Synthesis, Physicochemical, Thermal and Antioxidative Properties of Zn(II) Coordination Compounds with Pyrazole-Type Ligand
Abstract
:1. Introduction
2. Results and Discussion
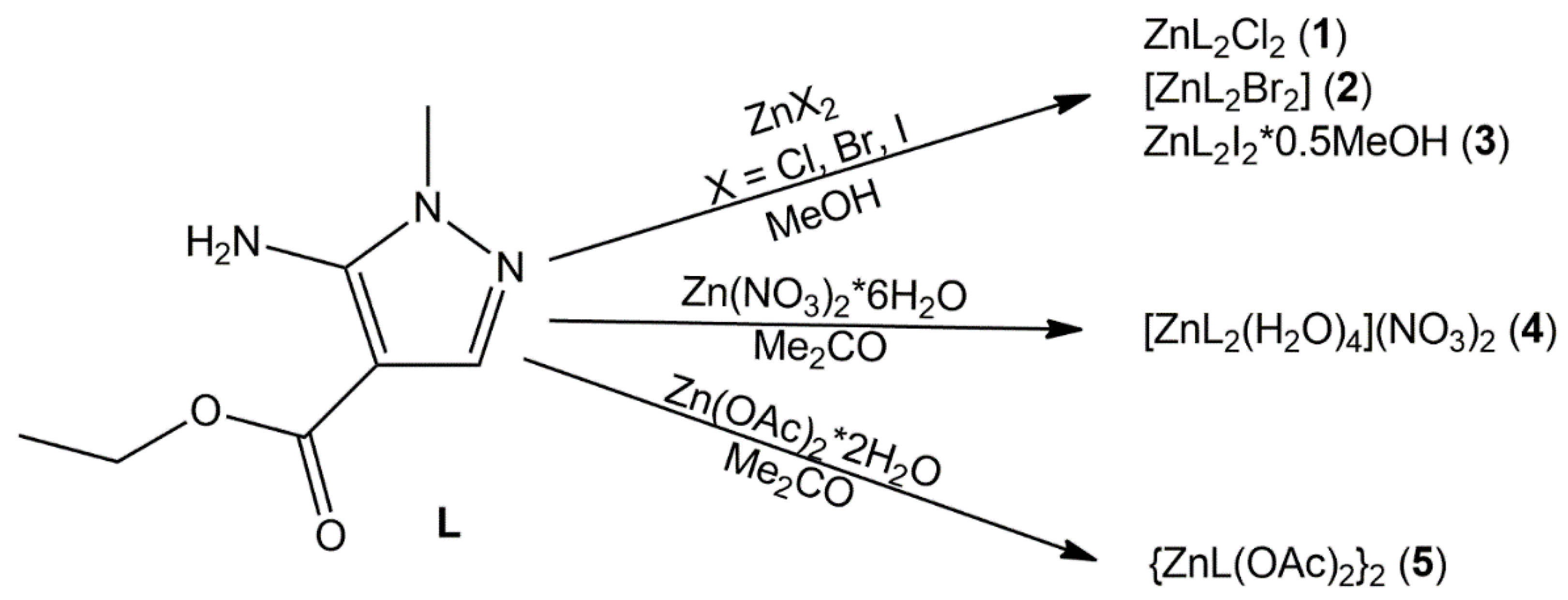
2.1. Syntheses and Physicochemical Properties of the Complexes
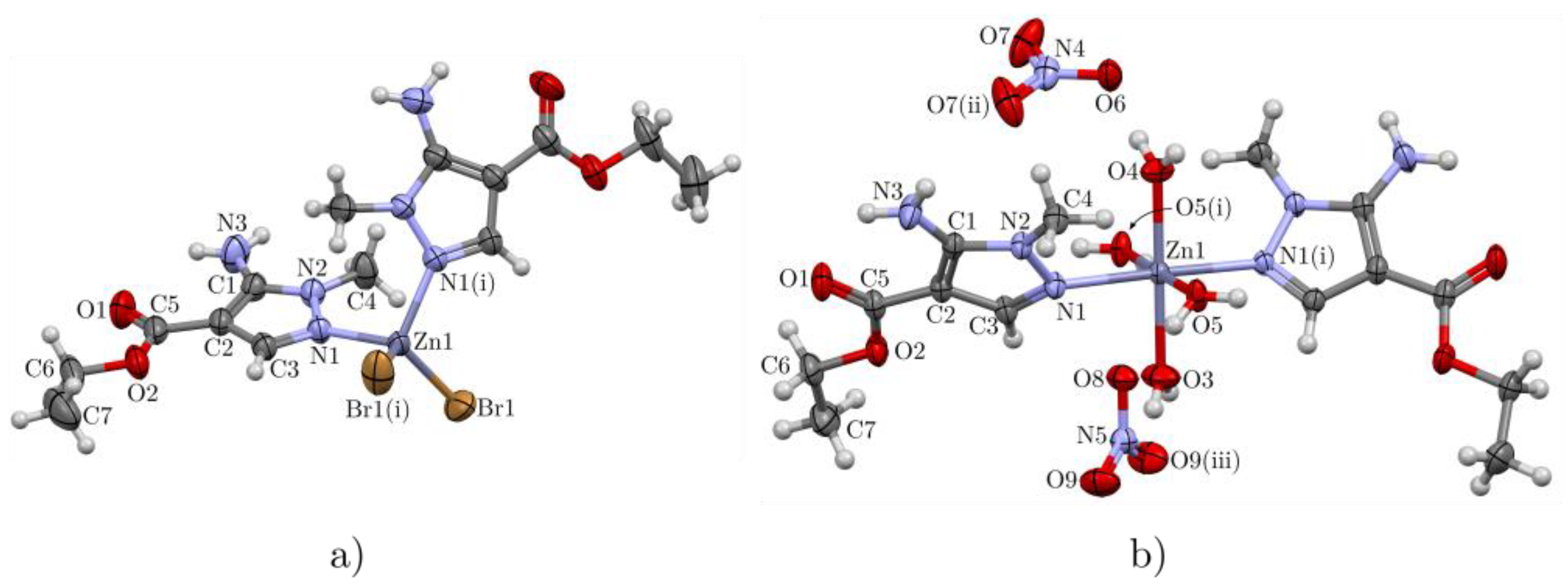
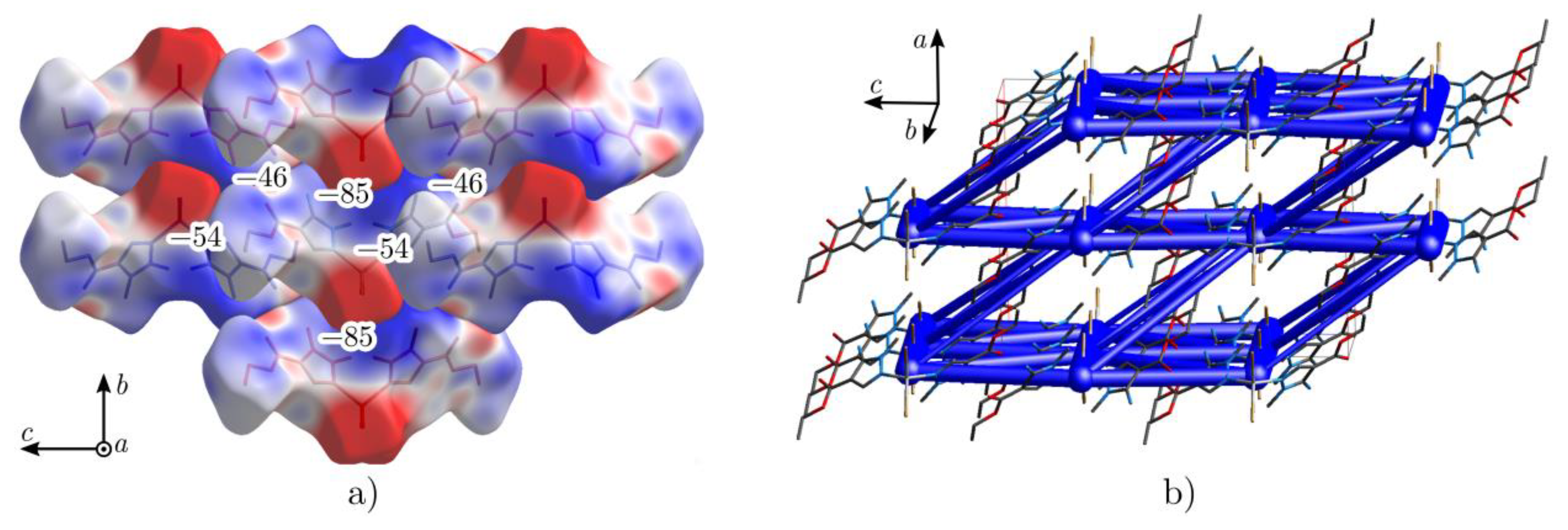
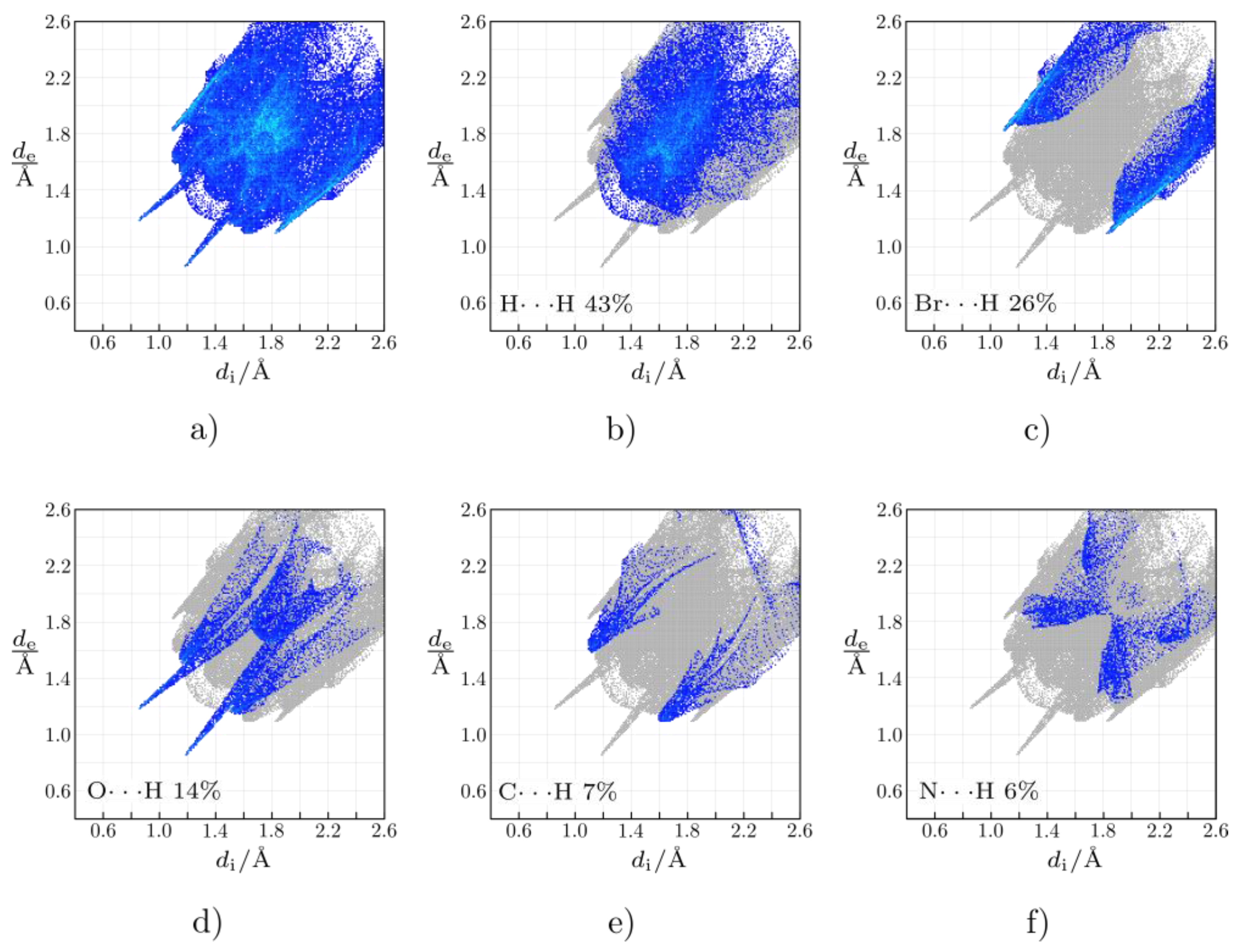
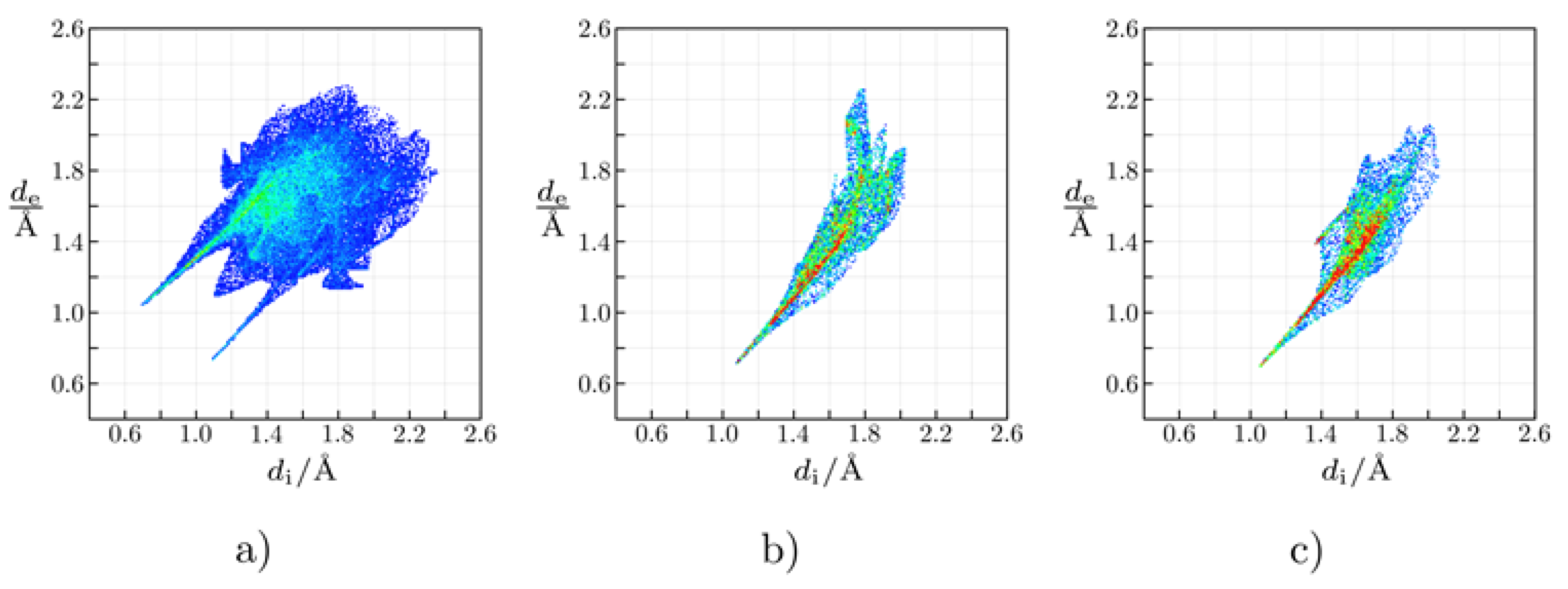
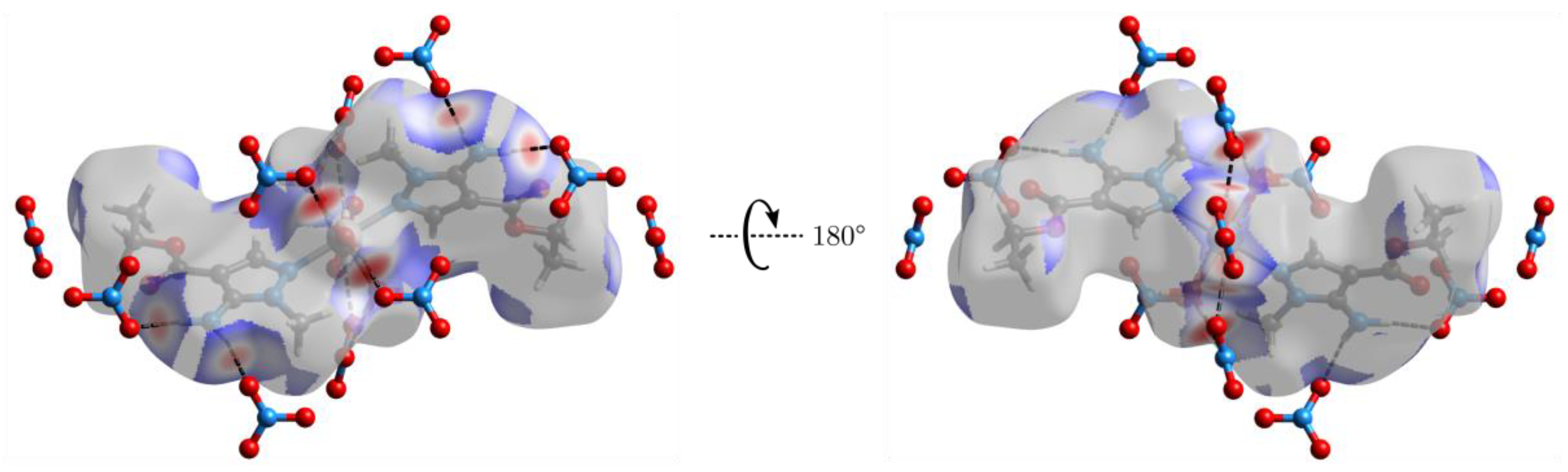
2.2. Crystal Structure of Complexes 2 and 4
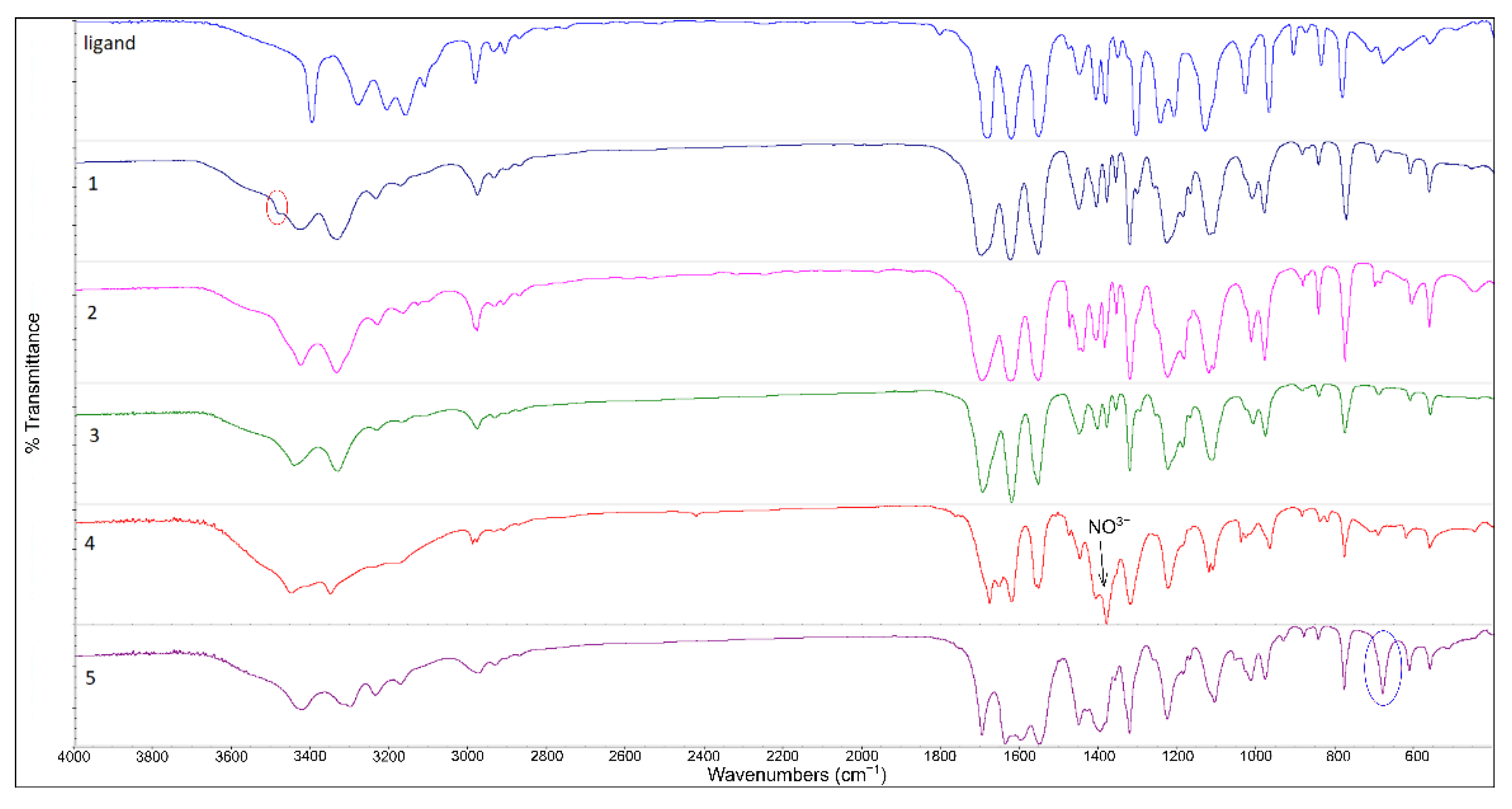
2.3. FTIR Spectra of the Complexes
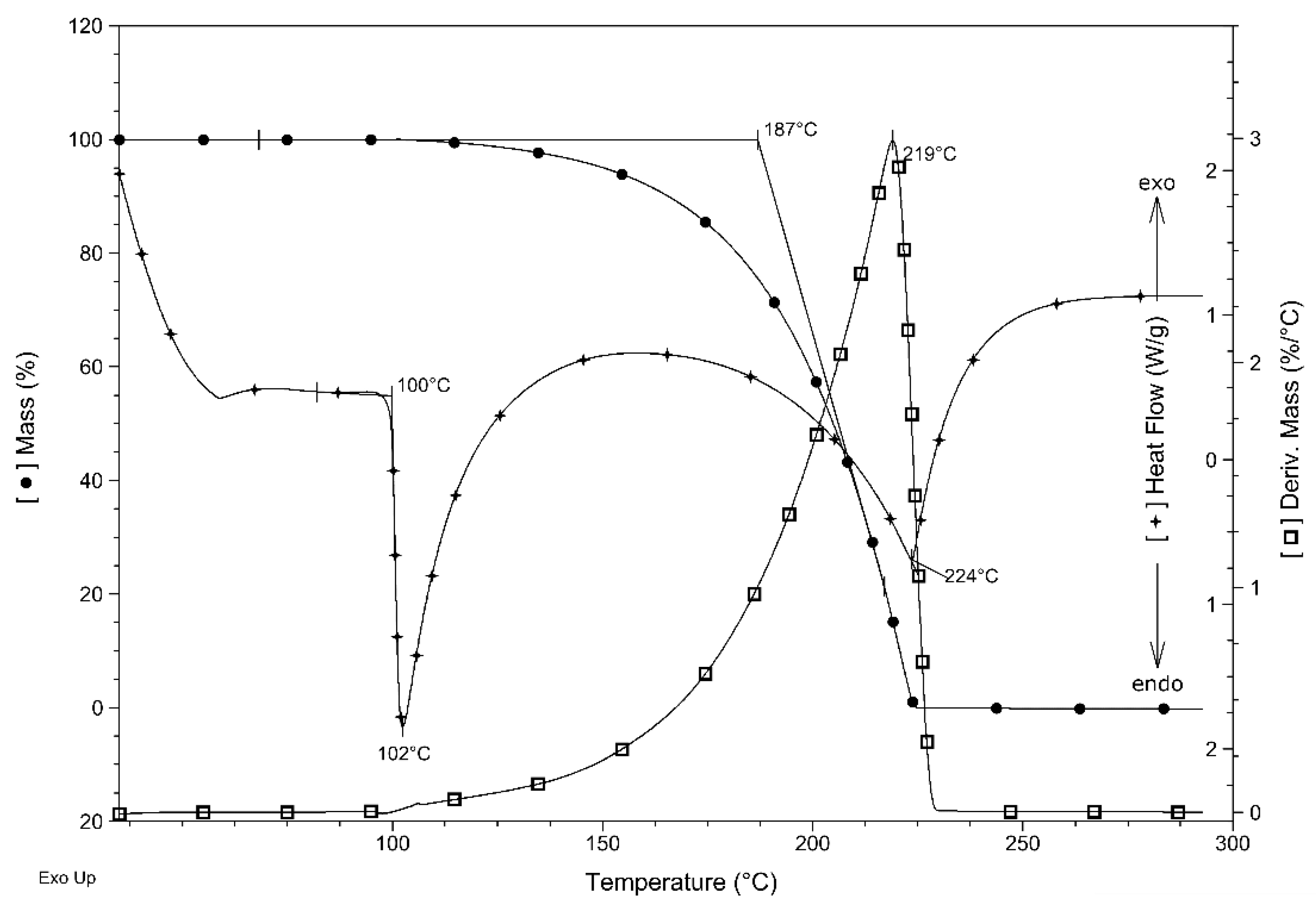
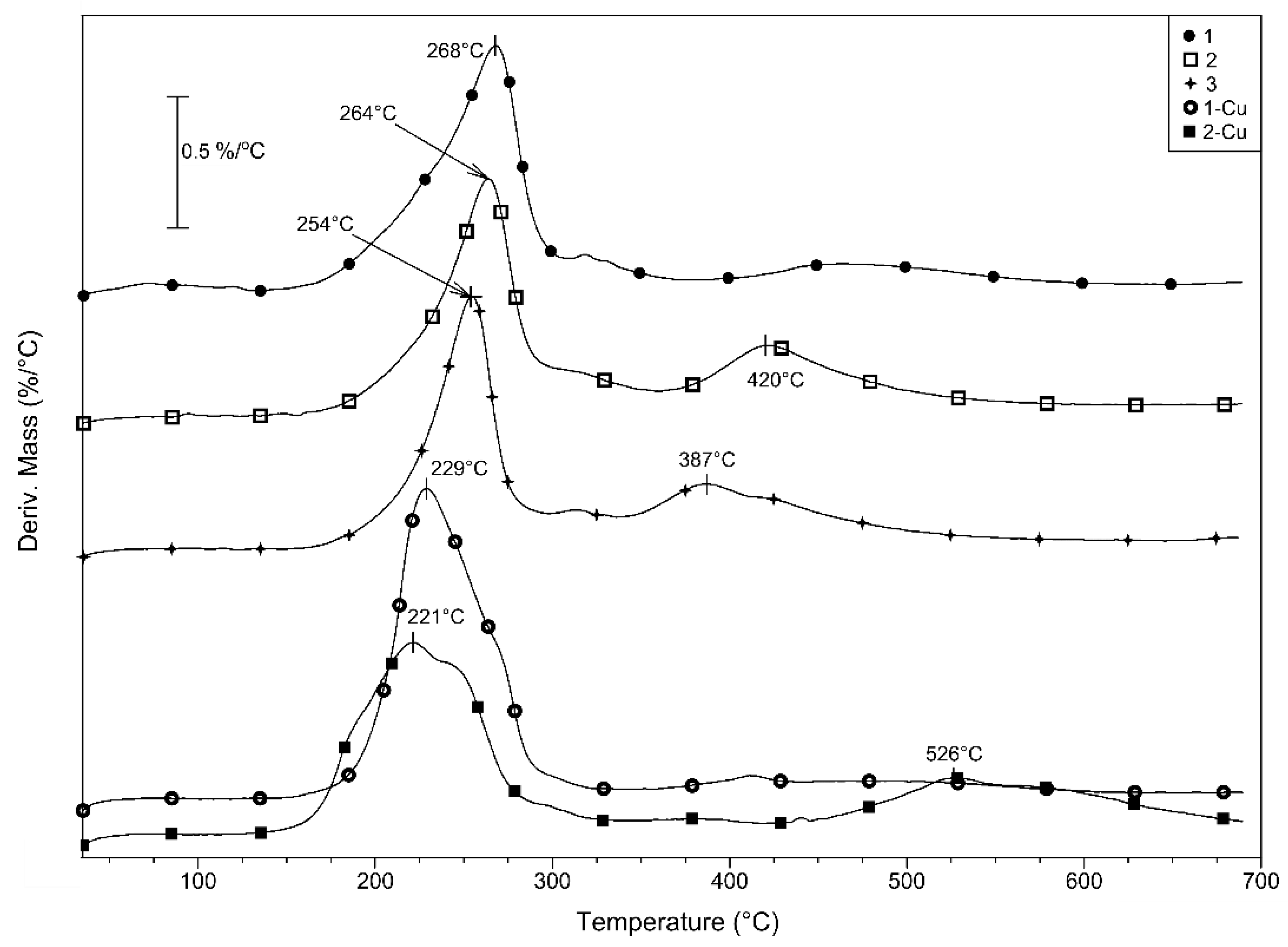
2.4. Thermal Properties
2.5. Antioxidative Capacity
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Neutralization of DPPH Radical
3.3. Preparation of the Coordination Compounds
3.3.1. Zn(L)2Cl2 (1)
3.3.2. [Zn(L)2Br2] (2)
3.3.3. Zn(L)2I2·0.5MeOH (3)
3.3.4. [Zn(L)2(H2O)4](NO3)2 (4)
3.3.5. {ZnL(OAc)2}2 (5)
3.3.6. Crystal Structure Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matiadis, D.; Karagiaouri, M.; Mavroidi, B.; Nowak, K.E.; Katsipis, G.; Pelecanou, M.; Pantazaki, A.; Sagnou, M. Synthesis and Antimicrobial Evaluation of a Pyrazoline-Pyridine Silver(I) Complex: DNA-Interaction and Anti-Biofilm Activity. BioMetals 2021, 34, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.; Delancey, E.; Ramey, H.; Williams, C.; Alsharif, Z.A.; Al-khattabi, H.; Ontko, A.; Gilmore, D.; Alam, M.A. Synthesis and Antimicrobial Studies of Novel Derivatives of 4-(4-Formyl-3-phenyl-1H-pyrazol-1-yl)Benzoic Acid as Potent Anti-Acinetobacter Baumannii Agents. Bioorg. Med. Chem. Lett. 2017, 27, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules 2012, 17, 4962–4971. [Google Scholar] [CrossRef] [PubMed]
- Mǎruţescu, L.; Calu, L.; Chifiriuc, M.C.; Bleotu, C.; Daniliuc, C.G.; Fǎlcescu, D.; Kamerzan, C.M.; Badea, M.; Olar, R. Synthesis, Physico-Chemical Characterization, Crystal Structure and Influence on Microbial and Tumor Cells of Some Co(II) Complexes with 5,7-Dimethyl-1,2,4-triazolo[1,5-a]pyrimidine. Molecules 2017, 22, 1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenchappa, R.; Bodke, Y.D. Synthesis, Analgesic and Anti-Inflammatory Activity of Benzofuran Pyrazole Heterocycles. Chem. Data Coll. 2020, 28, 100453. [Google Scholar] [CrossRef]
- Ragab, F.A.; Abdel Gawad, N.M.; Georgey, H.H.; Said, M.F. Synthesis of Novel 1,3,4-Trisubstituted Pyrazoles as Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2013, 63, 645–654. [Google Scholar] [CrossRef]
- Gökhan-Kelekçi, N.; Yabanoǧlu, S.; Küpeli, E.; Salgin, U.; Özgen, Ö.; Uçar, G.; Yeşilada, E.; Kendi, E.; Yeşilada, A.; Bilgin, A.A. A New Therapeutic Approach in Alzheimer Disease: Some Novel Pyrazole Derivatives as Dual MAO-B Inhibitors and Antiinflammatory Analgesics. Bioorg. Med. Chem. 2007, 15, 5775–5786. [Google Scholar] [CrossRef]
- Badaweya, E.-S.A.M.; El-Ashmaweyb, I.M. Nonsteroidal Antiinflammatory Agents-Part 1: Antiinflammatory, Analgesic and Antipyretic Activity of Some New 1-(Pyrimidin-2-il)-3-pyrazolin-5-ones and 2-(Pyrimidin-2-yl)-l,2,4,5,6,7-hexahydro-3H-indazol-3-ones. Eur. J. Med. Chem. 1998, 33, 349–361. [Google Scholar] [CrossRef]
- Zhang, D.; Du, P.; Chen, J.; Guo, H.; Lu, X. Pyrazolate-Based Porphyrinic Metal-Organic Frameworks as Catechol Oxidase Mimic Enzyme for Fluorescent and Colorimetric Dual-Mode Detection of Dopamine with High Sensitivity and Specificity. Sens. Actuators B Chem. 2021, 341, 130000. [Google Scholar] [CrossRef]
- Hammouda, M.M.; Metwally, H.M.; Fekri, A.; van der Eycken, J. Synthesis and Molecular Modeling Studies on Novel C2 Alkylated Benzoazonine Scaffold and Corresponding 2-Pyrazoline Derivatives as Acetylcholinestrase Enzyme Inhibitors. Polycycl. Aromat. Compd. 2021, 41, 1223–1240. [Google Scholar] [CrossRef]
- Mede, R.; Gläser, S.; Suchland, B.; Schowtka, B.; Mandel, M.; Görls, H.; Krieck, S.; Schiller, A.; Westerhausen, M. Manganese(I)-Based CORMs with 5-Substituted 3-(2-Pyridyl)pyrazole Ligands. Inorganics 2017, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xu, T.; Fan, J.; Zhang, Q.; Ding, M.; Huang, M.; Deng, L.; Lu, Y.; Guo, Y. Natural Products-Based Pesticides: Design, Synthesis and Pesticidal Activities of Novel Fraxinellone Derivatives Containing N-Phenylpyrazole Moiety. Ind. Crop. Prod. 2018, 117, 50–57. [Google Scholar] [CrossRef]
- Graillot, V.; Tomasetig, F.; Cravedi, J.P.; Audebert, M. Evidence of the in Vitro Genotoxicity of Methyl-pyrazole Pesticides in Human Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2012, 748, 8–16. [Google Scholar] [CrossRef]
- da Silva, P.B.; Frem, R.C.G.; Netto, A.V.G.; Mauro, A.E.; Ferreira, J.G.; Santos, R.H.A. Pyrazolyl Coordination Polymers of Cadmium(II). Inorg. Chem. Commun. 2006, 9, 235–238. [Google Scholar] [CrossRef]
- Hosny, N.M. Solvothermal Synthesis, Thermal and Adsorption Properties of Metal-Organic Frameworks Zn and CoZn(DPB). J. Therm. Anal. Calorim. 2015, 122, 89–95. [Google Scholar] [CrossRef]
- Netto, A.V.G.; Frem, R.C.G.; Mauro, A.E.; Crespi, M.S.; Zorel, H.E. Synthesis, Spectral and Thermal Studies on Pyrazolate-bridged Palladium(II) Coordination Polymers. J. Therm. Anal. Calorim. 2007, 87, 789–792. [Google Scholar] [CrossRef]
- Yan, X.Q.; Wang, Z.C.; Qi, P.F.; Li, G.; Zhu, H.L. Design, Synthesis and Biological Evaluation of 2-H-pyrazole Derivatives Containing Morpholine Moieties as Highly Potent Small Molecule Inhibitors of APC–Asef Interaction. Eur. J. Med. Chem. 2019, 177, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, B.; Peng, R.; Liu, Q.; Tan, B.; Chu, S. Synthesis and Characterization of a New Energetic Salt 1H-Pyrazole-1-carboxamidine Dinitramide and its Thermal Properties. J. Therm. Anal. Calorim. 2016, 124, 1431–1439. [Google Scholar] [CrossRef]
- Serushkin, V.; Sinditskii, V.P.; Hoang, T.H.; Filatov, S.A.; Shipulina, A.S.; Dalinger, I.L.; Shakhnes, A.K.; Sheremetev, A.B. Thermal and Combustion Behavior of Novel Oxygen-Rich Energetic Pyrazoles. J. Therm. Anal. Calorim. 2018, 132, 127–142. [Google Scholar] [CrossRef]
- Barta Holló, B.; Vojinović Ješić, L.S.; Radanović, M.M.; Rodić, M.; Jaćimović, Ž.K.; Mészáros Szécsényi, K. Synthesis, Physicochemical, and Thermal Characterization of Coordination Compounds of Cu(II) with a Pyrazole-Type Ligand. J. Therm. Anal. Calorim. 2020, 142, 451–460. [Google Scholar] [CrossRef]
- Jaćimović, Ž.K.; Giester, G.; Kosović, M.; Bogdanović, G.A.; Novaković, S.B.; Leovac, V.M.; Latinović, N.; Barta Holló, B.; Mészáros Szécsényi, K. Pyrazole-Type Complexes with Ni(II) and Cu(II): Solvent Exchange Reactions in Coordination Compounds. J. Therm. Anal. Calorim. 2017, 127, 1501–1509. [Google Scholar] [CrossRef]
- Jaćimović, Ž.; Kosović, M.; Kastratović, V.; Barta Holló, B.; Mészáros Szécsényi, K.; Szilágyi, I.M.; Latinović, N.; Vojinović-Ješić, L.; Rodić, M. Synthesis and Characterization of Copper, Nickel, Cobalt, Zinc Complexes with 4-Nitro-3-Pyrazolecarboxylic Acid Ligand. J. Therm. Anal. Calorim. 2018, 133, 813–821. [Google Scholar] [CrossRef]
- Mészáros Szécsényi, K.; Leovac, V.M.; Jaćimović, K.; Češljević, V.I.; Kovács, A.; Pokol, G. Transition Metal Complexes with Pyrazole-Based Ligands Part 12. Characterisation and Thermal Decomposition of CuCl2 Complexes with Di- and Trisubstituted Pyrazoles. J. Therm. Anal. Calorim. 2001, 66, 573–581. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, Τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Gavezzotti, A. The “Sceptical Chymist”: Intermolecular Doubts and Paradoxes. CrystEngComm 2013, 15, 4027–4035. [Google Scholar] [CrossRef] [Green Version]
- Edwards, A.J.; Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Intermolecular Interactions in Molecular Crystals: What’s in a Name? Faraday Discuss. 2017, 203, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 64–79. [Google Scholar]
- Günzler, H.; Gremlich, H.U. IR-Spektroskopie: Eine Einführung, 4th Croatian ed.; Školska Knjiga: Zagreb, Croatia, 2006; pp. 166–194. [Google Scholar]
- Győryová, K.; Balek, V. Thermal Stability of New Zinc Acetate-Based Complex Compounds. J. Therm. Anal. Calorim. 1993, 40, 519–532. [Google Scholar] [CrossRef]
- NIST WebBook. Available online: https://webbook.nist.gov/ (accessed on 1 October 2021).
- Keter, F.K.; Darkwa, J. Perspective: The Potential of Pyrazole-Based Compounds in Medicine. BioMetals 2012, 25, 9–21. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mollanejad, K.; Asghari, S.; Jadidi, K. Diastereoselective Synthesis of Pyrrolo[1, 2-c]Imidazoles Using Chiral Thiohydantoins, Malononitrile, and Aldehydes and Evaluation of Their Antioxidant and Antibacterial Activities. J. Heterocycl. Chem. 2020, 57, 556–564. [Google Scholar] [CrossRef]
- Amir, A.; Kadhum, H.; Mohamad, A.B.; Al-Amiery, A.A.; Takriff, M.S. Molecules Antimicrobial and Antioxidant Activities of New Metal Complexes Derived from 3-Aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Jelača, S.; Zengin, G.; Mimica-Dukić, N.; Berežni, S.; Miljić, M.; Dajić Stevanović, Z. Alchemilla Vulgaris Agg. (Lady’s Mantle) from Central Balkan: Antioxidant, Anticancer and Enzyme Inhibition Properties. RSC Adv. 2019, 9, 37474–37483. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, I.G.; Apetrei, C.; Hadjipavlou-Litina, D. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System; Rigaku Corporation: Wroclaw, Poland, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy Frameworks: Insights into Interaction Anisotropy and the Mechanical Properties of Molecular Crystals. Chem. Commun. 2015, 51, 3735–3738. [Google Scholar] [CrossRef] [PubMed]




| Bonds | Length, Å | Bonds | Angle, ° | ||
|---|---|---|---|---|---|
| 2 | 4 | 2 | 4 | ||
| Zn1–N1 | 2.034 (2) | 2.1977 (12) | N1–Zn1–N1 i | 102.16 (14) | 178.34 (6) |
| Zn1–Br1 | 2.3498 (4) | – | N1–Zn1–Br1 | 108.13 (7) | – |
| Zn1–O3 | – | 2.1028 (18) | N1 i–Zn1–Br1 | 108.83 (7) | – |
| Zn1–O4 | – | 2.0655 (16) | Br1–Zn1–Br1 i | 119.39 (3) | – |
| Zn1–O5 | – | 2.0833 (10) | O5–Zn1–O5 i | – | 179.61 (6) |
| O1–C5 | 1.207 (4) | 1.2152 (19) | O4–Zn1–O3 | – | 180 |
| O2–C5 | 1.329 (4) | 1.3399 (19) | |||
| O2–C6 | 1.453 (4) | 1.4567 (19) | |||
| N1–C3 | 1.321 (4) | 1.3177 (19) | |||
| N1–N2 | 1.382 (3) | 1.3890 (16) | |||
| N2–C1 | 1.342 (4) | 1.3470 (18) | |||
| N2–C4 | 1.446 (4) | 1.4505 (18) | C5–O2–C6 | 117.2 (3) | 115.78 (12) |
| N3–C1 | 1.346 (4) | 1.340 (2) | C3–N1–N2 | 105.9 (2) | 104.79 (11) |
| C1–C2 | 1.401 (4) | 1.402 (2) | C3–N1–Zn1 | 129.2 (2) | 121.98 (10) |
| C2–C3 | 1.392 (4) | 1.398 (2) | N2–N1–Zn1 | 124.51 (18) | 127.08 (8) |
| C2–C5 | 1.449 (4) | 1.4466 (19) | C1–C2–C5 | 125.7 (3) | 124.68 (14) |
| C6–C7 | 1.479 (7) | 1.496 (3) | |||
| Label | n | Symmetry Operation | R/Å | E/kJ mol−1 | ||||
|---|---|---|---|---|---|---|---|---|
| Eele | Epol | Edis | Erep | Etot | ||||
| 1 | 2 | x, y, z | 7.47 | −64.7 | −19.1 | −33.5 | 29.3 | −84.8 |
| 2 | 2 | −x + 1/2, −y + 1/2, −z | 13.22 | −0.8 | −1.6 | −28.7 | 11.5 | −18.5 |
| 3 | 2 | −x + 1/2, −y + 1/2, −z | 12.89 | −20.9 | −6.0 | −16.5 | 12.2 | −30.2 |
| 4 | 4 | x + 1/2, y + 1/2, z | 8.37 | 8.0 | −7.6 | −14.4 | 6.7 | −4.4 |
| 5 | 2 | −x, −y, −z | 10.21 | −9.8 | −8.3 | −61.5 | 21.3 | −53.5 |
| 6 | 2 | −x, −y, −z | 13.00 | −43.5 | −12.4 | −14.0 | 22.9 | −46.4 |
| D—H⋯A | D—H (Å) | H⋯A (Å) | D⋯A (Å) | D—H···A (°) |
|---|---|---|---|---|
| O3—H3C⋯O9 i | 0.85 | 2.30 | 3.0894 (15) | 154.6 |
| O3—H3C⋯O9 ii | 0.85 | 2.38 | 3.018 (2) | 132.8 |
| O4—H4A⋯O6 iii | 0.84 | 1.94 | 2.7628 (11) | 167.0 |
| O5—H5A⋯O1 iv | 0.82 | 1.99 | 2.7943 (15) | 167.8 |
| O5—H5B⋯O8 | 0.87 | 1.86 | 2.7201 (13) | 171.6 |
| O5—H5B⋯N5 | 0.87 | 2.62 | 3.4537 (17) | 161.6 |
| N3—H3A⋯O7 v | 0.86 | 2.36 | 3.205 (2) | 166.9 |
| N3—H3B⋯O1 | 0.86 | 2.41 | 2.9539 (19) | 121.2 |
| N3—H3B⋯O7 vi | 0.86 | 2.31 | 3.0138 (18) | 139.2 |
| Parameter | [Zn(L)2Br2] (2) | [Zn(L)2(H2O)4](NO3)2 (4) |
|---|---|---|
| Crystal data | ||
| Chemical formula | C14H22Br2N6O4Zn | C14H30N8O14Zn |
| Mr | 563.57 | 599.83 |
| Crystal system | Monoclinic | Orthorhombic |
| Space group | C2/c | Ibca |
| a/Å | 14.9764 (6) | 7.95140 (10) |
| b/Å | 7.4712 (3) | 20.5418 (2) |
| c/Å | 20.3960 (9) | 29.7574 (3) |
| β/° | 91.476 (4) | 90 |
| V/Å3 | 2281.38 (16) | 4860.46 (9) |
| Z | 4 | 8 |
| Radiation type | Mo Kα | Cu Kα |
| µ/mm−1 | 4.61 | 2.17 |
| Crystal size, mm | 0.66 × 0.43 × 0.19 | 0.72 × 0.48 × 0.11 |
| Data collection | ||
| Absorption correction | Multi-scan | Analytical |
| Tmin, Tmax | 0.186, 1.000 | 0.341, 0.797 |
| Measured reflections | 9408 | 11,430 |
| Independent reflections | 2646 | 2317 |
| Observed reflections, [I > 2σ(I)] | 1998 | 2199 |
| Rint | 0.029 | 0.032 |
| (sin θ/λ)max/Å−1 | 0.685 | 0.609 |
| Refinement | ||
| R[F2 > 2σ(F2)] | 0.037 | 0.028 |
| wR(F2) | 0.090 | 0.080 |
| S | 1.02 | 1.06 |
| No. of reflections | 2646 | 2317 |
| No. of parameters | 126 | 174 |
| Δρmax, Δρmin, e Å−3 | 0.72, −0.84 | 0.32, −0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barta Holló, B.; Radanović, M.M.; Rodić, M.V.; Krstić, S.; Jaćimović, Ž.K.; Vojinović Ješić, L.S. Synthesis, Physicochemical, Thermal and Antioxidative Properties of Zn(II) Coordination Compounds with Pyrazole-Type Ligand. Inorganics 2022, 10, 20. https://doi.org/10.3390/inorganics10020020
Barta Holló B, Radanović MM, Rodić MV, Krstić S, Jaćimović ŽK, Vojinović Ješić LS. Synthesis, Physicochemical, Thermal and Antioxidative Properties of Zn(II) Coordination Compounds with Pyrazole-Type Ligand. Inorganics. 2022; 10(2):20. https://doi.org/10.3390/inorganics10020020
Chicago/Turabian StyleBarta Holló, Berta, Mirjana M. Radanović, Marko V. Rodić, Sanja Krstić, Željko K. Jaćimović, and Ljiljana S. Vojinović Ješić. 2022. "Synthesis, Physicochemical, Thermal and Antioxidative Properties of Zn(II) Coordination Compounds with Pyrazole-Type Ligand" Inorganics 10, no. 2: 20. https://doi.org/10.3390/inorganics10020020







