Copper (II)-Catalyzed Oxidation of Ascorbic Acid: Ionic Strength Effect and Analytical Use in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantitative Analysis of Copper (II) Ions Using Ascorbic Acid Oxidation as an Indicator Reaction
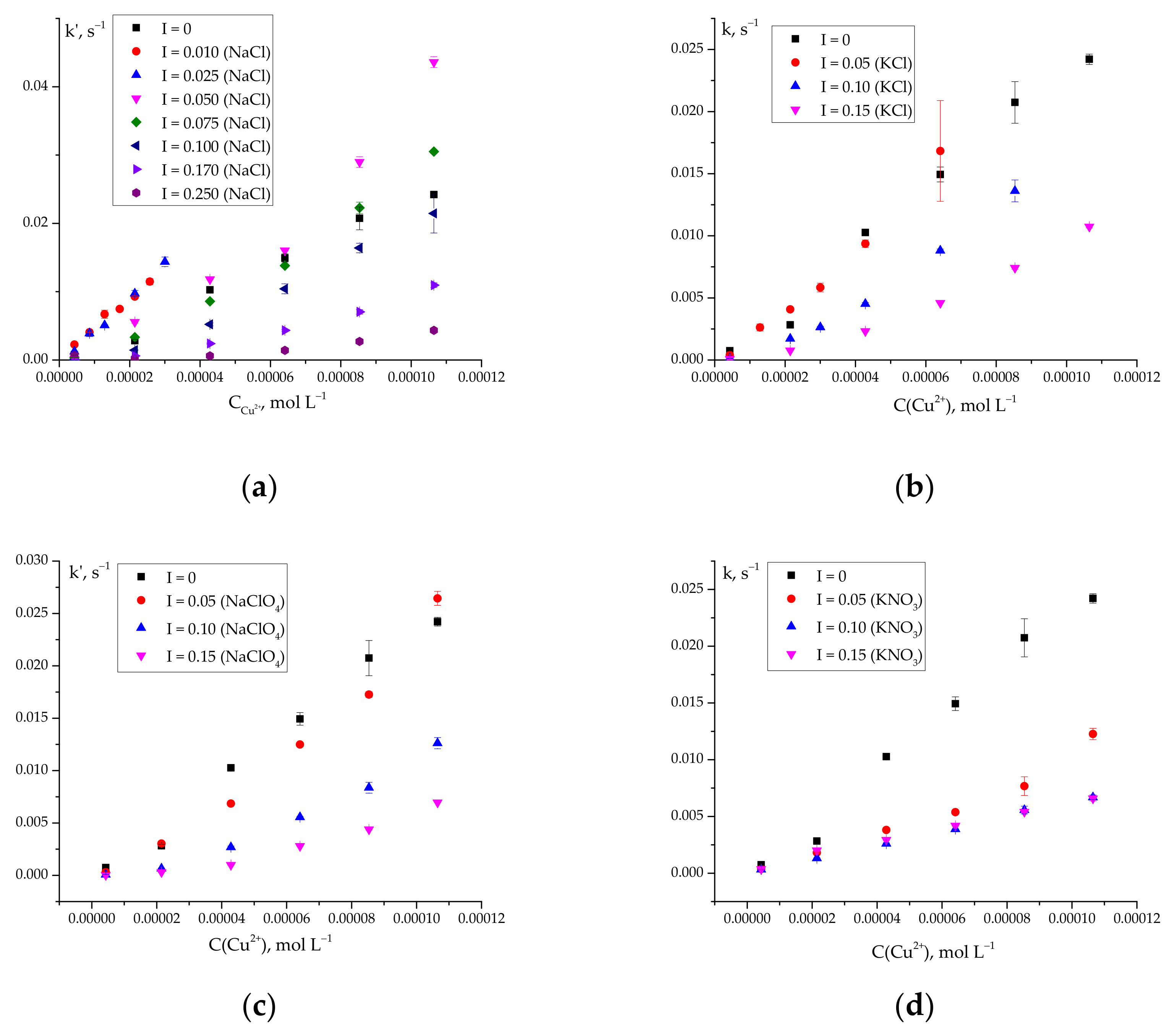
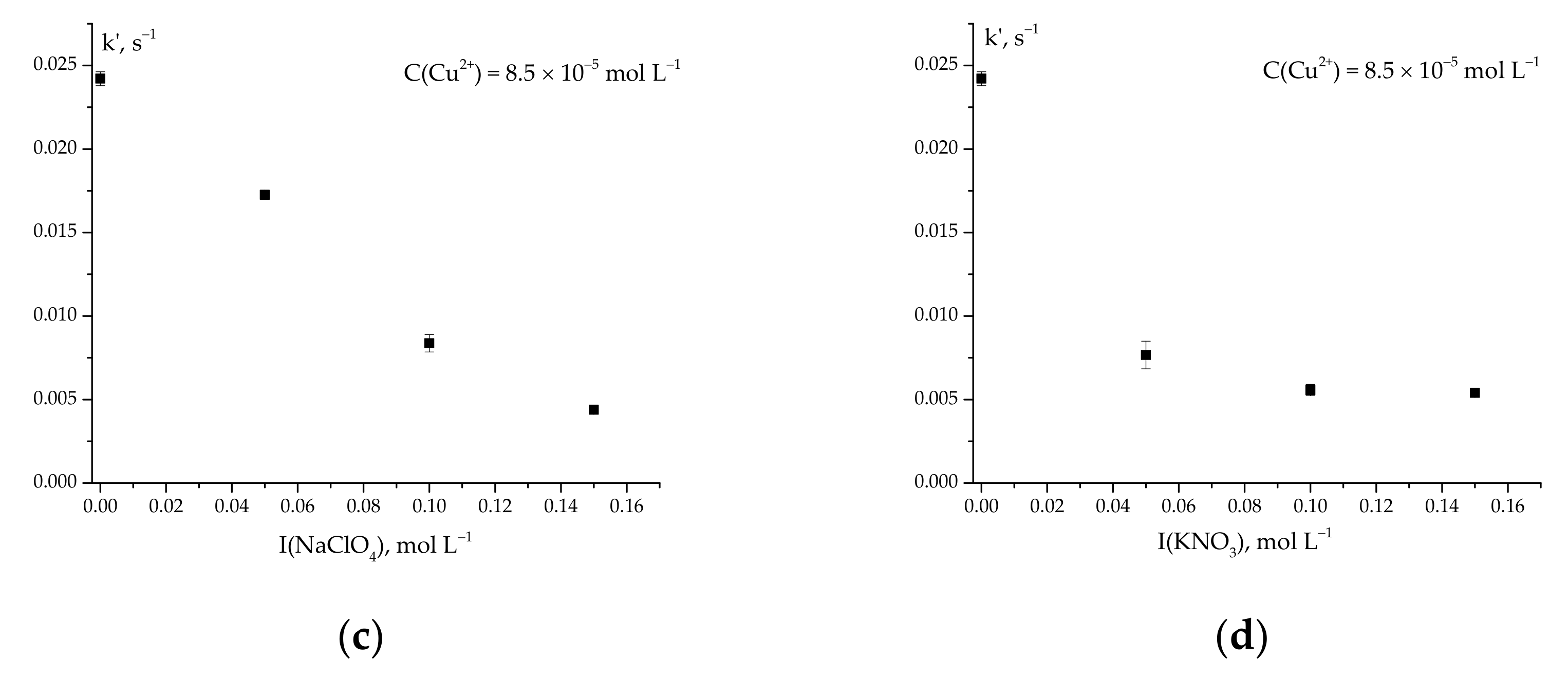
2.2. Effect of the Nature and Concentration of Background Electrolytes on the Rate of Ascorbic Acid Oxidation
k′ = 70 for CuCl+ and CuCl2
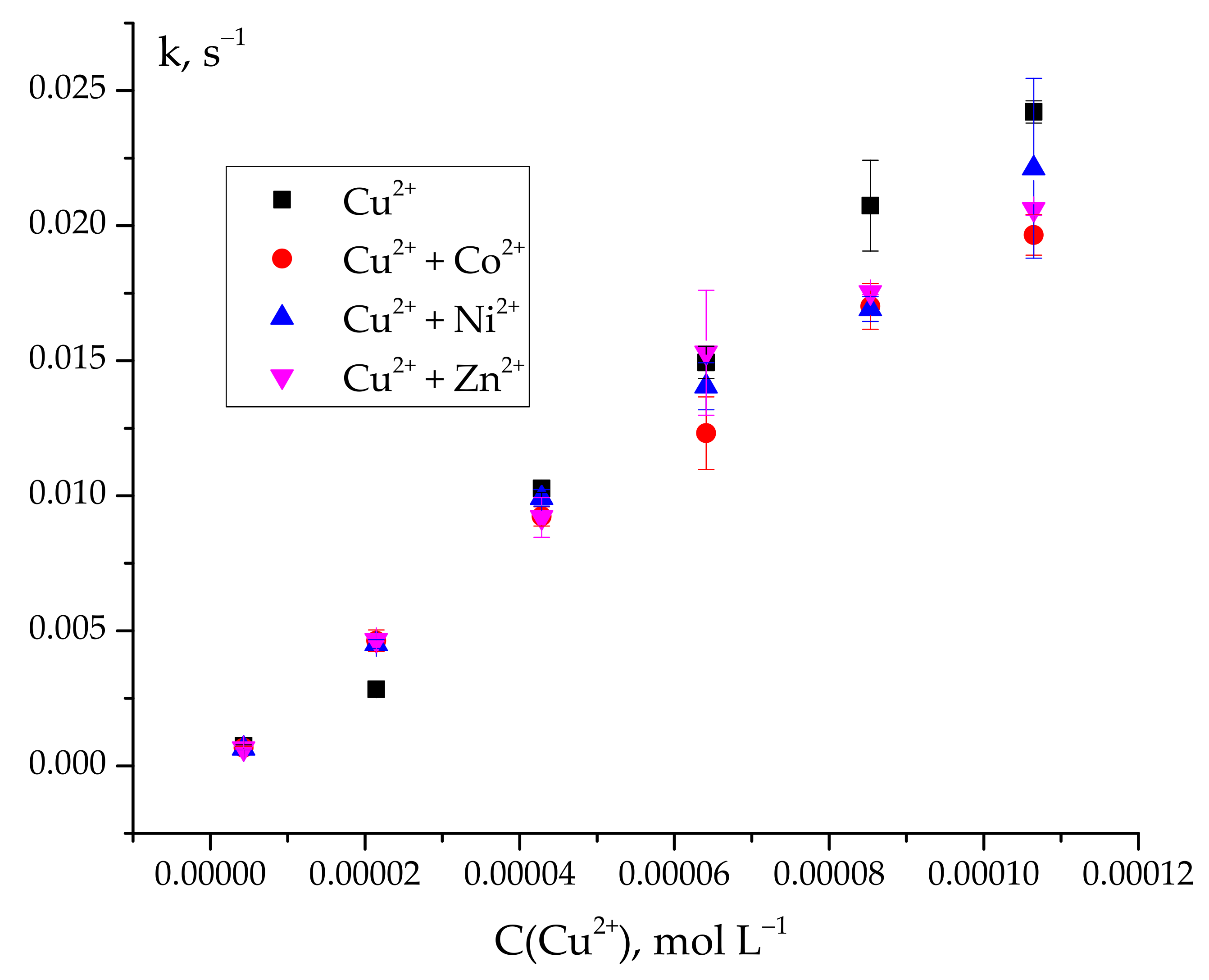
2.3. Selectivity of Copper (II) Quantitative Analysis Using Ascorbic Acid Oxidation as an Indicator Reaction
3. Materials and Methods
3.1. Chemicals
3.2. Copper (II) Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Osredkar, J.; Sustar, N. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, S3, 0495. [Google Scholar] [CrossRef] [Green Version]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaim, W.; Rall, J. Copper—A “Modern” Bioelement. Angew. Chem. Int. Ed. 1996, 35, 43–60. [Google Scholar] [CrossRef]
- Leal, P.P.; Hurd, C.L.; Sander, S.G.; Armstrong, E.; Fernandez, P.A.; Suhrhoff, T.J.; Roleda, M.Y. Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Sci. Rep. 2018, 8, 14763. [Google Scholar] [CrossRef]
- Luo, Y.; Rao, J.; Jia, Q. Heavy metal pollution and environmental risks in the water of Rongna River caused by natural AMD around Tiegelongnan copper deposit, Northern Tibet, China. PLoS ONE 2022, 17, e0266700. [Google Scholar] [CrossRef]
- Manne, R.; Kumaradoss, M.M.R.M.; Iska, R.S.R.; Devarajan, A.; Mekala, N. Water quality and risk assessment of copper content in drinking water stored in copper container. Appl. Water Sci. 2022, 12, 27. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Canc. 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Block, G. Vitamin C and cancer prevention: The epidemiologic evidence. Am. J. Clin. Nutr. 1991, 53, 270S–282S. [Google Scholar] [CrossRef] [Green Version]
- Patterson, R.E.; White, E.; Kristal, A.R.; Neuhouser, M.L.; Potter, J.D. Vitamin supplements and cancer risk: The epidemiologic evidence. Canc. Causes Control 1997, 8, 786–802. [Google Scholar] [CrossRef]
- Gey, K.F. Vitamins E plus C and interacting conutrients required for optimal health. BioFactors 1998, 7, 113–174. [Google Scholar] [CrossRef]
- Loria, C.M.; Klag, M.J.; Caulfield, L.E.; Whelton, P.K. Vitamin C status and mortality in US adults. Am. J. Clin. Nutr. 2000, 72, 139–145. [Google Scholar] [CrossRef]
- Fischer, A.P.; Miles, S.L. Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease Hypoxia Inducible Factor -1 alpha activity and reduce malignant potential in human melanoma. Biomed. Pharmacother. 2017, 86, 502–513. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Kononova, P.A.; Koshman, V.E.; Fedenok, L.G.; Polyakov, N.E. The Interplay of Ascorbic Acid with Quinones-Chelators—Influence on Lipid Peroxidation: Insight into Anticancer Activity. Antioxidants 2022, 11, 376. [Google Scholar] [CrossRef]
- Mumtaz, S.; Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Mughal, T.A.; Younas, M. Evaluation of antibacterial activity of vitamin C against human bacterial pathogens. Braz. J. Biol. 2021, 83, e247165. [Google Scholar] [CrossRef]
- Rosengrave, P.; Spencer, E.; Williman, J.; Mehrtens, J.; Morgan, S.; Doyle, T.; Van Der Heyden, K.; Morris, A.; Shaw, G.; Carr, A.C. Intravenous vitamin C administration to patients with septic shock: A pilot randomised controlled trial. Crit. Care 2022, 26, 26. [Google Scholar] [CrossRef]
- Muzaffar, S.N.; Saran, S.; Siddiqui, S.S. Vitamin C therapy in septic shock. Crit. Care 2022, 26, 87. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, O.; Baek, M.S.; Kim, W. Vitamin C for septic shock in previous randomized trials: Implications of erroneous dosing, timing, and duration. Crit. Care 2022, 26, 61. [Google Scholar] [CrossRef]
- Lavillegrand, J.; Raia, L.; Urbina, T.; Hariri, G.; Gabarre, P.; Bonny, V.; Bige, N.; Baudel, J.; Bruneel, A.; Dupre, T.; et al. Vitamin C improves microvascular reactivity and peripheral tissue perfusion in septic shock patients. Crit. Care 2022, 26, 25. [Google Scholar] [CrossRef]
- Yi, Y.; Wu, M.; Zhou, X.; Xiong, M.; Tan, Y.; Yu, H.; Liu, Z.; Wu, Y.; Zhang, Q. Ascorbic acid 2-glucoside preconditioning enhances the ability of bone marrow mesenchymal stem cells in promoting wound healing. Stem Cell Res. Ther. 2022, 13, 119. [Google Scholar] [CrossRef]
- Sampaio, I.; Quatroni, F.D.; Lins, P.M.P.; Nascimento, A.S.; Zucolotto, V. Modulation of beta-amyloid aggregation using ascorbic acid. Biochimie 2022, 200, 36–43. [Google Scholar] [CrossRef]
- Gamov, G.A.; Yarullin, D.N.; Gudyrina, M.A.; Pogodina, E.I.; Medvedeva, A.S.; Zavalishin, M.N. Protonation of L-Ascorbic Acid in an Aqueous Solution at T = 298.2 K, p = 0.1 MPa, and I = 0.10−5.0 mol L−1 (NaCl). J. Chem. Eng. Data 2022, 67, 1358–1364. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Zhang, Y.; Liu, Y.; Liang, J.; Liu, B.; Zhang, W. Generation of hydrogen peroxide and hydroxyl radical resulting from oxygen-dependent oxidation of L-ascorbic acid via copper redox-catalyzed reactions. RSC Adv. 2016, 6, 38541–38547. [Google Scholar] [CrossRef]
- Shen, J.; Griffiths, P.T.; Campbell, S.J.; Utinger, B.; Kalberer, M.; Paulson, S.E. Ascorbate oxidation by iron, copper and reactive oxygen species: Review, model development, and derivation of key rate constants. Sci. Rep. 2021, 11, 7417. [Google Scholar] [CrossRef]
- Mottola, H.A.; Perez-Bendito, D. Kinetic Determinations and Some Kinetic Aspects of Analytical Chemistry. Anal. Chem. 1994, 66, 131–162. [Google Scholar] [CrossRef] [PubMed]
- Crouch, S.R.; Cullen, T.F.; Scheeline, A.; Kirkor, E.S. Kinetic Determinations and Some Kinetic Aspects of Analytical Chemistry. Anal. Chem. 1998, 70, 53–106. [Google Scholar] [CrossRef]
- Cerda, V.; Gonzalez, A.; Danchana, K. From thermometric to spectrophotometric kinetic-catalytic methods of analysis. A review. Talanta 2017, 167, 733–746. [Google Scholar] [CrossRef]
- Campbell, S.J.; Utinger, B.; Lienhard, D.M.; Paulson, S.E.; Shen, J.; Griffiths, P.T.; Stell, A.C.; Kalberer, M. Development of a Physiologically Relevant Online Chemical Assay to Quantify Aerosol Oxidative Potential. Anal. Chem. 2019, 91, 13088–13095. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Mulholland, J.A.; Russell, A.G. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Godri, K.J.; Harrison, R.M.; Evans, T.; Baker, T.; Dunster, C.; Mudway, I.S.; Kelly, F.J. Increased Oxidative Burden Associated with Traffic Component of Ambient Particulate Matter at Roadside and Urban Background Schools Sites in London. PLoS ONE 2011, 6, e21961. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.T.; Mellado, J.M.R.; Medina, A. Rapid Electrochemical Determination of Antioxidant Capacity Using Glassy Carbon Electrodes Modified with Copper and Polyaniline. Application to Ascorbic and Gallic Acids. Bioint. Res. Appl. Chem. 2022, 13, 23. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, F.; Yin, L.; Qu, G.; Ma, D.; Leung, C.; Lu, L. Ratiometric fluorescent detection of pesticide based on split aptamer and magnetic separation. Sens. Actuat. B Chem. 2022, 367, 132045. [Google Scholar] [CrossRef]
- Cesario, D.; Furia, E.; Mazzone, G.; Beneduci, A.; De Luca, G.; Sicilia, E. Complexation of Al3+ and Ni2+ by L-Ascorbic Acid: An Experimental and Theoretical Investigation. J. Phys. Chem. A 2017, 121, 9773–9781. [Google Scholar] [CrossRef] [PubMed]
- Ritacca, A.G.; Malacaria, L.; Sicilia, E.; Furia, E.; Mazzone, G. Experimental and theoretical study of the complexation of Fe3+ and Cu2+ by L-ascorbic acid in aqueous solution. J. Mol. Liq. 2022, 355, 118973. [Google Scholar] [CrossRef]
- Anand, T.; Sivaraman, G.; Chellappa, D. Quinazoline copper(II) ensemble as turn-on fluorescence sensor for cysteine and chemodosimeter for NO. J. Photochem. Photobiol. A Chem. 2022, 281, 47–52. [Google Scholar] [CrossRef]
- Tian, M.; He, H.; Wang, B.; Wang, X.; Liu, Y.; Jiang, F. A reaction-based turn-on fluorescent sensor for the detection of Cu(II) with excellent sensitivity and selectivity: Synthesis, DFT calculations, kinetics and application in real water samples. Dyes Pigm. 2019, 165, 383–390. [Google Scholar] [CrossRef]
- Arslan, F.N.; Geyik, G.A.; Koran, K.; Ozen, F.; Aydin, D.; Elmas, S.N.K.; Gorgulu, A.O.; Yilmaz, I. Fluorescence “Turn On–Off” Sensing of Copper (II) Ions Utilizing Coumarin–Based Chemosensor: Experimental Study, Theoretical Calculation, Mineral and Drinking Water Analysis. J. Fluoresc. 2020, 30, 317–327. [Google Scholar] [CrossRef]
- Kim, G.; Choi, D.; Kim, C. A Benzothiazole-Based Fluorescence Turn-on Sensor for Copper(II). J. Fluoresc. 2021, 31, 1203–1209. [Google Scholar] [CrossRef]
- Silpcharu, K.; Soonthonhut, S.; Sukwattanasinitt, M.; Rashatasakhon, P. Fluorescent Sensor for Copper(II) and Cyanide Ions via the Complexation−Decomplexation Mechanism with Di(bissulfonamido)spirobifluorene. ACS Omega 2021, 6, 16696–16703. [Google Scholar] [CrossRef]
- Selvan, G.T.; Varadaraju, C.; Selvan, R.T.; Enoch, I.V.M.V.; Selvakumar, P.M. On/Off Fluorescent Chemosensor for Selective Detection of Divalent Iron and Copper Ions: Molecular Logic Operation and Protein Binding. ACS Omega 2018, 3, 7985–7992. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Xu, W.; Gan, K.; Xu, K.; Chen, Q.; Wei, W.; Wu, W. On the synthesis and performance of a simple colorimetric and fluorescent chemosensor for Cu2+ with good reversibility. Spectrochim. Acta A Mol. Biomol. Spec. 2022, 277, 121245. [Google Scholar] [CrossRef]
- Gauthama, B.U.; Narayana, B.; Sarojini, B.K.; Kodlady, S.N.; Sangappa, Y.; Kudva, A.K.; Raghu, V. A versatile rhodamine B-derived fluorescent probe for selective copper(II) sensing. Inorg. Chem. Comm. 2022, 141, 109501. [Google Scholar] [CrossRef]
- Sawminathan, S.; Munusamy, S.; Manickam, S.; Kulathulyera, S. A simple quinazolinone-isophorone based colorimetric chemosensor for the reversible detection of copper (II) and its application in real samples. J. Mol. Struct. 2022, 1257, 132633. [Google Scholar] [CrossRef]
- Heo, J.S.; Suh, B.; Kim, C. Selective detection of Cu2+ by benzothiazole-based colorimetric chemosensor: A DFT study. J. Chem. Sci. 2022, 134, 43. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. Water-soluble coumarin based sequential colorimetric and fluorescence on-off chemosensor for copper(II) and cyanide ions in water. Opt. Mater. 2022, 127, 112275. [Google Scholar] [CrossRef]
- Giri, P.K.; Samanta, S.S.; Mudi, N.; Shyamal, M.; Misra, A. Highly Sensitive ‘on–off’ Pyrene Based AIEgen for Selective Sensing of Copper (II) Ions in Aqueous Media. J. Fluoresc. 2022, 32, 1059–1071. [Google Scholar] [CrossRef]
- Lv, M.; Bian, L.; Wu, W.; Xu, Z.; Wang, Y.; Zhao, X.; Xu, Z.; Fan, Y.; Yan, L. Thiocarbazone-appended coumarin: An easily accessible ratiometric fluorescent chemosensor for multianalyte (Zn2+ and Cu2+) systems. Corol. Techn. 2022, 138, 157–167. [Google Scholar] [CrossRef]
- Isaad, J.; El Achari, A. Sequential colorimetric sensor for copper (II) and cyanide ions via the complexation−decomplexation mechanism based on sugar pyrazolidine-3,5-dione. J. Mol. Struct. 2022, 1252, 132151. [Google Scholar] [CrossRef]
- Kim, B.; Choi, H. Determination of Copper by Atomic Absorption Spectrophotometry After Solid-Liquid Separation by Adsorption of its 1-Nitroso-2-Naphthol Complex onto Microcrystalline Benzophenone. Anal. Lett. 1999, 32, 995–1009. [Google Scholar] [CrossRef]
- Ouyang, Z.; Chen, Z.; Mou, H. Determination of copper by Flame Atomic Absorption Spectrometry using Flow Injection On-line Preconcentration with Double Micro-columns. Rev. Anal. Chem. 2010, 29, 51–58. [Google Scholar] [CrossRef]
- Sahan, S.; Sahin, U. Determination of Copper(II) Using Atomic Absorption Spectrometry and Eriochrome Blue Black R Loaded Amberlite XAD-1180 Resin. Clean Soil Air Water 2010, 38, 485–491. [Google Scholar] [CrossRef]
- Bagherian, B.; Chamjangali, M.A.; Evari, H.S.; Ashrafi, M. Determination of copper(II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid–liquid microextraction technique. J. Anal. Sci. Tech. 2019, 10, 3. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of Detection in Spectroscopy. Spectroscopy 2003, 18, 112–114. [Google Scholar]
- Tan, M.; Sudjadi; Astuti, P.; Rohman, A. Validation and quantitative analysis of cadmium, chromium, copper, nickel, and lead in snake fruit by Inductively Coupled Plasma-Atomic Emission Spectroscopy. J. Appl. Pharm. Sci. 2018, 8, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Jameson, R.F.; Blackburn, N.J. Role of copper dimers and the participation of copper(III) in the copper-catalysed autoxidation of ascorbic acid. Part II. Kinetics and mechanism in 0.100 mol dm−3 potassium nitrate. J. Chem. Soc. Dalton Trans. 1976, 6, 534–541. [Google Scholar] [CrossRef]
- Davies, M.B. The copper(II) catalyzed reaction of L-ascorbinc acid with tris(oxalato)cobaltate(III) ion in aqueous solution. Inorg. Chim. Acta 1984, 92, 141–146. [Google Scholar] [CrossRef]
- Kriss, E.E.; Kurbatova, G.T. Vanadium(IV) complexation with ascorbic and dehydroascorbic acids. Zhurn. Neorg. Khim. 1976, 21, 2368–2374. (In Russian) [Google Scholar]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant metals with inorganic ligands Part 2: The Cu2+-OH−, Cl−, CO32−, SO42−, and PO43− systems (IUPAC Technical Report). Pure Appl. Chem. 2007, 79, 895–950. [Google Scholar] [CrossRef]
- Xiao, Z.; Gammons, C.H.; Williams-Jones, A.E. Experimental study of copper(I) chloride complexing in hydrothermal solutions at 40 to 300 °C and saturated water vapor pressure. Geochim. Cosmochim. Acta 1998, 62, 2949–2964. [Google Scholar] [CrossRef]
- Nicol, M.J. Kinetics of the oxidation of copper(I) by hydrogen peroxide in acidic chloride solutions. South Afr. J. Chem. 1982, 35, 77–79. [Google Scholar] [CrossRef]
- Yuan, X.; Pham, A.N.; Xing, G.; Rose, A.L.; Waite, T.D. Effects of pH, Chloride, and Bicarbonate on Cu(I) Oxidation Kinetics at Circumneutral pH. Environ. Sci. Technol. 2012, 46, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Davila, M.; Santana-Casiano, J.M.; Gonzalez, A.G.; Perez, N.; Millero, F.J. Oxidation of copper(I) in seawater at nanomolar levels. Marine Chem. 2009, 115, 118–124. [Google Scholar] [CrossRef]
- Van de Weert, M. Fluorescence Quenching to Study Protein-ligand Binding: Common Errors. J. Fluoresc. 2010, 20, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.chem.msu.su/rus/teaching/KINET2012/ (accessed on 13 June 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murekhina, A.E.; Yarullin, D.N.; Sovina, M.A.; Kitaev, P.A.; Gamov, G.A. Copper (II)-Catalyzed Oxidation of Ascorbic Acid: Ionic Strength Effect and Analytical Use in Aqueous Solution. Inorganics 2022, 10, 102. https://doi.org/10.3390/inorganics10070102
Murekhina AE, Yarullin DN, Sovina MA, Kitaev PA, Gamov GA. Copper (II)-Catalyzed Oxidation of Ascorbic Acid: Ionic Strength Effect and Analytical Use in Aqueous Solution. Inorganics. 2022; 10(7):102. https://doi.org/10.3390/inorganics10070102
Chicago/Turabian StyleMurekhina, Anastasia E., Daniil N. Yarullin, Maria A. Sovina, Pavel A. Kitaev, and George A. Gamov. 2022. "Copper (II)-Catalyzed Oxidation of Ascorbic Acid: Ionic Strength Effect and Analytical Use in Aqueous Solution" Inorganics 10, no. 7: 102. https://doi.org/10.3390/inorganics10070102






