Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Nickel Hydroxide
2.2. Conversion of Nickel Hydroxide into Nickel Oxide (NiO)
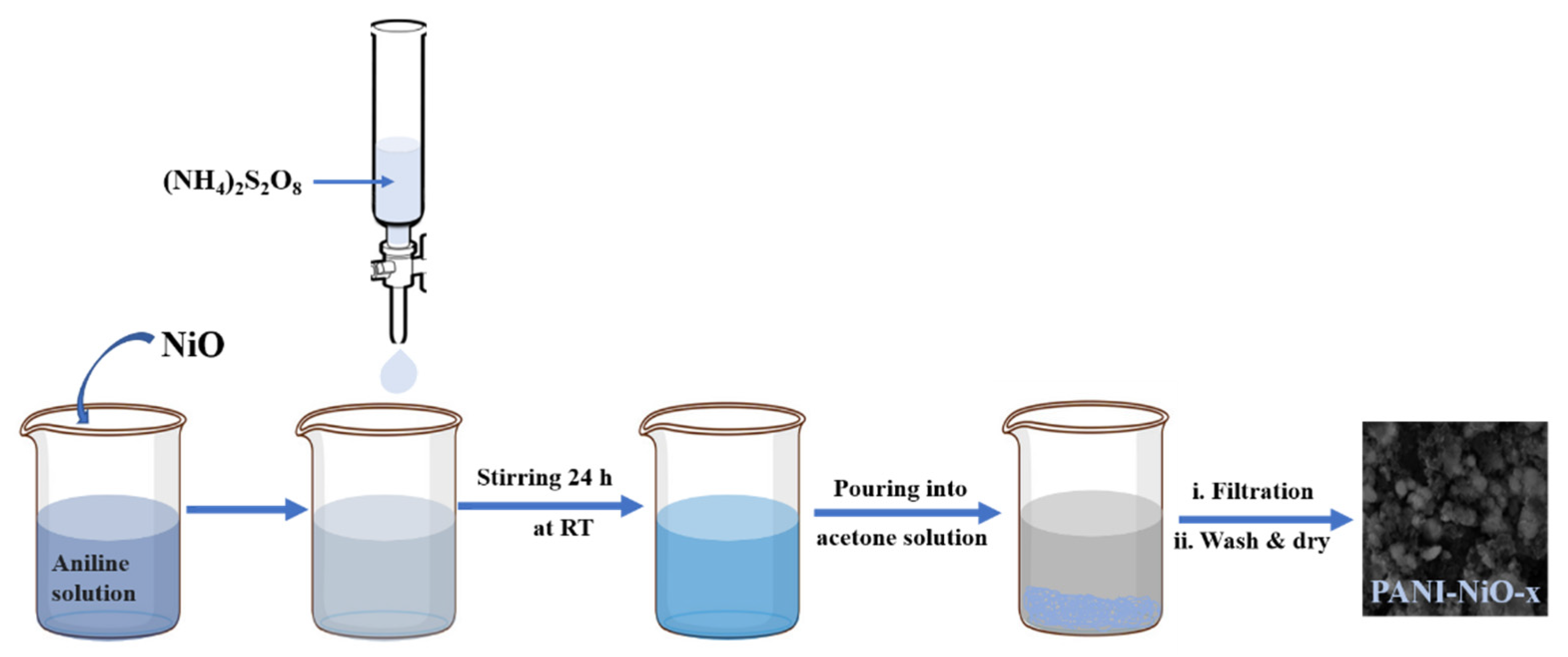
2.3. Synthesis of Polyaniline—Nickel Oxide Nanocomposite (PANI-NiO Nanocomposite)
2.4. Electrochemical Measurements
3. Results and Discussion
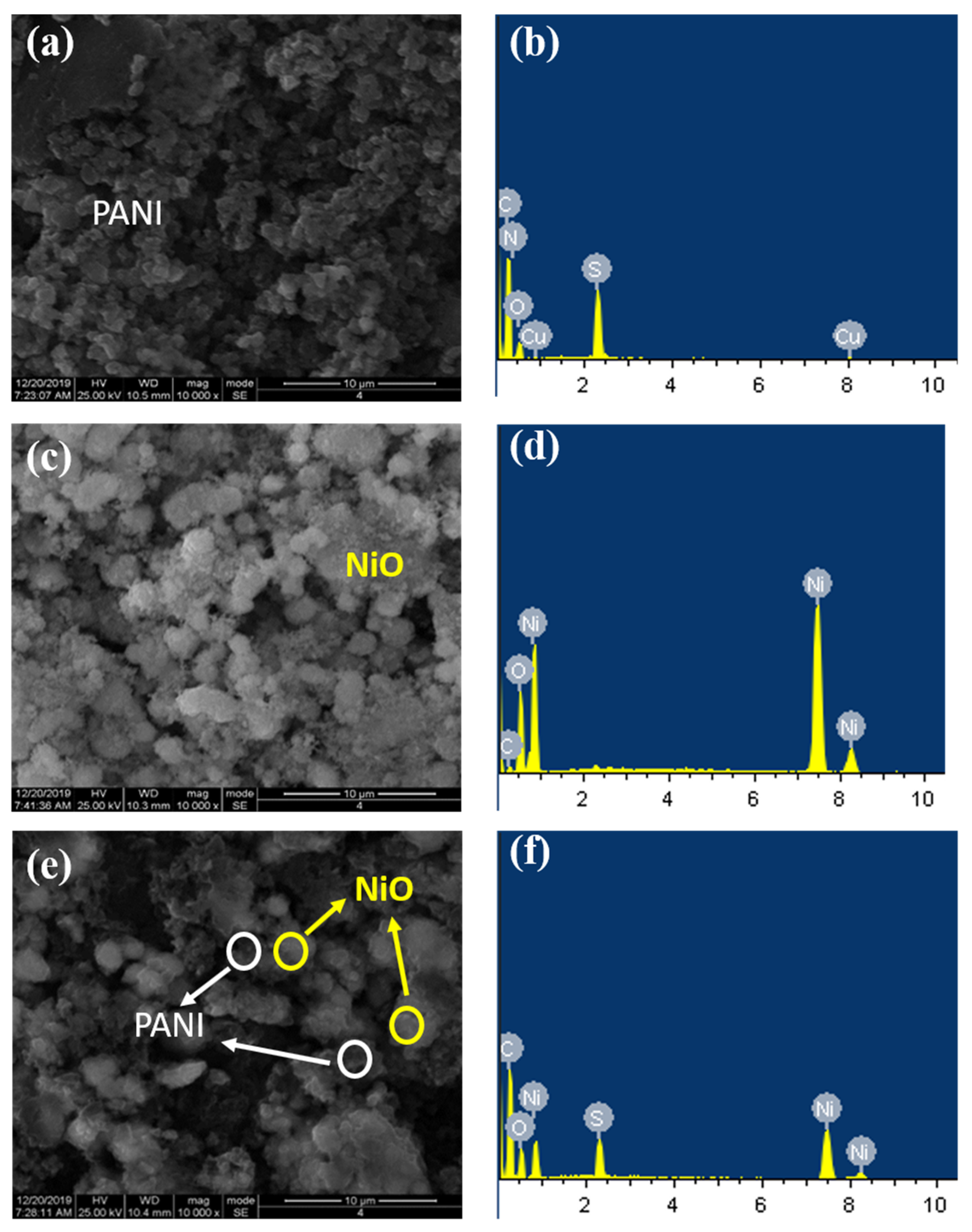
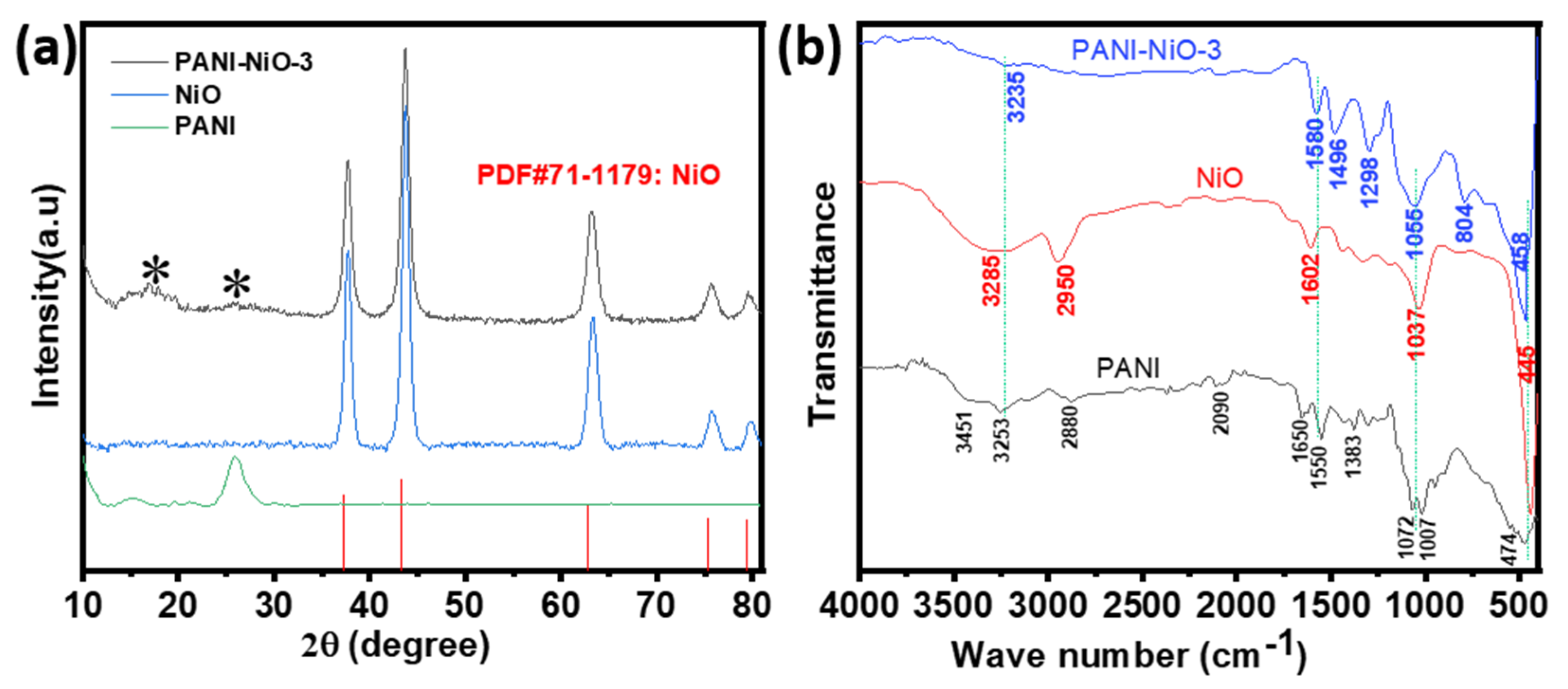
3.1. Synthesis, Morphology, and Structural Characterization
3.2. Energy Storage Performances in the 3-Electrode System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Cherusseri, J.; Choudhary, N.; Sambath Kumar, K.; Jung, Y.; Thomas, J. Recent trends in transition metal dichalcogenide based supercapacitor electrodes. Nanoscale Horiz. 2019, 4, 840–858. [Google Scholar] [CrossRef]
- Chhetri, K.; Subedi, S.; Muthurasu, A.; Ko, T.H.; Dahal, B.; Kim, H.Y. A review on nanofiber reinforced aerogels for energy storage and conversion applications. J. Energy Storage 2022, 46, 103927. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Heydari, H.; Abdolmaleki, A.; Hosseini, H. Nanostructured CuO/PANI composite as supercapacitor electrode material. Mater. Sci. Semicond. Process. 2015, 30, 157–161. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Natarajan, S.; Saud, S.; Kim, S.J.; Mok, Y.S. Activated carbon derived from cherry flower biowaste with a self-doped heteroatom and large specific surface area for supercapacitor and sodium-ion battery applications. Chemosphere 2022, 303, 135290. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mishra, K.; Hamsan, M.H.; Karim, W.O.; Abdullah, R.M.; Kadir, M.F.Z.; Abdulwahid, R.T. Fabrication of high performance energy storage EDLC device from proton conducting methylcellulose: Dextran polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 1137–1150. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Kim, T.; Subedi, S.; Dahal, B.; Chhetri, K.; Mukhiya, T.; Muthurasu, A.; Gautam, J.; Lohani, P.C.; Acharya, D.; Pathak, I.; et al. Homogeneous Elongation of N-Doped CNTs over Nano-Fibrillated Hollow-Carbon-Nanofiber: Mass and Charge Balance in Asymmetric Supercapacitors Is no Longer Problematic. Adv. Sci. 2022, 2200650. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Sun, F.; Dorrell, D.G. A review of supercapacitor modeling, estimation, and applications: A control/management perspective. Renew. Sustain. Energy Rev. 2018, 81, 1868–1878. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, J.; Eid, K.; Ali, R.; Fan, X.; Murtaza, G.; Faizan, M.; Laref, A.; Zheng, W.; Varma, R.S. Engineering of Transition Metal Sulfide Nanostructures as Efficient Electrodes for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Tiwari, A.P.; Mukhiya, T.; Muthurasu, A.; Ojha, G.P.; Lee, M.; Kim, T.; Chae, S.-H.; Kim, H.Y. Controlled Selenium Infiltration of Cobalt Phosphide Nanostructure Arrays from a Two-Dimensional Cobalt Metal–Organic Framework: A Self-Supported Electrode for Flexible Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Wu, H.B.; Yu, X.-Y.; Zhang, X.; Lou, X.W. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015, 6, 6694. [Google Scholar] [CrossRef] [PubMed]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-Friendly Synthesis of Cobalt Molybdenum Hydroxide 3d Nanostructures on Carbon Fabric Coupled with Cherry Flower Waste-Derived Activated Carbon for Quasi-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Chhetri, K.; Kim, H.; Ji, S.; Chae, S.-H.; Kim, T.; Kim, H.Y. Self-assembled polypyrrole hierarchical porous networks as the cathode and porous three dimensional carbonaceous networks as the anode materials for asymmetric supercapacitor. J. Energy Storage 2021, 33, 102080. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Sun, J.; Guo, L.; Sun, X.; Zhang, J.; Hou, L.; Li, L.; Yang, S.; Yuan, C. One-Dimensional Nanostructured Pseudocapacitive Materials: Design, Synthesis and Applications in Supercapacitors. Batter. Supercaps 2019, 2, 820–841. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Hsu, S.-H.; Wu, K.C.W.; Yamauchi, Y. Large-Scale Synthesis of Reduced Graphene Oxides with Uniformly Coated Polyaniline for Supercapacitor Applications. ChemSusChem 2014, 7, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.S.; Kim, K.M.; Park, N.-G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Jeyaranjan, A.; Sakthivel, T.S.; Neal, C.J.; Seal, S. Scalable ternary hierarchical microspheres composed of PANI/ rGO/CeO2 for high performance supercapacitor applications. Carbon 2019, 151, 192–202. [Google Scholar] [CrossRef]
- Ghosh, D.; Giri, S.; Mandal, A.; Das, C.K. Supercapacitor based on H+ and Ni2+ co-doped polyaniline–MWCNTs nanocomposite: Synthesis and electrochemical characterization. RSC Adv. 2013, 3, 11676–11685. [Google Scholar] [CrossRef]
- Chhetri, K.; Yadav, A.P. Electrochemical Behavior of Polyaniline (Pani) Doped with Different Nitrate Salts. J. Univ. Grant Commision 2018, 7, 14–24. [Google Scholar]
- Sumboja, A.; Wang, X.; Yan, J.; Lee, P.S. Nanoarchitectured current collector for high rate capability of polyaniline based supercapacitor electrode. Electrochim. Acta 2012, 65, 190–195. [Google Scholar] [CrossRef]
- Agudosi, E.S.; Abdullah, E.C.; Numan, A.; Mubarak, N.M.; Aid, S.R.; Benages-Vilau, R.; Gómez-Romero, P.; Khalid, M.; Omar, N. Fabrication of 3D binder-free graphene NiO electrode for highly stable supercapattery. Sci. Rep. 2020, 10, 11214. [Google Scholar] [CrossRef]
- Chapagain, A.; Acharya, D.; Das, A.K.; Chhetri, K.; Oli, H.B.; Yadav, A.P. Alkaloid of Rhynchostylis retusa as Green Inhibitor for Mild Steel Corrosion in 1 M H2SO4 Solution. Electrochem 2022, 3, 211–224. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.; Li, X.; Zhao, J.; Ma, J.; Liu, Y.; Lu, G. Preparation of NiO nanoparticles in microemulsion and its gas sensing performance. Mater. Lett. 2012, 68, 168–170. [Google Scholar] [CrossRef]
- Alagiri, M.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of NiO nanoparticles by sol–gel method. J. Mater. Sci. Mater. Electron. 2012, 23, 728–732. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Liu, Y.; Li, J.; Cui, H. One-pot solvothermal synthesis of size-controlled NiO nanoparticles. Adv. Powder Technol. 2019, 30, 861–868. [Google Scholar] [CrossRef]
- Bahari Molla Mahaleh, Y.; Sadrnezhaad, S.K.; Hosseini, D. NiO Nanoparticles Synthesis by Chemical Precipitation and Effect of Applied Surfactant on Distribution of Particle Size. J. Nanomater. 2008, 2008, 470595. [Google Scholar] [CrossRef] [Green Version]
- Al-Sehemi, A.G.; Al-Shihri, A.S.; Kalam, A.; Du, G.; Ahmad, T. Microwave synthesis, optical properties and surface area studies of NiO nanoparticles. J. Mol. Struct. 2014, 1058, 56–61. [Google Scholar] [CrossRef]
- Mohseni Meybodi, S.; Hosseini, S.A.; Rezaee, M.; Sadrnezhaad, S.K.; Mohammadyani, D. Synthesis of wide band gap nanocrystalline NiO powder via a sonochemical method. Ultrason. Sonochem. 2012, 19, 841–845. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, J.; Qiu, S.; Sun, L.; Xu, F.; Zhu, M.; Ouyang, L.; Sun, D. Thermal stability, decomposition and glass transition behavior of PANI/NiO composites. J. Therm. Anal. Calorim. 2009, 98, 533. [Google Scholar] [CrossRef]
- Shambharkar, B.H.; Umare, S.S. Synthesis and characterization of polyaniline/NiO nanocomposite. J. Appl. Polym. Sci. 2011, 122, 1905–1912. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Q.; Zhang, W.; Wang, Y.; Chen, W. Nano nickel oxide coated graphene/polyaniline composite film with high electrochemical performance for flexible supercapacitor. Electrochim. Acta 2016, 211, 1066–1075. [Google Scholar] [CrossRef]
- Singu, B.S.; Palaniappan, S.; Yoon, K.R. Polyaniline–nickel oxide nanocomposites for supercapacitor. J. Appl. Electrochem. 2016, 46, 1039–1047. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.-H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-derived nanoporous carbon nanocomposite wrapped with Co3O4-polyaniline as an efficient electrode material for an asymmetric supercapacitor. J. Electroanal. Chem. 2020, 856, 113670. [Google Scholar] [CrossRef]
- Bian, C.; Yu, A.; Wu, H. Fibriform polyaniline/nano-TiO2 composite as an electrode material for aqueous redox supercapacitors. Electrochem. Commun. 2009, 11, 266–269. [Google Scholar] [CrossRef]
- Chelliah, M.; Rayappan, J.B.B.; Krishnan, U.M. Synthesis and characterization of cerium oxide nanoparticles by hydroxide mediated approach. J. Appl. Sci. 2012, 12, 1734–1737. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, R.M.; Moopri Singer Pandiyarajan, S.; Saud, S.; Kim, S.J.; Mok, Y.S. Synergistic effects of nanocarbon spheres sheathed on a binderless CoMoO4 electrode for high-performance asymmetric supercapacitor. Dalton Trans. 2020, 49, 14506–14519. [Google Scholar] [CrossRef] [PubMed]
- Pilban Jahromi, S.; Pandikumar, A.; Goh, B.T.; Lim, Y.S.; Basirun, W.J.; Lim, H.N.; Huang, N.M. Influence of particle size on performance of a nickel oxide nanoparticle-based supercapacitor. RSC Adv. 2015, 5, 14010–14019. [Google Scholar] [CrossRef]
- Bhattarai, S.; Devkota, N.B.; Neupane, S.; Gupta, D.K.; Yadav, A.P. Synthesis and Characterization of Acid Doped Polyaniline for Super Capacitor Application. J. Nepal Chem. Soc. 2017, 37, 49–54. [Google Scholar] [CrossRef]
- Pandey, S.; Neupane, S.; Gupta, D.K.; Das, A.K.; Karki, N.; Singh, S.; Yadav, R.J.; Yadav, A.P. Ce-Doped PANI/Fe3O4 Nanocomposites: Electrode Materials for Supercapattery. Front. Chem. Eng. 2021, 3, 650301. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Chatterjee, K. Synthesis and characterization of nano-dimensional nickelous oxide (NiO) semiconductor. J. Phys. Sci. 2009, 13, 245–250. [Google Scholar]
- Atta, A.; Abdelhamied, M.M.; Essam, D.; Shaban, M.; Alshammari, A.H.; Rabia, M. Structural and physical properties of polyaniline/silver oxide/silver nanocomposite electrode for supercapacitor applications. Int. J. Energy Res. 2022, 46, 6702–6710. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Raghu, M.S.; Kumar, K.Y.; Rao, S.; Aravinda, T.; Prasanna, B.P.; Prashanth, M.K. Fabrication of polyaniline–few-layer MoS2 nanocomposite for high energy density supercapacitors. Polym. Bull. 2018, 75, 4359–4375. [Google Scholar] [CrossRef]

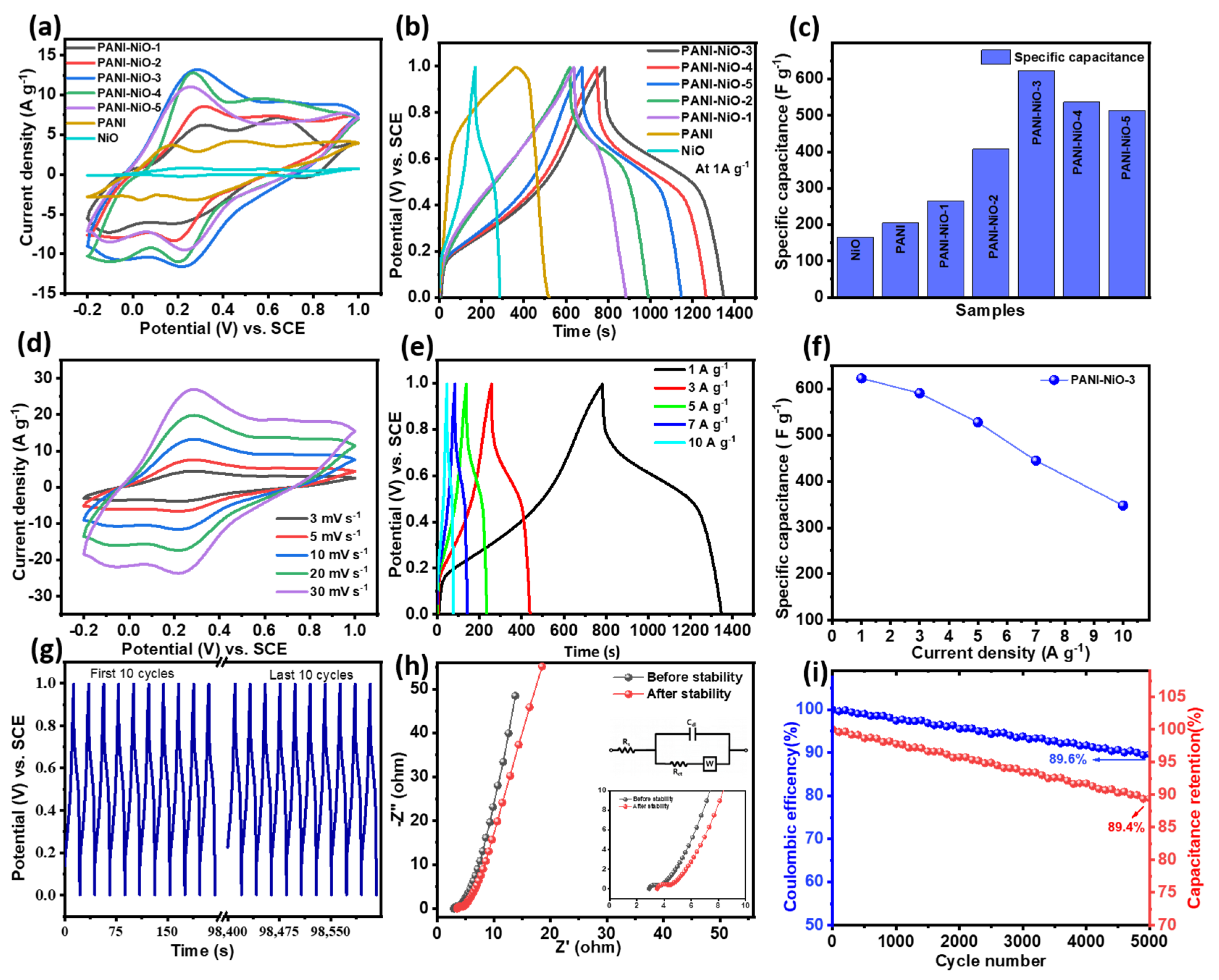
| Electrode Materials | PANI | NiO | PANI-NiO-1 | PANI-NiO-2 | PANI-NiO-3 | PANI-NiO-4 | PANI-NiO-5 |
|---|---|---|---|---|---|---|---|
| Specific capacitance (F g−1) | 205 | 165 | 265 | 407 | 623 | 537 | 514 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. https://doi.org/10.3390/inorganics10060086
Gautam KP, Acharya D, Bhatta I, Subedi V, Das M, Neupane S, Kunwar J, Chhetri K, Yadav AP. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics. 2022; 10(6):86. https://doi.org/10.3390/inorganics10060086
Chicago/Turabian StyleGautam, Krishna Prasad, Debendra Acharya, Indu Bhatta, Vivek Subedi, Maya Das, Shova Neupane, Jyotendra Kunwar, Kisan Chhetri, and Amar Prasad Yadav. 2022. "Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application" Inorganics 10, no. 6: 86. https://doi.org/10.3390/inorganics10060086









