29.5 (9.3) ** | 42.4 (13.3) ** | 493 (154.7) ** | 356 (111.7) ** |
| Material | U251 μg/mL (μM) | MRC-5 μg/mL (μM) |
|---|---|---|
| MSN-H1[Ir] * | 617 (207.6) ** | >2000 (>672.7) ** |
| MSN-H2[Ir] * | 843 (264.6) ** | >2000 (>672.7) ** |
| [Ir]2 *** | 163 (196.1) ** | >2000 (>672.7) ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knežević, N.Ž.; Ilić, N.; Kaluđerović, G.N. Mesoporous Silica Nanoparticles for pH-Responsive Delivery of Iridium Metallotherapeutics and Treatment of Glioblastoma Multiforme. Inorganics 2022, 10, 250. https://doi.org/10.3390/inorganics10120250
Knežević NŽ, Ilić N, Kaluđerović GN. Mesoporous Silica Nanoparticles for pH-Responsive Delivery of Iridium Metallotherapeutics and Treatment of Glioblastoma Multiforme. Inorganics. 2022; 10(12):250. https://doi.org/10.3390/inorganics10120250
Chicago/Turabian StyleKnežević, Nikola Ž., Nebojša Ilić, and Goran N. Kaluđerović. 2022. "Mesoporous Silica Nanoparticles for pH-Responsive Delivery of Iridium Metallotherapeutics and Treatment of Glioblastoma Multiforme" Inorganics 10, no. 12: 250. https://doi.org/10.3390/inorganics10120250