Recent Advances in Lanthanide Metal–Organic Framework Thin Films Based on Eu, Tb, Gd: Preparation and Application as Luminescent Sensors and Light-Emitting Devices
Abstract
:1. Introduction
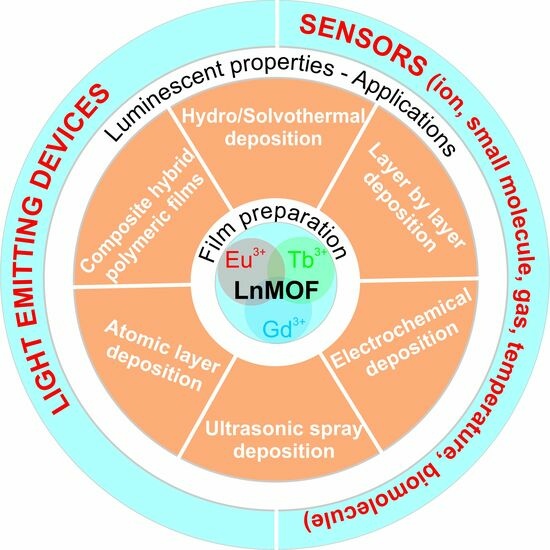
2. Preparation Methods of LnMOF-TFs
- Casted MOF-TFs—solvothermally synthesized nanocrystalline powders are cast onto a pretreated substrate;
- SURMOFs—Surface-supported Metal–Organic Frameworks—fabricated using the layer-by-layer method, where the orientation and film thickness can be easily and precisely controlled;
- Electrochemically (electrophoretically) deposited MOF films;
- Ultrasonic spray-deposited MOF;
- MOF-TFs made by using vapor–solid synthesis—ALD/MLD.
2.1. Solvo/Hydrothermal Deposition
2.1.1. In Situ Direct Growth
2.1.2. In Situ Secondary Growth
2.2. Layer-by-Layer Deposition
2.3. Electrochemical Deposition
2.3.1. Cathodic Electrodeposition
2.3.2. Anodic Electrodeposition
2.3.3. Electrophoretic Deposition
2.4. Ultrasonic Spray Deposition
2.5. Atomic Layer Deposition/Molecular Layer Deposition
2.6. Composite Hybrid Films
2.6.1. Nanoparticle-Based Films
2.6.2. Mixed Matrix Membranes
2.6.3. Polymeric-Based Hybrid Films
2.6.4. Postsynthetic Modification
2.7. Differences between Pure and Composite Films
3. Luminescent Properties of LnMOF-TFs
3.1. Structure of Ligands Involved in Coordination with Lanthanide Ions
3.2. Lanthanide-Centered Luminescence
3.3. Ligand-Based Luminescence
3.4. Guest-Induced Luminescence
4. Light-Emitting Devices
5. Application of LnMOF Films as Sensors
5.1. Cations Sensing
5.2. Anions Sensing
5.3. Small Molecules, Gas, and Vapor Sensing
5.4. Temperature Sensing
5.5. Sensing of Biomolecules
6. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, F.; Drake, H.F.; Feng, L.; Powell, J.A.; Wang, K.Y.; Yan, T.H.; Zhou, H.C. Metal–Organic Frameworks as Versatile Platforms for Organometallic Chemistry. Inorganics 2021, 9, 27. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Jonnalagadda, S.B. The Pioneering Role of Metal-Organic Framework-5 in Ever-Growing Contemporary Applications—A Review. RSC Adv. 2022, 12, 14282–14298. [Google Scholar] [CrossRef]
- Ellis, J.E.; Crawford, S.E.; Kim, K.J. Metal-Organic Framework Thin Films as Versatile Chemical Sensing Materials. Mater. Adv. 2021, 2, 6169–6196. [Google Scholar] [CrossRef]
- Song, X.Z.; Song, S.Y.; Zhang, H.J. Luminescent Lanthanide Metal-Organic Frameworks. Struct. Bond. 2015, 163, 109–144. [Google Scholar] [CrossRef]
- Zhuang, Z.; Liu, D. Conductive MOFs with Photophysical Properties: Applications and Thin-Film Fabrication; Springer: Singapore, 2020; Volume 12, ISBN 0123456789. [Google Scholar]
- Bai, W.; Li, S.; Ma, J.; Cao, W.; Zheng, J. Ultrathin 2D Metal-Organic Framework (Nanosheets and Nanofilms)-Based: X D-2D Hybrid Nanostructures as Biomimetic Enzymes and Supercapacitors. J. Mater. Chem. A 2019, 7, 9086–9098. [Google Scholar] [CrossRef]
- Xu, G.; Yamada, T.; Otsubo, K.; Sakaida, S.; Kitagawa, H. Facile “Modular Assembly” for Fast Construction of a Highly Oriented Crystalline MOF Nanofilm. J. Am. Chem. Soc. 2012, 134, 16524–16527. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, C.H. Metal-Organic Framework Thin Films: Fabrication, Modification, and Patterning. Processes 2020, 8, 377. [Google Scholar] [CrossRef]
- Soni, S.; Bajpai, P.K.; Arora, C. A Review on Metal-Organic Framework: Synthesis, Properties and Application. Charact. Appl. Nanomater. 2020, 3, 551. [Google Scholar] [CrossRef]
- Meyer, L.V.; Schönfeld, F.; Buschbaum, K.M. Lanthanide Based Tuning of Luminescence in MOFs and Dense Frameworks—From Mono- and Multimetal Systems to Sensors and Films. Chem. Commun. 2014, 50, 8093–8108. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Chen, C.; Xu, C.; Ma, J.; Yang, L.; Wang, W.; Dou, W.; Liu, W. Function-Oriented: The Construction of Lanthanide MOF Luminescent Sensors Containing Dual-Function Urea Hydrogen-Bond Sites for Efficient Detection of Picric Acid. Chem. A Eur. J. 2019, 25, 1090–1097. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Fu, Z.-H.; Xu, G. Metal-Organic Framework Nanosheets: Preparation and Applications. Coord. Chem. Rev. 2019, 388, 79–106. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.; Zhang, H.; Wang, A.; Lo, W.S.; Dong, Q.; Wong, N.; Povinelli, C.; Shao, Y.; Chereddy, S.; et al. A Metal–Organic Framework Thin Film for Selective Mg2+ Transport. Angew. Chem. Int. Ed. 2019, 58, 15313–15317. [Google Scholar] [CrossRef]
- Liu, T.Y.; Yuan, H.G.; Liu, Y.Y.; Ren, D.; Su, Y.C.; Wang, X. Metal-Organic Framework Nanocomposite Thin Films with Interfacial Bindings and Self-Standing Robustness for High Water Flux and Enhanced Ion Selectivity. ACS Nano 2018, 12, 9253–9265. [Google Scholar] [CrossRef]
- De Luna, P.; Liang, W.; Mallick, A.; Shekhah, O.; García De Arquer, F.P.; Proppe, A.H.; Todorović, P.; Kelley, S.O.; Sargent, E.H.; Eddaoudi, M. Metal-Organic Framework Thin Films on High-Curvature Nanostructures Toward Tandem Electrocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 31225–31232. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Bahrami, M.; Nabavizadeh, S.M. ZIF-8 Nanoparticles Thin Film at an Oil-Water Interface as an Electrocatalyst for the Methanol Oxidation Reaction without the Application of Noble Metals. New J. Chem. 2019, 43, 15811–15822. [Google Scholar] [CrossRef]
- Fang, X.; Zong, B.; Mao, S. Metal–Organic Framework-Based Sensors for Environmental Contaminant Sensing. Nano-Micro Lett. 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Chocarro-Ruiz, B.; Pérez-Carvajal, J.; Avci, C.; Calvo-Lozano, O.; Alonso, M.I.; Maspoch, D.; Lechuga, L.M. A CO2 Optical Sensor Based on Self-Assembled Metal-Organic Framework Nanoparticles. J. Mater. Chem. A 2018, 6, 13171–13177. [Google Scholar] [CrossRef]
- Elmehrath, S.; Nguyen, H.L.; Karam, S.M.; Amin, A. BioMOF-Based Anti-Cancer Drug Delivery Systems. Nanomaterials 2023, 13, 953. [Google Scholar] [CrossRef]
- Zhao, S.N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef]
- Liao, Z.; Xia, T.; Yu, E.; Cui, Y. Luminescent Metal–Organic Framework Thin Films: From Preparation to Biomedical Sensing Applications. Crystals 2018, 8, 338. [Google Scholar] [CrossRef]
- Ramya, A.R.; Sharma, D.; Natarajan, S.; Reddy, M.L.P. Highly Luminescent and Thermally Stable Lanthanide Coordination Polymers Designed from 4-(Dipyridin-2-Yl)Aminobenzoate: Efficient Energy Transfer from Tb3+ to Eu3+ in a Mixed Lanthanide Coordination Compound. Inorg. Chem. 2012, 51, 8818–8826. [Google Scholar] [CrossRef]
- Ramya, A.R.; Varughese, S.; Reddy, M.L.P. Tunable White-Light Emission from Mixed Lanthanide (Eu3+, Gd3+, Tb3+) Coordination Polymers Derived from 4-(Dipyridin-2-Yl)Aminobenzoate. Dalton Trans. 2014, 43, 10940–10946. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, L.; Yue, B. Luminescent Properties and Recent Progress in Applications of Lanthanide Metal-Organic Frameworks. Chin. Chem. Lett. 2023, 34, 108009. [Google Scholar] [CrossRef]
- Gomez, G.E.; Roncaroli, F. Photofunctional Metal-Organic Framework Thin Films for Sensing, Catalysis and Device Fabrication. Inorganica Chim. Acta 2020, 513, 119926. [Google Scholar] [CrossRef]
- Seethalekshmi, S.; Ramya, A.R.; Reddy, M.L.P.; Varughese, S. Lanthanide Complex-Derived White-Light Emitting Solids: A Survey on Design Strategies. J. Photochem. Photobiol. C Photochem. Rev. 2017, 33, 109–131. [Google Scholar] [CrossRef]
- Tang, T.; Liu, M.; Chen, Z.; Wang, X.; Lai, C.; Ding, L.; Zeng, C. Highly Sensitive Luminescent Lanthanide Metal–Organic Framework Sensor for L-Kynurenine. J. Rare Earths 2022, 40, 415–420. [Google Scholar] [CrossRef]
- Liu, W.; Li, D.; Wang, F.; Chen, X.; Wang, X.; Tian, Y. A Luminescent Lanthanide MOF as Highly Selective and Sensitive Fluorescent Probe for Nitrobenzene and Fe3+. Opt. Mater. 2022, 123, 111895. [Google Scholar] [CrossRef]
- Min, J.; Qu, X.L.; Yan, B. Tb Post-Functionalized La (III) Metal Organic Framework Hybrid Probe for Simple and Highly Sensitive Detection of Acetaldehyde. Sens. Actuators B Chem. 2019, 300, 126985. [Google Scholar] [CrossRef]
- Li, C.; Zeng, C.; Chen, Z.; Jiang, Y.; Yao, H.; Yang, Y.; Wong, W.T. Luminescent Lanthanide Metal-Organic Framework Test Strip for Immediate Detection of Tetracycline Antibiotics in Water. J. Hazard. Mater. 2020, 384, 121498. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.L.; Yan, B. Ln(III)-Functionalized Metal-Organic Frameworks Hybrid System: Luminescence Properties and Sensor for Trans, Trans-Muconic Acid as a Biomarker of Benzene. Inorg. Chem. 2018, 57, 7815–7824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.W.; Zhang, F.Q.; Zhang, X.M. Single Component Lanthanide Hybrids Based on Metal-Organic Framework for Near-Ultraviolet White Light LED. ACS Appl. Mater. Interfaces 2016, 8, 24123–24130. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Yan, B. Tunable Multi-Color Luminescence and White Emission in Lanthanide Ion Functionalized Polyoxometalate-Based Metal–Organic Frameworks Hybrids and Fabricated Thin Films. J. Alloys Compd. 2019, 777, 415–422. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Du, S. Tunable Luminescence and White Light Emission of Mixed Lanthanide-Organic Frameworks Based on Polycarboxylate Ligands. J. Mater. Chem. C 2016, 4, 3364–3374. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D. Lanthanide-Functionalized Metal-Organic Frameworks as Ratiometric Luminescent Sensors. J. Mater. Chem. C 2020, 8, 12739–12754. [Google Scholar] [CrossRef]
- Ji, G.; Liu, J.; Gao, X.; Sun, W.; Wang, J.; Zhao, S.; Liu, Z. A Luminescent Lanthanide MOF for Selectively and Ultra-High Sensitively Detecting Pb2+ Ions in Aqueous Solution. J. Mater. Chem. A 2017, 5, 10200–10205. [Google Scholar] [CrossRef]
- Zhao, Y.; Wan, M.Y.; Bai, J.P.; Zeng, H.; Lu, W.; Li, D. PH-Modulated Luminescence Switching in a Eu-MOF: Rapid Detection of Acidic Amino Acids. J. Mater. Chem. A 2019, 7, 11127–11133. [Google Scholar] [CrossRef]
- Wu, K.Y.; Qin, L.; Fan, C.; Cai, S.L.; Zhang, T.T.; Chen, W.H.; Tang, X.Y.; Chen, J.X. Sequential and Recyclable Sensing of Fe3+ and Ascorbic Acid in Water with a Terbium(Iii)-Based Metal-Organic Framework. Dalton Trans. 2019, 48, 8911–8919. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Xiao, W.; He, M.; Yu, J.; Bai, Y.; Guan, Y. Optical Determination of Nitro Phenol via Ratiometric Emission from Tb:Eu-MOFs: Chemical Synthesis and Spectral Response. J. Photochem. Photobiol. A Chem. 2020, 389, 112194. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Liu, K.; Wang, B. Luminescent Mixed-Crystal Ln-MOF Thin Film for the Recognition and Detection of Pharmaceuticals. Sens. Actuators B Chem. 2018, 257, 931–935. [Google Scholar] [CrossRef]
- Yang, X.; Lin, X.; Zhao, Y.; Zhao, Y.S.; Yan, D. Lanthanide Metal–Organic Framework Microrods: Colored Optical Waveguides and Chiral Polarized Emission. Angew. Chem. Int. Ed. 2017, 56, 7853–7857. [Google Scholar] [CrossRef]
- Guo, H.; Wu, N.; Xue, R.; Liu, H.; Li, L.; Wang, M.-Y.; Yao, W.-Q.; Li, Q.; Yang, W. Multifunctional Ln-MOF Luminescent Probe Displaying Superior Capabilities for Highly Selective Sensing of Fe3+ and Al3+ Ions and Nitrotoluene. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124094. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, P.; Liu, J.; Chen, X.; Guo, X.; Jin, H.; Chai, J.; Wang, L.; Fan, Y. Multi-Responsive Luminescent Sensor Based on Three Dimensional Lanthanide Metal-Organic Framework. New J. Chem. 2018, 42, 19485–19493. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, C.C.; Zhang, X.W.; Ren, X.Y.; Yu, B.; Wang, P.; Zhao, Z.X.; Fu, H. A New Eu-MOF for Ratiometrically Fluorescent Detection toward Quinolone Antibiotics and Selective Detection toward Tetracycline Antibiotics. Chin. Chem. Lett. 2022, 33, 1353–1357. [Google Scholar] [CrossRef]
- Dou, Z.; Yu, J.; Xu, H.; Cui, Y.; Yang, Y.; Qian, G. Preparation and Thiols Sensing of Luminescent Metal-Organic Framework Films Functionalized with Lanthanide Ions. Microporous Mesoporous Mater. 2013, 179, 198–204. [Google Scholar] [CrossRef]
- Liu, X.; Fu, W.; Bouwman, E. One-Step Growth of Lanthanoid Metal-Organic Framework (MOF) Films under Solvothermal Conditions for Temperature Sensing. Chem. Commun. 2016, 52, 6926–6929. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Yan, B. Determination of Urinary N-Acetylneuraminic Acid for Early Diagnosis of Lung Cancer by a Boric Acid Covalently Functionalized Lanthanide MOFs and Its Intelligent Visual Molecular Robot Application. Sens. Actuators B Chem. 2021, 349, 130736. [Google Scholar] [CrossRef]
- Balderas, J.U.; Navarro, D.; Vargas, V.; Tellez-Cruz, M.M.; Carmona, S.; Falcony, C. Ultrasonic Spray Deposition as a New Route to Luminescent MOF Film Synthesis. J. Lumin. 2019, 212, 322–327. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Spanopoulos, I.; Trikalitis, P.N.; Eddaoudi, M. Liquid Phase Epitaxial Growth of Heterostructured Hierarchical MOF Thin Films. Chem. Commun. 2017, 53, 6191–6194. [Google Scholar] [CrossRef] [PubMed]
- Mártire, A.P.; Segovia, G.M.; Azzaroni, O.; Rafti, M.; Marmisollé, W. Layer-by-Layer Integration of Conducting Polymers and Metal Organic Frameworks onto Electrode Surfaces: Enhancement of the Oxygen Reduction Reaction through Electrocatalytic Nanoarchitectonics. Mol. Syst. Des. Eng. 2019, 4, 893–900. [Google Scholar] [CrossRef]
- Kim, K.J.; Zhang, Y.; Kreider, P.B.; Chong, X.; Wang, A.X.; Ohodnicki, P.R.; Baltrus, J.P.; Chang, C.H. Nucleation and Growth of Oriented Metal-Organic Framework Thin Films on Thermal SiO2 Surface. Thin Solid Film. 2018, 659, 24–35. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, C.A.; Chen, R.; Sheng, L.; Wang, J. Comparison of Fabrication Methods of Metal-Organic Framework Optical Thin Films. Nanomaterials 2018, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yan, B. Polymer Hybrid Thin Films Based on Rare Earth Ion-Functionalized MOF: Photoluminescence Tuning and Sensing as a Thermometer. Dalton Trans. 2015, 44, 1875–1881. [Google Scholar] [CrossRef]
- Duan, T.W.; Yan, B. Hybrids Based on Lanthanide Ions Activated Yttrium Metal-Organic Frameworks: Functional Assembly, Polymer Film Preparation and Luminescence Tuning. J. Mater. Chem. C 2014, 2, 5098–5104. [Google Scholar] [CrossRef]
- Gu, Z.G.; Chen, Z.; Fu, W.Q.; Wang, F.; Zhang, J. Liquid-Phase Epitaxy Effective Encapsulation of Lanthanide Coordination Compounds into MOF Film with Homogeneous and Tunable White-Light Emission. ACS Appl. Mater. Interfaces 2015, 7, 28585–28590. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, W.; Yan, B. Multi-Step Tandem Functionalization Assembly of MOFs-Based Hybrid Polymeric Films for Color Tuning Luminescence and Responsive Sensing on Organic Vapors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129416. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF Thin Films: Existing and Future Applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Shan, Y.; Du, M.; Pang, H. Synthesis and Application of Metal-Organic Framework Films. Coord. Chem. Rev. 2021, 444, 214060. [Google Scholar] [CrossRef]
- Liu, J.; Wöll, C. Surface-Supported Metal-Organic Framework Thin Films: Fabrication Methods, Applications, and Challenges. Chem. Soc. Rev. 2017, 46, 5730–5770. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal-Organic Framework Membranes: From Synthesis to Separation Application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Li, W. Metal–Organic Framework Membranes: Production, Modification, and Applications. Prog. Mater. Sci. 2019, 100, 21–63. [Google Scholar] [CrossRef]
- Li, C.; Zhang, F.; Li, X.; Zhang, G.; Yang, Y. A Luminescent Ln-MOF Thin Film for Highly Selective Detection of Nitroimidazoles in Aqueous Solutions Based on Inner Filter Effect. J. Lumin. 2019, 205, 23–29. [Google Scholar] [CrossRef]
- Ji, Y.; Qian, W.; Yu, Y.; An, Q.; Liu, L.; Zhou, Y.; Gao, C. Recent Developments in Nanofiltration Membranes Based on Nanomaterials. Chin. J. Chem. Eng. 2017, 25, 1639–1652. [Google Scholar] [CrossRef]
- Sahoo, S.; Mondal, S.; Sarma, D. Luminescent Lanthanide Metal Organic Frameworks (LnMOFs): A Versatile Platform towards Organomolecule Sensing. Coord. Chem. Rev. 2022, 470, 214707. [Google Scholar] [CrossRef]
- Crivello, C.; Sevim, S.; Graniel, O.; Franco, C.; Pane, S.; Puigmarti-Luis, J.; Munoz-Rojas, D. Advanced Technologies for the Fabrication of MOF Thin Films. Mater. Horiz. 2021, 8, 168–178. [Google Scholar] [CrossRef]
- Jia, P.; Wang, Z.; Zhang, Y.; Zhang, D.; Gao, W.; Su, Y.; Li, Y.; Yang, C. Selective Sensing of Fe3+ Ions in Aqueous Solution by a Biodegradable Platform Based Lanthanide Metal Organic Framework. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118084. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, R.; Wang, X.; Liu, F.; Gao, Y.; Guan, R.; Chen, Y. Flexible and Washable CDs@Eu-MOFs/PVDF Multifunctional Thin Films as Highly Selective Sensing for Nitrobenzene and 4-Nitrophenol. Inorg. Chem. Commun. 2023, 149, 110423. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Y.; Qiu, S.; Lercher, A.J.; Zhang, H. Coordination Modulation Induced Synthesis of Nanoscale Eu 1-Tbxmetal-Organic Frameworks for Luminescent Thin Films. Adv. Mater. 2010, 22, 4190–4192. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, S.; Cai, D.; Liu, C. Fabrication of ITO Glass Supported Tb-MOF Film for Sensing Organic Solvent. Inorg. Chem. Commun. 2014, 41, 29–32. [Google Scholar] [CrossRef]
- Ozer, R.R.; Hinestroza, J.P. One-Step Growth of Isoreticular Luminescent Metal-Organic Frameworks on Cotton Fibers. RSC Adv. 2015, 5, 15198–15204. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Streckova, M.; Medvecky, L.; Sopcak, T.; Shepa, I.; Kovalcikova, A.; Lisnichuk, M.; Kolev, H. Transformation of Amorphous Terbium Metal–Organic Framework on Terbium Oxide TbOx(111) Thin Film on Pt(111) Substrate: Structure of TbxOy Film. Nanomaterials 2022, 12, 2817. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Kovalcikova, A.; Molcanova, Z.; Podobova, M.; Medvecky, L. Preparation and Characterization of Isostructural Lanthanide Eu/Gd/Tb Metal-Organic Framework Thin Films for Luminescent Applications. Appl. Surf. Sci. 2021, 542, 148731. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, T.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. In Situ Secondary Growth of Eu(III)-Organic Framework Film for Fluorescence Sensing of Sulfur Dioxide. Sens. Actuators B Chem. 2018, 260, 63–69. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gu, Z.Y.; Jiang, D.Q.; Li, Y.; Wang, H.F.; Yan, X.P. In Situ Hydrothermal Growth of Metal-Organic Framework 199 Films on Stainless Steel Fibers for Solid-Phase Microextraction of Gaseous Benzene Homologues. Anal. Chem. 2009, 81, 9771–9777. [Google Scholar] [CrossRef]
- Luo, R.; Fu, H.; Li, Y.; Xing, Q.; Liang, G.; Bai, P.; Guo, X.; Lyu, J.; Tsapatsis, M. In Situ Fabrication of Metal–Organic Framework Thin Films with Enhanced Pervaporation Performance. Adv. Funct. Mater. 2023, 33, 2213221. [Google Scholar] [CrossRef]
- Brunckova, H.; Mudra, E.; Rocha, L.; Nassar, E.; Nascimento, W.; Kolev, H.; Lisnichuk, M.; Kovalcikova, A.; Molcanova, Z.; Strečkova, M.; et al. Nanostructure and Luminescent Properties of Bimetallic Lanthanide Eu/Gd, Tb/Gd and Eu/Tb Coordination Polymers. Inorganics 2021, 9, 77. [Google Scholar] [CrossRef]
- Shekhah, O. Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs). Materials 2010, 3, 1302–1315. [Google Scholar] [CrossRef]
- Marets, N.; Kanno, S.; Ogata, S.; Ishii, A.; Kawaguchi, S.; Hasegawa, M. Lanthanide-Oligomeric Brush Films: From Luminescence Properties to Structure Resolution. ACS Omega 2019, 4, 15512–15520. [Google Scholar] [CrossRef]
- Brower, L.J.; Gentry, L.K.; Napier, A.L.; Anderson, M.E. Tailoring the Nanoscale Morphology of HKUST-1 Thin Films via Codeposition and Seeded Growth. Beilstein J. Nanotechnol. 2017, 8, 2307–2314. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Arslan, H.K.; Grosjean, S.; Hagendorn, T.; Gliemann, H.; Bräse, S.; Wöll, C. Post-Synthetic Modification of Metal-Organic Framework Thin Films Using Click Chemistry: The Importance of Strained C-C Triple Bonds. Langmuir 2013, 29, 15958–15964. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Zhang, F.; Chu, T.; Yang, Y. A Novel Lanthanide MOF Thin Film: The Highly Performance Self-Calibrating Luminescent Sensor for Detecting Formaldehyde as an Illegal Preservative in Aquatic Product. Sens. Actuators B Chem. 2017, 251, 667–673. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Wu, Y.-N.; Ma, X.; Li, F.; Zhang, B.; Wang, Y.; Lei, Z.; Zhang, Z. From Powder to Cloth: Facile Fabrication of Dense MOF-76(Tb) Coating onto Natural Silk Fiber for Feasible Detection of Copper Ions. Chem. Eng. J. 2018, 350, 637–644. [Google Scholar] [CrossRef]
- Chen, D.H.; Sedykh, A.E.; Gomez, G.E.; Neumeier, B.L.; Santos, J.C.C.; Gvilava, V.; Maile, R.; Feldmann, C.; WÖll, C.; Janiak, C.; et al. SURMOF Devices Based on Heteroepitaxial Architectures with White-Light Emission and Luminescent Thermal-Dependent Performance. Adv. Mater. Interfaces 2020, 7, 2000929. [Google Scholar] [CrossRef]
- Chen, D.H.; Haldar, R.; Neumeier, B.L.; Fu, Z.H.; Feldmann, C.; Wöll, C.; Redel, E. Tunable Emission in Heteroepitaxial Ln-SURMOFs. Adv. Funct. Mater. 2019, 29, 1903086. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, G.; Yao, H.; Wang, Y.; Chu, T.; Yang, Y. A Europium (III) Based Nano-Flake MOF Film for Efficient Fluorescent Sensing of Picric Acid. Microchim. Acta 2017, 184, 1207–1213. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Chu, T.; Wang, Z.; Li, W.; Yang, Y. A Facile Fabrication of Electrodeposited Luminescent MOF Thin Films for Selective and Recyclable Sensing of Nitroaromatic Explosives. Analyst 2016, 141, 4502–4510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Wang, S.; Rao, Z.; Yang, Y. A Luminescent Terbium-Succinate MOF Thin Film Fabricated by Electrodeposition for Sensing of Cu2+ in Aqueous Environment. Sens. Actuators B Chem. 2015, 220, 779–787. [Google Scholar] [CrossRef]
- Li, W.J.; Feng, J.F.; Lin, Z.J.; Yang, Y.L.; Yang, Y.; Wang, X.S.; Gao, S.Y.; Cao, R. Patterned Growth of Luminescent Metal-Organic Framework Films: A Versatile Electrochemically-Assisted Microwave Deposition Method. Chem. Commun. 2016, 52, 3951–3954. [Google Scholar] [CrossRef]
- Feng, J.F.; Yang, X.; Gao, S.Y.; Shi, J.; Cao, R. Facile and Rapid Growth of Nanostructured Ln-BTC Metal-Organic Framework Films by Electrophoretic Deposition for Explosives Sensing in Gas and Cr 3+ Detection in Solution. Langmuir 2017, 33, 14238–14243. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Hao, S.; Wen, J.; Li, S.; Peng, W.; Huang, R.; Xu, X. Electrochemical Fabrication of Metal–Organic Frameworks Membranes and Films: A Review. Microporous Mesoporous Mater. 2020, 305, 110322. [Google Scholar] [CrossRef]
- Li, W.J.; Tu, M.; Cao, R.; Fischer, R.A. Metal-Organic Framework Thin Films: Electrochemical Fabrication Techniques and Corresponding Applications & Perspectives. J. Mater. Chem. A 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochemical Deposition of Metal-Organic Framework Films and Their Applications. J. Mater. Chem. A 2020, 8, 7569–7587. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, T.; Yu, M.; Liu, H.; Yang, Y. One Step Cathodically Electrodeposited [Tb2(BDC)3(H2O)4]n Thin Film as a Luminescent Probe for Cu2+ Detection. RSC Adv. 2014, 4, 58178–58183. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Chu, T.; Yu, M.; Yang, Y. An Electrodeposited Lanthanide MOF Thin Film as a Luminescent Sensor for Carbonate Detection in Aqueous Solution. J. Mater. Chem. C 2014, 2, 8683–8690. [Google Scholar] [CrossRef]
- Liu, H.; Chu, T.; Rao, Z.; Wang, S.; Yang, Y.; Wong, W.T. The Tunable White-Light and Multicolor Emission in An Electrodeposited Thin Film of Mixed Lanthanide Coordination Polymers. Adv. Opt. Mater. 2015, 3, 1545–1550. [Google Scholar] [CrossRef]
- Alizadeh, S.; Nematollahi, D. Convergent and Divergent Paired Electrodeposition of Metal-Organic Framework Thin Films. Sci. Rep. 2019, 9, 14325. [Google Scholar] [CrossRef]
- Hauser, J.L.; Tso, M.; Fitchmun, K.; Oliver, S.R.J. Anodic Electrodeposition of Several Metal Organic Framework Thin Films on Indium Tin Oxide Glass. Cryst. Growth Des. 2019, 19, 2358–2365. [Google Scholar] [CrossRef]
- Li, W.J.; Lü, J.; Gao, S.Y.; Li, Q.H.; Cao, R. Electrochemical Preparation of Metal-Organic Framework Films for Fast Detection of Nitro Explosives. J. Mater. Chem. A 2014, 2, 19473–19478. [Google Scholar] [CrossRef]
- Hod, I.; Bury, W.; Karlin, D.M.; Deria, P.; Kung, C.W.; Katz, M.J.; So, M.; Klahr, B.; Jin, D.; Chung, Y.W.; et al. Directed Growth of Electroactive Metal-Organic Framework Thin Films Using Electrophoretic Deposition. Adv. Mater. 2014, 26, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.F.; Gao, S.Y.; Shi, J.; Liu, T.F.; Cao, R. C-QDs@UiO-66-(COOH)2 Composite Film via Electrophoretic Deposition for Temperature Sensing. Inorg. Chem. 2018, 57, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, H.; Zhitomirsky, I.; Zhu, S. Preparation of Metal-Organic Framework Films by Electrophoretic Deposition Method. Mater. Lett. 2015, 142, 19–22. [Google Scholar] [CrossRef]
- Feng, J.F.; Gao, S.Y.; Liu, T.F.; Shi, J.; Cao, R. Preparation of Dual-Emitting Ln@UiO-66-Hybrid Films via Electrophoretic Deposition for Ratiometric Temperature Sensing. ACS Appl. Mater. Interfaces 2018, 10, 6014–6023. [Google Scholar] [CrossRef]
- Aceituno Melgar, V.M.; Kwon, H.T.; Kim, J. Direct Spraying Approach for Synthesis of ZIF-7 Membranes by Electrospray Deposition. J. Memb. Sci. 2014, 459, 190–196. [Google Scholar] [CrossRef]
- Bai, X.J.; Chen, D.; Li, L.L.; Shao, L.; He, W.X.; Chen, H.; Li, Y.N.; Zhang, X.M.; Zhang, L.Y.; Wang, T.Q.; et al. Fabrication of MOF Thin Films at Miscible Liquid-Liquid Interface by Spray Method. ACS Appl. Mater. Interfaces 2018, 10, 25960–25966. [Google Scholar] [CrossRef]
- Li, Y.N.; Wang, S.; Zhou, Y.; Bai, X.J.; Song, G.S.; Zhao, X.Y.; Wang, T.Q.; Qi, X.; Zhang, X.M.; Fu, Y. Fabrication of Metal-Organic Framework and Infinite Coordination Polymer Nanosheets by the Spray Technique. Langmuir 2017, 33, 1060–1065. [Google Scholar] [CrossRef]
- Giedraityte, Z.; Sundberg, P.; Karppinen, M. Flexible Inorganic-Organic Thin Film Phosphors by ALD/MLD. J. Mater. Chem. C 2015, 3, 12316–12321. [Google Scholar] [CrossRef]
- Silva, R.M.; Carlos, L.D.; Rocha, J.; Silva, R.F. Luminescent Thin Films of Eu-Bearing UiO-66 Metal Organic Framework Prepared by ALD/MLD. Appl. Surf. Sci. 2020, 527, 146603. [Google Scholar] [CrossRef]
- Feng, J.F.; Liu, T.F.; Shi, J.; Gao, S.Y.; Cao, R. Dual-Emitting UiO-66(Zr&Eu) Metal-Organic Framework Films for Ratiometric Temperature Sensing. ACS Appl. Mater. Interfaces 2018, 10, 20854–20861. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, Y.; Liu, D.; Li, Z.; Li, H. Luminescence Modulation: Via Cation-π Interaction in a Lanthanide Assembly: Implications for Potassium Detection. J. Mater. Chem. C 2018, 6, 1944–1950. [Google Scholar] [CrossRef]
- Yang, D.; Liu, D.; Tian, C.; Wang, S.; Li, H. Flexible and Transparent Films Consisting of Lanthanide Complexes for Ratiometric Luminescence Thermometry. J. Colloid Interface Sci. 2018, 519, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Sheng, D.; Yu, Y.; Wang, Y.; Zhao, L.; Lu, J.; Li, Y.; Li, D.; Dou, J.; Duan, J.; et al. Tunable Light Emission and Multiresponsive Luminescent Sensitivities in Aqueous Solutions of Two Series of Lanthanide Metal-Organic Frameworks Based on Structurally Related Ligands. ACS Appl. Mater. Interfaces 2019, 11, 7914–7926. [Google Scholar] [CrossRef]
- Bai, K.P.; Zhou, L.J.; Yang, G.P.; Cao, M.X.; Wang, Y.Y. Luminescence Sensing of Fe3+ and Nitrobenzene by Three Isostructural Ln–MOFs Assembled by a Phenyl-Dicarboxylate Ligand. ChemistrySelect 2019, 4, 12794–12800. [Google Scholar] [CrossRef]
- Xu, J.; Jia, L.; Jin, N.; Ma, Y.; Liu, X.; Wu, W.; Liu, W.; Tang, Y.; Zhou, F. Fixed-Component Lanthanide-Hybrid-Fabricated Full-Color Photoluminescent Films as Vapoluminescent Sensors. Chem. A Eur. J. 2013, 19, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, R.; Fan, J.; Liu, H.; Sun, T.; Wang, P.; Yang, Y. Lanthanide Coordination Polymer-Based Composite Films for Selective and Highly Sensitive Detection of Cr2O72– in Aqueous Media. Inorg. Chem. 2019, 58, 15118–15125. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, H.; Xia, Z.; Ren, C.; Han, J.; Sun, W.; Wei, Q.; Xie, G.; Chen, S. A Robust TbIII-MOF for Ultrasensitive Detection of Trinitrophenol: Matched Channel Dimensions and Strong Host-Guest Interactions. Inorg. Chem. 2019, 58, 8198–8207. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Zeng, C.H.; Chu, T.S.; Wang, H.M.; Yang, Y.Y.; Tong, Y.X.; Su, C.Y.; Wong, W.T. A Novel Highly Luminescent LnMOF Film: A Convenient Sensor for Hg2+ Detecting. J. Mater. Chem. A 2013, 1, 11312–11319. [Google Scholar] [CrossRef]
- Ma, W.P.; Yan, B. Lanthanide Functionalized MOF Thin Films as Effective Luminescent Materials and Chemical Sensors for Ammonia. Dalton Trans. 2020, 49, 15663–15671. [Google Scholar] [CrossRef] [PubMed]
- Campagnol, N.; Rezende Souza, E.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Luminescent Terbium-Containing Metal–Organic Framework Films: New Approaches for the Electrochemical Synthesis and Application as Detectors for Explosives. Chem. Commun. 2014, 50, 12680–12683. [Google Scholar] [CrossRef]
- Wang, X.; Batra, K.; Clavier, G.; Maurin, G.; Ding, B.; Tissot, A.; Serre, C. Ln-MOF Based Ratiometric Luminescent Sensor for the Detection of Potential COVID-19 Drugs. Chem. A Eur. J. 2023, 29, e202203136. [Google Scholar] [CrossRef]
- Feng, T.; Ye, Y.; Liu, X.; Cui, H.; Li, Z.; Zhang, Y.; Liang, B.; Li, H.; Chen, B. A Robust Mixed-Lanthanide PolyMOF Membrane for Ratiometric Temperature Sensing. Angew. Chem. Int. Ed. 2020, 59, 21752–21757. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, J.; Gao, Y. Decoding and Quantitative Detection of Antibiotics by a Luminescent Mixed-Lanthanide-Organic Framework. Front. Environ. Sci. Eng. 2022, 16, 154. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, H.; Chu, T.; Zhang, G.; Wang, Y.; Yang, Y. A Lanthanide MOF Thin-Film Fixed with Co3O4 Nano-Anchors as a Highly Efficient Luminescent Sensor for Nitrofuran Antibiotics. Chem. A Eur. J. 2017, 23, 10293–10300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, M.; Yang, Y.; Meng, H.; Wang, Q.; Li, C.; Li, G. Luminescence-Colour-Changing Sensing toward Neurological Drug Carbamazepine in Water and Biofluids Based on White Light-Emitting CD/Ln-MOF/PVA Test Papers. J. Mater. Chem. C 2021, 9, 8683–8693. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, D.; Lu, Y.; Sun, W.Y. Photoluminescent Metal-Organic Frameworks and Their Application for Sensing Biomolecules. J. Mater. Chem. A 2019, 7, 22744–22767. [Google Scholar] [CrossRef]
- Simões, R.; Rodrigues, J.; Granadeiro, C.M.; Rino, L.; Neto, V.; Monteiro, T.; Gonçalves, G. Boosting the Optical Properties of Polylactic Acid/ Lanthanide-Based Metal-Organic Framework Composites. Mater. Today Chem. 2023, 29, 101436. [Google Scholar] [CrossRef]











| Eu/Gd/Tb/MOF | Deposition Method | Used Substrate | CIE Coordinates (x; y) | Eu3+ → τ (ms) | Tb3+ → τ (ms) | η (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Tb-HMA Eu-HMA Tb10Eu1-HMA Tb10Gd1-HMA | ECD ECD ECD ECD | FTO FTO FTO FTO | - - 0.330; 0.340 - | - 0.273 - - | 0.286 - 0.735 0.449 | 38.42 4.66 11.41 47.79 | [95] [95] [95] [95] |
| Eu-MOF-L@PBMA | PSM | glass | 0.293; 0.299 | - | - | - | [56] |
| Tb/Eu/Gd-BTC SURMOF | LBL | quartz | 0.331; 0.329 | - | - | - | [83] |
| Eu0.25Gd0.5Tb0.25-BTC Eu0.25Gd0.25Tb0.5-BTC Eu0.5Gd0.25Tb0.25-BTC | HT green HT green HT green | Pt/SiO2/Si Pt/SiO2/Si Pt/SiO2/Si | 0.520; 0.300 0.590; 0.300 0.280; 0.310 | 0.220 0.440 1.170 | 0.210 0.220 0.530 | 7.7 21.5 30.3 | [72] [72] [72] |
| LnMOF Film | Growth/Coating Method | Substrate | Target Analyte | Film Thickness (μm) | Ref. |
|---|---|---|---|---|---|
| Eu-BQDC | ST/ECD | ITO | Hg2+ | 10 | [116] |
| Tb-BDC | ECD | FTO | Cu2+ | 2 | [93] |
| Tb-SA | ST/ECD | FTO | Cu2+ | 1 | [87] |
| Tb-BTC | LBL | Silk fiber | Cu2+ | 10 | [82] |
| Tb-BTC | ST/EPD | Zn plate | Cr3+, NB; TNT | 55 | [89] |
| Eu0.24Tb0.76-BHM-COOH-PLA | ST/drop-cast | glass | Fe3+ | - | [66] |
| Eu-HBPTC | ST/ECD | FTO | CO32− | - | [94] |
| Tb-CPON-PMMA | ST/SC | glass | Cr2O72− | - | [114] |
| Tb-BTC | ST/DC | ITO | Organic solvents | 7 | [69] |
| Eu-NDC@HPAN | HT/LBL | HPAN | Formaldehyde | 2–4 | [81] |
| Eu-BDC-NH2 | ST | glass | Gaseous SO2 | 1 | [73] |
| Eu@UMOF-LA | PSM | Al2O3 | NH3 | 50 | [117] |
| Eu-MOF-L@PBMA | PSM | glass | Organic vapors | - | [56] |
| Tb-BTC | ECD | Al | DNT | - | [118] |
| Eu-TDC | ECD | FTO | Nitrophenols | 7 | [86] |
| Eu-NDC | ECD | FTO | PA, TNP | 0.05 | [85] |
| CDs@Eu-PHEN-BTEC/PVDF | ST | PVDF | NB, 4-NP | - | [67] |
| Gd0.9Tb0.1HL | ST | Gd2O3 | Ratiom. thermometer | 10 | [46] |
| UiO-66(Zr&Eu)/PVDF | HT;/DC | glass | Smart thermometer | 30 | [108] |
| Eu0.5Tb0.5(L)1@PMMA | ST | glass | Ratiom. thermometer | - | [110] |
| Eu0.1Tb0.9-BTC | ST/DC | ITO | Pharmaceuticals | 4 | [40] |
| Eu-TDC MMMs | ST | ITO | Pharmaceuticals NIABs | - | [62] |
| Eu0.047Tb0.953H2L@PVDF | ST/SC | glass | COVID-19 Favipiravir | - | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunckova, H.; Mudra, E.; Shepa, I. Recent Advances in Lanthanide Metal–Organic Framework Thin Films Based on Eu, Tb, Gd: Preparation and Application as Luminescent Sensors and Light-Emitting Devices. Inorganics 2023, 11, 376. https://doi.org/10.3390/inorganics11100376
Brunckova H, Mudra E, Shepa I. Recent Advances in Lanthanide Metal–Organic Framework Thin Films Based on Eu, Tb, Gd: Preparation and Application as Luminescent Sensors and Light-Emitting Devices. Inorganics. 2023; 11(10):376. https://doi.org/10.3390/inorganics11100376
Chicago/Turabian StyleBrunckova, Helena, Erika Mudra, and Ivan Shepa. 2023. "Recent Advances in Lanthanide Metal–Organic Framework Thin Films Based on Eu, Tb, Gd: Preparation and Application as Luminescent Sensors and Light-Emitting Devices" Inorganics 11, no. 10: 376. https://doi.org/10.3390/inorganics11100376