Research Progress on Preparation Methods of Skutterudites
Abstract
:1. Introduction
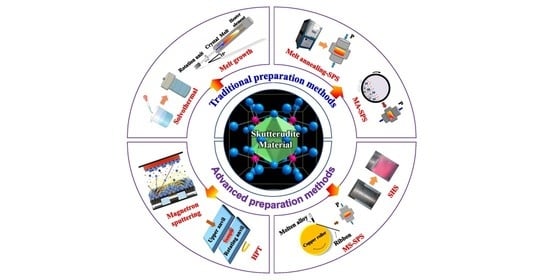
2. Traditional Preparation Methods
2.1. Melt Growth Method
2.2. Solvothermal Method
2.3. Solid Phase Reaction Method
2.4. Mechanical Alloying Method
3. New Preparation Methods
3.1. Melt Spinning
3.2. High-Temperature and High-Pressure Method
3.3. Pulsed Laser Deposition
3.4. Magnetron Sputtering
3.5. Molecular Beam Epitaxy (MBE)
3.6. Self-Spreading High-Temperature Synthetic (SHS)
3.7. Microwave Sintering
3.8. High Pressure Torsion (HPT)
4. Conclusions
- (1)
- We need to further understand the principles of some preparation methods of TE materials and theoretically understand the reaction mechanism of the preparation process, thus providing an important theoretical basis for further optimization of the preparation process;
- (2)
- We need to further optimize the existing advanced preparation process, such as process parameters and process flow;
- (3)
- The key to the large-scale production of TE materials is to further explore novel preparation processes. This new preparation process has the advanced characteristics of being less time- and energy-consuming and being more environment-friendly. The prepared TE materials have high density, are of uniform composition and provide stable performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, M.; Calvin, K.; Thomson, A.; Clarke, L.; Bond-Lamberty, B.; Sands, R.; Smith, S.J.; Janetos, A.; Edmonds, J. Implications of limiting CO2 concentrations for land use and energy. Science 2009, 324, 1183–1186. [Google Scholar] [CrossRef]
- Denilson, B.E.S. An energy and exergy analysis of a high-efficiency engine trigeneration system for a hospital: A case study methodology based on annual energy demand profiles. Energy Build. 2014, 76, 185–198. [Google Scholar]
- Ioffe, A.F.; Stil’bans, L.S.; Iordanishvili, E.K.; Stavitskaya, T.S.; Gelbtuch, A.; Vineyard, G. Semiconductor Thermoelements and Thermoelectric Cooling. Phys. Today 1959, 12, 42. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Wang, X.Y.; Liu, Y.J.; Cao, F.; Zhao, L.D.; Zhang, Q. Development on thermoelectric materials. J. Chin. Ceram. Soc. 2018, 46, 288–305. [Google Scholar]
- Qin, D.; Cui, B.; Yin, L.; Zhao, X.; Zhang, Q.; Cao, J.; Cai, W.; Sui, J. Tin Acceptor Doping Enhanced Thermoelectric Performance of n-Type Yb Single-Filled Skutterudites via Reduced Electronic Thermal Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 25133–25139. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Zhang, Z.W.; Liu, C.Y.; Gao, J.; Ye, Z.Y.; Chen, C.G.; Peng, Y.; Bai, X.B.; Miao, L. Substantial thermoelectric enhancement achieved by manipulating the band structure and dislocations in Ag and La co-doped SnTe. J. Adv. Ceram. 2021, 10, 860–870. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Anbalagan, R.; Bursik, J.; Kerber, M.; Schafler, E.; Zehetbauer, M.; Bauer, E.; Rogl, P. Direct SPD-processing to achieve high-ZT skutterudites. Acta Mater. 2018, 159, 352–363. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zou, M.C.; Zhang, W.Y.; Yi, D.; Lan, J.L.; Nan, C.-W.; Lin, Y.-H. Electrical and thermal transport behaviours of high-entropy perovskite thermoelectric oxides. J. Adv. Ceram. 2021, 10, 377–384. [Google Scholar] [CrossRef]
- Slack, G.A. CRC Handbook of Thermoelectric; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Xia, X.; Huang, X.; Li, X.; Gu, M.; Qiu, P.; Liao, J.; Tang, Y.; Bai, S.; Chen, L. Preparation and structural evolution of Mo/SiOx protective coating on CoSb3-based filled skutterudite thermoelectric material. J. Alloys Compd. 2014, 604, 94–99. [Google Scholar] [CrossRef]
- Drevet, R.; Aranda, L.; Petitjean, C.; David, N.; Veys-Renaux, D.; Berthod, P. Oxidation Behavior of the Skutterudite Material Ce0.75Fe3CoSb12. Oxid. Met. 2019, 91, 767–779. [Google Scholar] [CrossRef]
- Schmidt, R.D.; Case, E.D.; Ni, J.E.; Sakamoto, J.S.; Trejo, R.M.; Lara-Curzio, E.; Payzant, E.A.; Kirkham, M.J.; Peascoe-Meisner, R.A. The temperature dependence of thermal expansion for p-type Ce0.9Fe3.5Co0.5Sb12 and n-type Co0.95Pd0.05Te0.05Sb3 skutterudite thermoelectric materials. Philos. Mag. 2012, 92, 1261–1286. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Wang, B.; Li, W.; Che, P. A comparative study on the thermoelectric properties of CoSb3 prepared by hydrothermal and solvothermal route. J. Alloys Compd. 2019, 772, 770–774. [Google Scholar] [CrossRef]
- Ghosh, S.; Shankar, G.; Karati, A.; Werbach, K.; Rogl, G.; Rogl, P.; Bauer, E.; Murty, B.S.; Suwas, S.; Mallik, R.C. Enhanced Thermoelectric Performance in the Ba0.3Co4Sb12/InSb Nanocomposite Originating from the Minimum Possible Lattice Thermal Conductivity. ACS Appl. Mater. Interfaces 2020, 12, 48729–48740. [Google Scholar] [CrossRef]
- Wang, S.; Salvador, J.R.; Yang, J.; Wei, P.; Duan, B.; Yang, J. High-performance n-type YbxCo4Sb12: From partially filled skutterudites towards composite thermoelectrics. NPG Asia Mater. 2016, 8, e285. [Google Scholar] [CrossRef] [Green Version]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Peranio, N.; Bauer, E.; Zehetbauer, M.; Eibl, O. n-Type skutterudites (R;Ba;Yb)yCo4Sb12 (R=Sr; La; Mm; DD.; SrMm; SrDD) approaching ZT≈2.0. Acta Mater. 2014, 63, 30–43. [Google Scholar] [CrossRef]
- Zong, P.-A.; Hanus, R.; Dylla, M.; Tang, Y.; Liao, J.; Zhang, Q.; Snyder, G.J.; Chen, L. Skutterudite with graphene-modified grain-boundary complexion enhances zT enabling high-efficiency thermoelectric device. Energy Environ. Sci. 2017, 10, 183–191. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Zhu, J.-L.; Tong, X.; Niu, S.; Zhao, W.-Y. A review of CoSb3-based skutterudite thermoelectric materials. J. Adv. Ceram. 2020, 9, 647–673. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Z.; Tong, X.; Xia, A.; Xu, D.; Lei, Y.; Yu, J.; Tang, D.; Ruan, X.; Zhao, W. Synergistic Optimization of Electrical-Thermal-Mechanical Properties of the In-Filled CoSb3 Material by Introducing Bi0.5Sb1.5Te3 Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 23894–23904. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Wang, A.Q.; Jia, X.P.; Wang, F.B.; Yang, A.L.; Huang, H.L.; Zuo, G.H.; Wang, L.B.; Deng, L. Optimization of thermoelectric properties of CoSb3 materials by increasing the complexity of chemical structure. J. Alloys Compd. 2020, 843, 156063. [Google Scholar] [CrossRef]
- Matsubara, M.; Asahi, R. Optimization of filler elements in CoSb3-based skutterudites for high-performance n-type thermoelectric materials. J. Electron. Mater. 2015, 45, 1669–1678. [Google Scholar] [CrossRef]
- Tong, X.; Liu, Z.Y.; Zhu, J.L.; Yang, T.; Wang, Y.G.; Xia, A.L. Research progress of p-type Fe-based skutterudite thermoelectric materials. Front. Mater. Sci. 2021, 15, 317–333. [Google Scholar] [CrossRef]
- Ghosh, S.; Valiyaveettil, S.M.; Shankar, G.; Maity, T.; Chen, K.-H.; Biswas, K.; Suwas, S.; Mallik, R.C. Enhanced thermoelectric properties of in-filled Co4Sb12 with InSb nanoinclusions. ACS Appl. Energy Mater. 2020, 3, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.Y.; Liu, Z.Y.; Sun, Z.G.; Zhang, Q.J.; Wei, P.; Mu, X.; Zhou, H.Y.; Li, C.C.; Ma, S.F.; He, D.Q.; et al. Superparamagnetic enhancement of thermoelectric performance. Nature 2017, 549, 247–251. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Liu, Z.Y.; Wei, P.; Zhang, Q.J.; Zhu, W.T.; Su, X.L.; Tang, X.F.; Yang, J.H.; Liu, Y.; Shi, J.; et al. Magnetoelectric interaction and transport behaviors in magnetic nanocomposite thermoelectric materials. Nat. Nanotechnol. 2017, 12, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Zhu, J.L.; Wei, P.; Zhu, W.T.; Zhao, W.Y.; Xia, A.L.; Xu, D.; Lei, Y.; Yu, J. Candidate for magnetic doping agent and high-temperature thermoelectric performance enhancer: Hard magnetic m-type BaFe12O19 nanometer suspension. ACS Appl. Mater. Interfaces 2019, 11, 45875–45884. [Google Scholar] [CrossRef]
- Liang, T.; Su, X.; Yan, Y.; Zheng, G.; Zhang, Q.; Chi, H.; Tang, X.; Uher, C. Ultra-fast synthesis and thermoelectric properties of Te doped skutterudites. J. Mater. Chem. A 2014, 2, 17914–17918. [Google Scholar] [CrossRef]
- Pillaca, M.; Harder, O.; Miller, W.; Gille, P. Forced convection by Inclined Rotary Bridgman method for growth of CoSb3 and FeSb2 single crystals from Sb-rich solutions. J. Cryst. Grow. 2017, 475, 346–353. [Google Scholar] [CrossRef]
- Caillat, T.; Fleurial, J.-P.; Borshchevsky, A. Bridgman-solution crystal growth and characterization of the skutterudite compounds CoSb3 and RhSb3. J. Cryst. Grow. 1996, 166, 722–726. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Li, X.; Zhong, H. Microstructure and thermoelectric properties of doped p-type CoSb3 under TGZM effect. J. Cryst. Grow. 2017, 466, 56–63. [Google Scholar] [CrossRef]
- Qin, Z.; Cai, K.F.; Chen, S.; Du, Y. Preparation and electrical transport properties of In filled and Te-doped CoSb3 skutterudite. J. Mater. Sci. 2013, 24, 4142–4147. [Google Scholar] [CrossRef]
- Kumar, M.U.; Swetha, R.; Kumari, L. Structural and Optical Studies on Strontium-Filled CoSb3 Nanoparticles Via a Solvo-/Hydrothermal Method. J. Electron. Mater. 2021, 50, 1735–1741. [Google Scholar] [CrossRef]
- Gharleghi, A.; Pai, Y.-H.; Lin, F.-H.; Liu, C.-J. Low thermal conductivity and rapid synthesis of n-type cobalt skutterudite via a hydrothermal method. J. Mater. Chem. C 2014, 2, 4213–4220. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.G.; Lee, H.; Wang, D.Z.; Ren, Z.F.; Fleurial, J.P.; Gogna, P. New Directions for Low-Dimensional Thermoelectric Materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, W.-Y.; Wei, P.; Tang, D.-G.; Zhang, Q.-J. Effects of excess Sb on thermoelectric properties of barium and indium double-filled iron-based p-type skutterudite materials. J. Electron. Mater. 2012, 41, 1414–1420. [Google Scholar] [CrossRef]
- Wan, S.; Huang, X.; Qiu, P.; Shi, X.; Chen, L. Compound Defects and Thermoelectric Properties of Self-Charge Compensated Skutterudites SeyCo4Sb12-xSex. ACS Appl. Mater. Interfaces 2017, 9, 22713–22724. [Google Scholar] [CrossRef]
- Yang, K.; Cheng, H.; Hng, H.H.; Ma, J.; Mi, J.L.; Zhao, X.B.; Zhu, T.J.; Zhang, Y.B. Synthesis and thermoelectric properties of double-filled skutterudites CeyYb0.5−yFe1.5Co2.5Sb12. J. Alloys Compd. 2009, 467, 528–532. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Z.H.; Xie, H.Q. Enhanced thermoelectric properties of CoSb3-based skutterudites by filling Se as electronegative element. Mater. Res. Express 2018, 6, 2053. [Google Scholar] [CrossRef]
- Wan, S.; Qiu, P.F.; Huang, X.Y.; Song, Q.F.; Bai, S.Q.; Shi, X.; Chen, L.D. Synthesis and Thermoelectric Properties of Charge-Compensated SyPdxCo4-xSb12 Skutterudites. ACS Appl. Mater. Interfaces 2017, 10, 625–634. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Wang, G.; Chi, H.; Zhou, X.; Tang, X.; Zhang, Q.; Uher, C. Structure and Transport Properties of Double-Doped CoSb2.75Ge0.25–xTex (x = 0.125–0.20) with in Situ Nanostructure. Chem. Mater. 2011, 23, 2948–2955. [Google Scholar] [CrossRef]
- Taha, M.A.; Youness, R.A.; Zawrah, M.F. Review on nanocomposites fabricated by mechanical alloying. Int. J. Min. Met. Mater. 2019, 26, 1047–1058. [Google Scholar] [CrossRef]
- Ur, S.-C.; Kwon, J.-C.; Kim, I.-H. Thermoelectric properties of Fe-doped CoSb3 prepared by mechanical alloying and vacuum hot pressing. J. Alloys Compd. 2007, 442, 358–361. [Google Scholar] [CrossRef]
- Liu, W.-S.; Zhang, B.-P.; Li, J.-F.; Zhao, L.-D. Thermoelectric property of fine-grained CoSb3 skutterudite compound fabricated by mechanical alloying and spark plasma sintering. J. Phys. D 2007, 40, 566–572. [Google Scholar] [CrossRef]
- Lin, B.L.; Tang, X.F.; Qi, Q.; Zhang, Q.J. Preparation and thermal transport properties of CoSb3 nano-compounds. Acta Phys. Sin. 2004, 53, 3130–3135. [Google Scholar]
- Trivedi, V.; Battabyal, M.; Balasubramanian, P.; Muralikrishna, G.M.; Jain, P.K.; Gopalan, R. Microstructure and doping effect on the enhancement of the thermoelectric properties of Ni doped Dy filled CoSb3 skutterudites. Sustain. Energy Fuels 2018, 2, 2687–2697. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Yubuta, K.; Puchegger, S.; Bauer, E.; Raju, C.; Mallik, R.C.; Rogl, P. In-doped multifilled n-type skutterudites with ZT=1.8. Acta Mater. 2015, 95, 201–211. [Google Scholar] [CrossRef]
- Thomas, R.; Rao, A.; Chauhan, N.S.; Vishwakarma, A.; Singh, N.K.; Soni, A. Melt spinning: A rapid and cost effective approach over ball milling for the production of nanostructured p-type Si80Ge20 with enhanced thermoelectric properties. J. Alloys Compd. 2019, 781, 344–350. [Google Scholar] [CrossRef]
- Kim, T.S.; Chun, B.S. Thermoelectric Properties of n-Typen-type 90%Bi2Te3+10%Bi2Se3 Thermoelectric Materials Produced by Melt Spinning Method and Sintering. Mater. Sci. Forum 2007, 534–536, 161–164. [Google Scholar] [CrossRef]
- Jie, Q.; Zhou, J.; Dimitrov, I.K. Thermoelectric properties of non-equilibrium synthesized Ce0.9Fe3CoSb12 filled skutterudites. MRS Proc. 2010, 1267, 55–60. [Google Scholar] [CrossRef]
- Son, G.; Lee, K.H.; Choi, S.-M. Enhanced Thermoelectric Properties of Melt-Spun p-Type Yb0.9Fe3CoSb12. J. Electron. Mater. 2016, 46, 2839–2843. [Google Scholar] [CrossRef]
- Tan, H.; Guo, L.; Wang, G.; Wu, H.; Shen, X.; Zhang, B.; Lu, X.; Wang, G.; Zhang, X.; Zhou, X. Synergistic Effect of Bismuth and Indium Codoping for High Thermoelectric Performance of Melt Spinning SnTe Alloys. ACS Appl. Mater. Interfaces 2019, 11, 23337–23345. [Google Scholar] [CrossRef]
- Thompson, D.R.; Liu, C.; Ellison, N.D.; Salvador, J.R.; Meyer, M.S.; Haddad, D.B.; Wang, H.; Cai, W. Improved thermoelectric performance of n-type Ca and Ca-Ce filled skutterudites. J. Appl. Phys. 2014, 116, 243701. [Google Scholar] [CrossRef]
- Sun, H.; Jia, X.; Lv, P.; Deng, L.; Guo, X.; Zhang, Y.; Sun, B.; Liu, B.; Ma, H. Improved thermoelectric performance of Te-doped and CNT dispersed CoSb3 skutterudite bulk materials via HTHP. RSC Adv. 2015, 5, 61324–613429. [Google Scholar] [CrossRef]
- Sun, H.; Jia, X.; Deng, L.; Lv, P.; Guo, X.; Sun, B.; Zhang, Y.; Liu, B.; Ma, H. Impacts of both high pressure and Te-Se double-substituted skutterudite on the thermoelectric properties prepared by HTHP. J. Alloys Compd. 2014, 615, 1056–1059. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.B.; Li, D.N.; Deng, L.; Jia, X.P.; Ma, H.A. Effects of pressure and ions doping on the optimization of double filled CoSb3 thermoelectric materials. Mater. Lett. 2019, 237, 49–52. [Google Scholar] [CrossRef]
- Kong, L.; Jia, X.; Zhang, Y.; Sun, B.; Liu, B.; Liu, H.; Wang, C.; Liu, B.; Chen, J.; Ma, H. N-type Ba0.3Ni0.15Co3.85Sb12 skutterudite: High pressure processing technique and thermoelectric properties. J. Alloys Compd. 2018, 734, 36–42. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, X.; Ma, H. The thermoelectric properties of CoSb3 compound doped with Te and Sn synthesized at different pressure. Mod. Phys. Lett. B 2017, 31, 1750261. [Google Scholar] [CrossRef]
- Deng, L.; Jia, X.P.; Su, T.C.; Jiang, Y.P.; Zheng, S.Z.; Guo, X.; Ma, H.A. The enhanced thermoelectric properties of Ba0.25Pb0.05Co4Sb11.5Te0.5 alloys prepared by HPHT at different pressure. Mater. Lett. 2011, 65, 1582–1594. [Google Scholar] [CrossRef]
- De, V.J.C.; Lee, D.; Shin, H.; Namuco, S.B.; Hwang, I.; Sarmago, R.V.; Song, J.H. Influence of deposition conditions on the growth of micron-thick highly c-axis textured superconducting GdBa2Cu3O7-delta films on SrTiO3 (100). J. Vac. Sci. Technol. 2018, 36, 031506. [Google Scholar]
- Sarath, K.S.R.; Alyamani, A.; Graff, J.W.; Tritt, T.M.; Alshareef, H.N. Pulsed laser deposition and thermoelectric properties of In- and Yb-doped CoSb3 skutterudite thin films. J. Mater. Res. 2011, 26, 1836–1841. [Google Scholar] [CrossRef]
- Jelínek, M.; Zeipl, R.; Kocourek, T.; Remsa, J.; Navrátil, J. Thermoelectric nanocrystalline YbCoSb laser prepared layers. Appl. Phys. A 2016, 122, 155. [Google Scholar] [CrossRef]
- Masarrat, A.; Bhogra, A.; Meena, R.; Bala, M.; Singh, R.; Barwal, V.; Dong, C.L.; Chen, C.L.; Som, T.; Kumar, A.; et al. Effect of Fe ion implantation on the thermoelectric properties and electronic structures of CoSb3 thin films. RSC Adv. 2019, 9, 36113–36122. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Fan, P.; Wei, M.; Zheng, Z.-H.; Zhang, X.-H.; Ma, H.-L.; Luo, J.-T.; Liang, G.-X. Effects of Ag-doped content on the microstructure and thermoelectric properties of CoSb3 thin films. Thin Solid Films 2019, 679, 49–54. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Li, F.; Li, F.; Li, Y.-Z.; Fan, P.; Luo, J.-T.; Liang, G.-X.; Fan, B.; Zhong, A.-H. Thermoelectric properties of co-sputtered CoSb3 thin films as a function of stoichiometry. Thin Solid Films 2017, 632, 88–92. [Google Scholar] [CrossRef]
- Li, Y.D.; Zheng, Z.H.; Fan, P.; Luo, J.T.; Liang, G.X.; Huang, B.X. Thermoelectric Characterization of Ti and In Double-Doped Cobalt Antimony Thin Films. Mater. Sci. Forum. 2016, 847, 143–147. [Google Scholar] [CrossRef]
- Zhou, J.M. Development of molecular beam epitaxy in China. Physics 2021, 50, 843–848. [Google Scholar]
- Goodhue, W.G.; Reeder, R.E.; Vineis, C.J.; Calawa, S.D.; Dauplaise, H.M.; Vangala, S.; Walsh, M.P.; Harman, T.C. High-output-power densities from molecular beam epitaxy grown n- and p-type PbTeSe-based thermoelectrics via improved contact metallization. J. Appl. Phys. 2012, 111, 104501. [Google Scholar] [CrossRef]
- Daniel, M.V.; Brombacher, C.; Beddies, G.; Jöhrmann, N.; Hietschold, M.; Johnson, D.C.; Aabdin, Z.; Peranio, N.; Eibl, O.; Albrecht, M. Structural properties of thermoelectric CoSb3 skutterudite thin films prepared by molecular beam deposition. J. Alloys Compd. 2015, 624, 216–225. [Google Scholar] [CrossRef]
- Peranio, N.; Eibl, O.; Bäßler, S.; Nielsch, K.; Klobes, B.; Hermann, R.P.; Daniel, M.; Albrecht, M.; Görlitz, H.; Pacheco, V.; et al. From thermoelectric bulk to nanomaterials: Current progress for Bi2Te3and CoSb3. Phys. Status Solidi A 2016, 213, 739–749. [Google Scholar] [CrossRef]
- Makogon, Y.N.; Pavlova, E.P.; Sidorenko, S.I.; Shkarban’, R.A.; Figurnaya, E.V. Effect of Sb content on the phase composition of CoSbx nanofilms grown on a heated substrate. Inorg. Mater. 2014, 50, 431–436. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, J.; Fan, W.; Zhang, H.; Chen, S.; Wu, Y.; Tang, X.; Xu, B. Energy-Efficient Synthesis and Superior Thermoelectric Performance of Sb-doped Mg2Si0.3Sn0.7 Solid Solutions by Rapid Thermal Explosion. Mater. Res. Bull. 2020, 128, 110885. [Google Scholar] [CrossRef]
- Liu, R.; Tan, X.; Ren, G.; Liu, Y.; Zhou, Z.; Liu, C.; Lin, Y.; Nan, C. Enhanced Thermoelectric Performance of Te-Doped Bi2Se3−xTex Bulks by Self-Propagating High-Temperature Synthesis. Crystals 2017, 7, 257. [Google Scholar] [CrossRef] [Green Version]
- Roslyakov, S.I.; Kovalev, D.Y.; Rogachev, A.S. Solution Combustion Synthesis: Dynamics of Phase Formation for Highly Porous Nickel. Dokl. Phys. Chem. 2013, 449, 48–51. [Google Scholar] [CrossRef]
- Su, X.; Fu, F.; Yan, Y.; Zheng, G.; Liang, T.; Zhang, Q.; Cheng, X.; Yang, D.; Chi, H.; Tang, X.; et al. Self-propagating high-temperature synthesis for compound thermoelectrics and new criterion for combustion processing. Nat. Commun. 2014, 5, 4908. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, R.; Sun, Y.-Y.; Chen, F.; Zhao, K.; Zhu, T.; Bai, S.; Chen, L. Self-propagation high-temperature synthesis of half-Heusler thermoelectric materials:reaction mechanism and applicability. J. Mater. Chem. 2018, 6, 19470–19478. [Google Scholar] [CrossRef]
- Xing, Y.F.; Liu, R.H.; Liao, J.C.; Zhang, Q.H.; Xia, X.G.; Wang, C.; Huang, H.; Chu, J.; Gu, M.; Zhu, T.J.; et al. High-efficiency half-Heusler thermoelectric modules enabled by self-propagating synthesis and topologic structure optimization. Energy Environ. Sci. 2019, 12, 3390–3399. [Google Scholar] [CrossRef]
- Kruszewski, M.J.; Cymerman, K.; Zybała, R.; Chmielewski, M.; Kowalczyk, M.; Zdunek, J.; Ciupiński, Ł. High homogeneity and ultralow lattice thermal conductivity in Se/Te-doped skutterudites obtained by self-propagating high-temperature synthesis and pulse plasma sintering. J. Alloys Compd. 2022, 909, 164796. [Google Scholar] [CrossRef]
- Biswas, K.; Muir, S.; Subramanian, M.A. Rapid microwave synthesis of indium filled skutterudites: An energy efficient route to high performance thermoelectric materials. Mater. Res. Bull. 2011, 46, 2288–2290. [Google Scholar] [CrossRef]
- Thiruppathi, K.; Raghuraman, S.; Mohan, R.R. Densification Studies on Aluminum-Based Brake Lining Composite Processed by Microwave and Spark Plasma Sintering. Powder Metall. Met. Ceram. 2021, 60, 44–51. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, W.; Zheng, R.; Li, Y.; Wan, R.; Chen, W.; Ma, L.; Zhou, H.; Chu, P.K. Rapid synthesis; microstructure; and thermoelectric properties of skutterudites. J. Alloys Compd. 2019, 806, 537–542. [Google Scholar] [CrossRef]
- Lei, Y.; Gao, W.; Zheng, R.; Li, Y.; Chen, W.; Zhang, L.; Wan, R.; Zhou, H.; Liu, Z.; Chu, P.K. Ultrafast Synthesis of Te-Doped CoSb3 with Excellent Thermoelectric Properties. ACS Appl. Energy Mater. 2019, 2, 4477–4485. [Google Scholar] [CrossRef]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia, R.N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Gao, W.S.; Li, Y.; Wan, R.D.; Chen, W.; Zheng, R.; Ma, L.Q.; Zhou, H.W. Structure and thermoelectric performance of Ti-filled and Te-doped skutterudite TixCo4Sb11.5Te0.5 bulks fabricated by combination of microwave synthesis and spark plasma sintering. Mater. Lett. 2018, 233, 166–169. [Google Scholar] [CrossRef]
- Zehetbauer, M.J.; Zhu, Y.T. Bulk Nanostructured Materials; VCH Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Royanian, E.; Bauer, E.; Horky, J.; Setman, D.; Schafler, E.; Zehetbauer, M. Dependence of thermoelectric behaviour on severe plastic deformation parameters: A case study on p-type skutterudite DD0.60Fe3CoSb12. Acta Mater. 2013, 61, 6778–6789. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Heinrich, P.; Bauer, E.; Kumar, P.; Peranio, N.; Eibl, O.; Horky, J.; Zehetbauer, M.; Rogl, P. New bulk p-type skutterudites DD0.7Fe2.7Co1.3Sb12-xXx (X = Ge, Sn) reaching ZT > 1.3. Acta Mater. 2015, 91, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Rogl, G.; Aabdin, Z.; Schafler, E. Effect of HPT processing on the structure, thermoelectric and mechanical properties of Sr0.07Ba0.07Yb0.07Co4Sb12. J. Alloys Compd. 2012, 537, 183–189. [Google Scholar] [CrossRef]
- Rogl, G.; Ghosh, S.; Renk, O.; Yubuta, K.; Grytsiv, A.; Schafler, E.; Zehetbauer, M.; Mallik, R.C.; Bauer, E.; Rogl, P. HPT production of large bulk skutterudites. J. Alloys Compd. 2021, 854, 156678. [Google Scholar] [CrossRef]













| Traditional Preparation Method | Skutterudites | Preparation Time | ZT | Grain Size | References |
|---|---|---|---|---|---|
| Melt growth | CoSb3 | 840 h | — | — | [28] |
| CoSb3 | 40 h | 0.47 | 20–200 μm | [30] | |
| Solvothermal | InxCo4Sb12 | 24 h | 0.92 | 1–5 μm | [31] |
| SryCoSb3 | 24 h | — | 50–120 nm | [32] | |
| CoSb3 | 12 h | 0.016 (RT) | 50–100 nm | [33] | |
| Solid phase reaction | BaInFe3.7Co0.3Sb12+m | 192 h | 0.63 | 2–10 μm | [35] |
| SeyCo4–−xPdxSb12−2ySe2y | 60 h | 0.9 | 1–4 μm | [38] | |
| SyPdxCo4−xSb12 | 72 h | 0.85 | 1–3 μm | [39] | |
| CoSb2.75Ge0.25−xTex | 198 h | 1.1 | 5–40 nm | [40] | |
| MA | FexCo4−xSb12 | 100 h | 0.3 | 20–80 nm | [42] |
| Dy0.4Co3.2Ni0.8Sb12 | 10 h | 1.4 | 40–200 nm | [45] | |
| Advanced Preparation Method | Component | Time | ZT | Grain Size | References |
| MS | Ce0.9Fe3CoSb12 | 30 h | 0.7 | 1–4 μm | [49] |
| Yb0.9Fe3CoSb12 | 12 h | 0.7 | 100–300 nm | [50] | |
| YbxBayCo4Sb12 | 30 min | 1.1 | — | [52] | |
| HTHP | In0.05Ba0.15Co4Sb11.5Te0.5 | — | 1.23 | 1–3 μm | [55] |
| Ba0.3Ni0.15Co3.85Sb12 | 25 min | 0.91 | — | [56] | |
| CoSb2.75Te0.20Sn0.05 | 30 min | 1.17 | — | [57] | |
| Pulsed Laser Deposition | In0.2Yb0.2Co4Sb12 | 20 ns | — | 90–110 nm | [60] |
| Yb0.19Co4Sb12 | 20 ns | 0.05 (RT) | 50–80 nm | [61] | |
| CoSb3 | 20 ns | — | 50–100 nm | [62] | |
| Magnetron sputtering | CoSb3 (Ag sputter) | 15 min | 2.97×10−4 | — | [63] |
| CoSb3 (Co-excess or Sb-excess) | 30 min | 6.9×10−4 | — | [65] | |
| CoSb3(In and Ti sputter) | 1–30 min | 2.32×10−4 | — | [66] | |
| MBE | CoSby | 10 min | — | 50–150 nm | [69] |
| Ybz(CoSb3)4 | 1–3 h | >1 | — | [70] | |
| CoSbx | — | — | 30 nm | [71] | |
| SHS | CoSb2.85Te0.15 | 10–20 min (SHS-PAS) | 0.98 | 1–2 μm | [27] |
| Co4Sb10.8Te0.6Se0.6 | 10–20 min (SHS-PPS) | 1.1 | — | [78] | |
| Microwave sintering | In0.2Co4Sb12 | 4 min | 0.9 | — | [79] |
| Co4Sb11.9−xTexSe0.1 | 5 min | 0.81 | 1–6 μm | [81] | |
| CoSb3−xTex | 5 min | 1.06 | 2–5 μm | [82] | |
| TixCo4Sb11.5Te0.5 | 5 min | 0.6 | 1–4 μm | [84] | |
| HPT-SPD | DD0.7Fe2.7Co1.3Sb11.7Ge0.1 | ~15 min | >1 | 15–300 nm | [87] |
| DD0.7Fe2.7Co1.3Sb11.8Sn0.2 | ~15 min | 1.4 | 15–300 nm | [87] | |
| Sr0.07Ba0.07Yb0.07Co4Sb12 | ~15 min | 1.8 | 5–250 nm | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wang, M.; Liu, Z. Research Progress on Preparation Methods of Skutterudites. Inorganics 2022, 10, 106. https://doi.org/10.3390/inorganics10080106
Zhao C, Wang M, Liu Z. Research Progress on Preparation Methods of Skutterudites. Inorganics. 2022; 10(8):106. https://doi.org/10.3390/inorganics10080106
Chicago/Turabian StyleZhao, Chengyu, Minhua Wang, and Zhiyuan Liu. 2022. "Research Progress on Preparation Methods of Skutterudites" Inorganics 10, no. 8: 106. https://doi.org/10.3390/inorganics10080106