The Effect of Cation Incorporation on the Elastic and Vibrational Properties of Mixed Lead Chloride Perovskite Single Crystals
Abstract
:1. Introduction
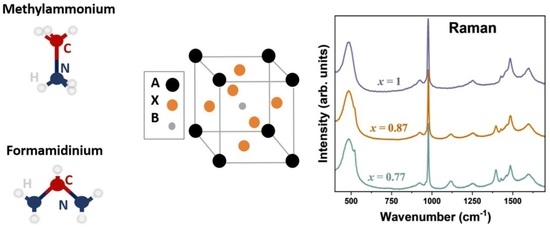
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Single Crystals Synthesis
3.3. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Sieradzki, A. Layered Lead Iodide of [Methylhydrazinium]2PbI4 with a Reduced Band Gap: Thermochromic Luminescence and Switchable Dielectric Properties Triggered by Structural Phase Transitions. Chem. Mater. 2019, 31, 8563–8575. [Google Scholar] [CrossRef]
- Li, X.; Hoffman, J.M.; Kanatzidis, M.G. The 2D halide perovskite rulebook: How the spacer influences everything from the structure to optoelectronic device efficiency. Chem. Rev. 2021, 121, 2230–2291. [Google Scholar] [CrossRef] [PubMed]
- Mączka, M.; Zarȩba, J.K.; Gągor, A.; Stefańska, D.; Ptak, M.; Roleder, K.; Kajewski, D.; Soszyński, A.; Fedoruk, K.; Sieradzki, A. [Methylhydrazinium]2PbBr4, a Ferroelectric Hybrid Organic-Inorganic Perovskite with Multiple Nonlinear Optical Outputs. Chem. Mater. 2021, 33, 2331–2342. [Google Scholar] [CrossRef]
- Mączka, M.; Gągor, A.; Stroppa, A.; Gonçalves, J.N.; Zaręba, J.K.; Stefańska, D.; Pikul, A.; Drozd, M.; Sieradzki, A. Two-dimensional metal dicyanamide frameworks of BeTriMe[M(dca)3(H2O)] (BeTriMe = benzyltrimethylammonium; dca = dicyanamide; M = Mn2+, Co2+, Ni2+): Coexistence of polar and magnetic orders and nonlinear optical threshold temperature sensing. J. Mater. Chem. C 2020, 8, 11735–11747. [Google Scholar] [CrossRef]
- Mączka, M.; Zienkiewicz, J.A.; Ptak, M. Comparative Studies of Phonon Properties of Three-Dimensional Hybrid Organic-Inorganic Perovskites Comprising Methylhydrazinium, Methylammonium, and Formamidinium Cations. J. Phys. Chem. C 2022, 126, 4048–4056. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Šimėnas, M.; Balčiū Nas, S.; Gągor, A.; Pieniążek, A.; Tolborg, K.; Kinka, M.; Klimavicius, V.; Svirskas, Š.N.; Kalendra, V.; Ptak, M.; et al. Mixology of MA1- xEAxPbI3Hybrid Perovskites: Phase Transitions, Cation Dynamics, and Photoluminescence. Chem. Mater. 2022, 34, 10104–10112. [Google Scholar] [CrossRef]
- Glazer, A.M. Simple ways of determining perovskite structures. Acta Crystallogr. Sect. A 1975, 31, 756–762. [Google Scholar] [CrossRef]
- Petrov, A.A.; Goodilin, E.A.; Tarasov, A.B.; Lazarenko, V.A.; Dorovatovskii, P.V.; Khrustalev, V.N. Formamidinium iodide: Crystal structure and phase transitions. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 569–572. [Google Scholar] [CrossRef]
- Ruan, S.; McMeekin, D.P.; Fan, R.; Webster, N.A.S.; Ebendorff-Heidepriem, H.; Cheng, Y.B.; Lu, J.; Ruan, Y.; McNeill, C.R. Raman Spectroscopy of Formamidinium-Based Lead Halide Perovskite Single Crystals. J. Phys. Chem. C 2020, 124, 2265–2272. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Park, N.G. Organometal perovskite light absorbers toward a 20% efficiency low-cost solid-state mesoscopic solar cell. J. Phys. Chem. Lett. 2013, 4, 2423–2429. [Google Scholar] [CrossRef]
- Li, Z.; Klein, T.R.; Kim, D.H.; Yang, M.; Berry, J.J.; Van Hest, M.F.A.M.; Zhu, K. Scalable fabrication of perovskite solar cells. Nat. Rev. Mater. 2018, 3, 18017. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; Van Schilfgaarde, M.; Walsh, A. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef]
- Frost, J.M.; Walsh, A. What Is Moving in Hybrid Halide Perovskite Solar Cells? Acc. Chem. Res. 2016, 49, 528–535. [Google Scholar] [CrossRef]
- Egger, D.A.; Rappe, A.M.; Kronik, L. Hybrid Organic-Inorganic Perovskites on the Move. Acc. Chem. Res. 2016, 49, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Hu, H.; Zhang, J.; Lv, S.; Yu, Y.; Wei, F.; Qin, T.; Xu, H.; Liu, Z.; Cui, G. NH2CH=NH2PbI3: An alternative organolead iodide perovskite sensitizer for mesoscopic solar cells. Chem. Mater. 2014, 26, 1485–1491. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Pellet, N.; Gao, P.; Gregori, G.; Yang, T.Y.; Nazeeruddin, M.K.; Maier, J.; Grätzel, M. Mixed-organic-cation perovskite photovoltaics for enhanced solar-light harvesting. Angew. Chem. Int. Ed. 2014, 53, 3151–3157. [Google Scholar] [CrossRef]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, K.; Yang, J.; Chen, X.; Xie, X.; Li, B.; Zhang, Z.; Liu, L.; Shan, C.; Shen, D. High-Performance Planar-Type Ultraviolet Photodetector Based on High-Quality CH3NH3PbCl3 Perovskite Single Crystals. ACS Appl. Mater. Interfaces 2019, 11, 34144–34150. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, F.H.; Ko, J.H. Structural Phase Transitions and Thermal Degradation Process of MAPbCl3 Single Crystals Studied by Raman and Brillouin Scattering. Materials 2022, 15, 8151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Naqvi, F.; Ko, J.H.; Kim, T.; Ahn, C. Acoustic Anomalies and the Critical Slowing-Down Behavior of MAPbCl3 Single Crystals Studied by Brillouin Light Scattering. Materials 2022, 15, 3692. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, A.A.; Yamilova, O.R.; Gutsev, L.G.; Sagdullina, D.K.; Novikov, A.V.; Ramachandran, B.R.; Stevenson, K.J.; Aldoshin, S.M.; Troshin, P.A. Highly sensitive and selective ammonia gas sensor based on FAPbCl3lead halide perovskites. J. Mater. Chem. C 2021, 9, 2561–2568. [Google Scholar] [CrossRef]
- Wang, J.; Peng, J.; Sun, Y.; Liu, X.; Chen, Y.; Liang, Z. FAPbCl3 Perovskite as Alternative Interfacial Layer for Highly Efficient and Stable Polymer Solar Cells. Adv. Electron. Mater. 2016, 2, 1600329. [Google Scholar] [CrossRef]
- Gong, J.; Li, X.; Guo, P.; Zhang, I.; Huang, W.; Lu, K.; Cheng, Y.; Schaller, R.D.; Marks, T.J.; Xu, T. Energy-distinguishable bipolar UV photoelectron injection from LiCl-promoted FAPbCl3 perovskite nanorods. J. Mater. Chem. A 2019, 7, 13043–13049. [Google Scholar] [CrossRef]
- Govinda, S.; Kore, B.P.; Swain, D.; Hossain, A.; De, C.; Guru Row, T.N.; Sarma, D.D. Critical Comparison of FAPbX3 and MAPbX3 (X = Br and Cl): How Do They Differ? J. Phys. Chem. C 2018, 122, 13758–13766. [Google Scholar] [CrossRef]
- Sharma, V.K.; Mukhopadhyay, R.; Mohanty, A.; García Sakai, V.; Tyagi, M.; Sarma, D.D. Influence of the Halide Ion on the A-Site Dynamics in FAPbX3(X = Br and Cl). J. Phys. Chem. C 2022, 126, 7158–7168. [Google Scholar] [CrossRef]
- Nations, S.; Gutsev, L.; Ramachandran, B.; Aldoshin, S.; Duan, Y.; Wang, S. First-principles study of the defect-activity and optical properties of FAPbCl3. Mater. Adv. 2022, 3, 3897–3905. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Alarco, J.A.; Wang, H.; Du, A.; Tesfamichael, T.; Ostrikov, K.K. Ab initio atomistic insights into lead-free formamidinium based hybrid perovskites for photovoltaics and optoelectronics. Comput. Mater. Sci. 2019, 169, 109118. [Google Scholar] [CrossRef]
- Pachori, S.; Agarwal, R.; Shukla, A.; Rani, U.; Verma, A.S. Mechanically stable with highly absorptive formamidinium lead halide perovskites [(HC(NH2)2PbX3; X = Br, Cl]: Recent advances and perspectives. Int. J. Quantum Chem. 2021, 121, e26671. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S. Il High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef]
- Charles, B.; Dillon, J.; Weber, O.J.; Islam, M.S.; Weller, M.T. Understanding the stability of mixed A-cation lead iodide perovskites. J. Mater. Chem. A 2017, 5, 22495–22499. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and cesium hybridization for photo- and moisture-stable perovskite solar cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cationsinto perovskite solar cells improvesphotovoltaic performance. Science 2016, 354, 203–206. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Weber, O.J.; Charles, B.; Weller, M.T. Phase behaviour and composition in the formamidinium-methylammonium hybrid lead iodide perovskite solid solution. J. Mater. Chem. A 2016, 4, 15375–15382. [Google Scholar] [CrossRef]
- Jesper Jacobsson, T.; Correa-Baena, J.P.; Pazoki, M.; Saliba, M.; Schenk, K.; Grätzel, M.; Hagfeldt, A. Exploration of the compositional space for mixed lead halogen perovskites for high efficiency solar cells. Energy Environ. Sci. 2016, 9, 1706–1724. [Google Scholar] [CrossRef]
- Yang, Z.; Chueh, C.C.; Liang, P.W.; Crump, M.; Lin, F.; Zhu, Z.; Jen, A.K.Y. Effects of formamidinium and bromide ion substitution in methylammonium lead triiodide toward high-performance perovskite solar cells. Nano Energy 2016, 22, 328–337. [Google Scholar] [CrossRef]
- Pisanu, A.; Ferrara, C.; Quadrelli, P.; Guizzetti, G.; Patrini, M.; Milanese, C.; Tealdi, C.; Malavasi, L. The FA1−xMAxPbI3 System: Correlations among Stoichiometry Control, Crystal Structure, Optical Properties, and Phase Stability. J. Phys. Chem. C 2017, 121, 8746–8751. [Google Scholar] [CrossRef]
- Alonso, M.I.; Charles, B.; Francisco-López, A.; Garriga, M.; Weller, M.T.; Goñi, A.R. Spectroscopic ellipsometry study of FAxMA1−xPbI3 hybrid perovskite single crystals. J. Vac. Sci. Technol. B 2019, 37, 062901. [Google Scholar] [CrossRef]
- Francisco-López, A.; Charles, B.; Alonso, M.I.; Garriga, M.; Campoy-Quiles, M.; Weller, M.T.; Goñi, A.R. Phase Diagram of Methylammonium/Formamidinium Lead Iodide Perovskite Solid Solutions from Temperature-Dependent Photoluminescence and Raman Spectroscopies. J. Phys. Chem. C 2020, 124, 3448–3458. [Google Scholar] [CrossRef]
- Chen, T.; Chen, W.L.; Foley, B.J.; Lee, J.; Ruff, J.P.C.; Ko, J.Y.P.; Brown, C.M.; Harriger, L.W.; Zhang, D.; Park, C.; et al. Origin of long lifetime of band-edge charge carriers in organic–inorganic lead iodide perovskites. Proc. Natl. Acad. Sci. USA 2017, 114, 7519–7524. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic-inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Chen, T.; Foley, B.J.; Park, C.; Brown, C.M.; Harriger, L.W.; Lee, J.; Ruff, J.; Yoon, M.; Choi, J.J.; Lee, S.H. Entropy-driven structural transition and kinetic trapping in formamidinium lead iodide perovskite. Sci. Adv. 2016, 2, e1601650. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S. Il Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Binek, A.; Hanusch, F.C.; Docampo, P.; Bein, T. Stabilization of the trigonal high-temperature phase of formamidinium lead iodide. J. Phys. Chem. Lett. 2015, 6, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Šimėnas, M.; Balčiū Nas, S.; Svirskas, Š.N.; Kinka, M.; Ptak, M.; Kalendra, V.; Gągor, A.; Szewczyk, D.; Sieradzki, A.; Grigalaitis, R.; et al. Phase Diagram and Cation Dynamics of Mixed MA1- xFA xPbBr3Hybrid Perovskites. Chem. Mater. 2021, 33, 5926–5934. [Google Scholar] [CrossRef]
- Baikie, T.; Barrow, N.S.; Fang, Y.; Keenan, P.J.; Slater, P.R.; Piltz, R.O.; Gutmann, M.; Mhaisalkar, S.G.; White, T.J. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 2015, 3, 9298–9307. [Google Scholar] [CrossRef]
- Maculan, G.; Sheikh, A.D.; Abdelhady, A.L.; Saidaminov, M.I.; Haque, M.A.; Murali, B.; Alarousu, E.; Mohammed, O.F.; Wu, T.; Bakr, O.M. CH3NH3PbCl3 Single Crystals: Inverse Temperature Crystallization and Visible-Blind UV-Photodetector. J. Phys. Chem. Lett. 2015, 6, 3781–3786. [Google Scholar] [CrossRef]
- Askar, A.M.; Karmakar, A.; Bernard, G.M.; Ha, M.; Terskikh, V.V.; Wiltshire, B.D.; Patel, S.; Fleet, J.; Shankar, K.; Michaelis, V.K. Composition-Tunable Formamidinium Lead Mixed Halide Perovskites via Solvent-Free Mechanochemical Synthesis: Decoding the Pb Environments Using Solid-State NMR Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zha, G.; Deng, C.; An, Y.; Mao, R.; Liu, Y.; Lu, Y.; Chen, Z. Refractive Index Dispersion of Organic–Inorganic Hybrid Halide Perovskite CH3NH3PbX3 (X = Cl, Br, I) Single Crystals. Cryst. Res. Technol. 2019, 54, 1900011. [Google Scholar] [CrossRef]
- Niemann, R.G.; Kontos, A.G.; Palles, D.; Kamitsos, E.I.; Kaltzoglou, A.; Brivio, F.; Falaras, P.; Cameron, P.J. Halogen Effects on Ordering and Bonding of CH3NH3+ in CH3NH3PbX3 (X = Cl, Br, I) Hybrid Perovskites: A Vibrational Spectroscopic Study. J. Phys. Chem. C 2016, 120, 2509–2519. [Google Scholar] [CrossRef]
- Kucharska, E.; Hanuza, J.; Ciupa, A.; Mączka, M.; Macalik, L. Vibrational properties and DFT calculations of formamidine-templated Co and Fe formates. Vib. Spectrosc. 2014, 75, 45–50. [Google Scholar] [CrossRef]
- Kontos, A.G.; Manolis, G.K.; Kaltzoglou, A.; Palles, D.; Kamitsos, E.I.; Kanatzidis, M.G.; Falaras, P. Halogen-NH2+ Interaction, Temperature-Induced Phase Transition, and Ordering in (NH2CHNH2)PbX3 (X = Cl, Br, I) Hybrid Perovskites. J. Phys. Chem. C 2020, 124, 8479–8487. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Goñi, A.R.; Frost, J.M.; Skelton, J.; Brivio, F.; Rodríguez-Martínez, X.; Weber, O.J.; Pallipurath, A.; Alonso, M.I.; Campoy-Quiles, M.; et al. Dynamic disorder, phonon lifetimes, and the assignment of modes to the vibrational spectra of methylammonium lead halide perovskites. Phys. Chem. Chem. Phys. 2016, 18, 27051–27066. [Google Scholar] [CrossRef]
- Maalej, A.; Abid, Y.; Kallel, A.; Daoud, A.; Lautié, A.; Romain, F. Phase transitions and crystal dynamics in the cubic perovskite-CH3NH3PbCl3. Solid State Commun. 1997, 103, 279–284. [Google Scholar] [CrossRef]
- MącZka, M.; Ptak, M.; Vasconcelos, D.L.M.; Giriunas, L.; Freire, P.T.C.; Bertmer, M.; Banys, J.; Simenas, M. NMR and Raman Scattering Studies of Temperature-and Pressure-Driven Phase Transitions in CH3NH2NH2PbCl3 Perovskite. J. Phys. Chem. C 2020, 124, 26999–27008. [Google Scholar] [CrossRef]
- Glaser, T.; Müller, C.; Sendner, M.; Krekeler, C.; Semonin, O.E.; Hull, T.D.; Yaffe, O.; Owen, J.S.; Kowalsky, W.; Pucci, A.; et al. Infrared Spectroscopic Study of Vibrational Modes in Methylammonium Lead Halide Perovskites. J. Phys. Chem. Lett. 2015, 6, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, W.; Yang, K.; Bi, J.; Feng, J.; Zhang, J.; Yan, Z.; Zhou, X.; Liu, C.; Ji, Y.; et al. A- or X-site mixture on mechanical properties of APbX3 perovskite single crystals. APL Mater. 2021, 9, 041112. [Google Scholar] [CrossRef]
- Sun, S.; Isikgor, F.H.; Deng, Z.; Wei, F.; Kieslich, G.; Bristowe, P.D.; Ouyang, J.; Cheetham, A.K. Factors Influencing the Mechanical Properties of Formamidinium Lead Halides and Related Hybrid Perovskites. ChemSusChem 2017, 10, 3740–3745. [Google Scholar] [CrossRef] [PubMed]








| Composition | Lattice Parameter (Å) | Unit-Cell Volume (Å3) | Refractive Index (n) | Density (kg/m3) |
|---|---|---|---|---|
| MAPbCl3 | 5.67 | 182.28 | 1.90 [58] | 3149 |
| MA0.87FA0.13PbCl3 | 5.68 | 183.25 | 1.93 | 3147 |
| MA0.77FA0.23PbCl3 | 5.69 | 184.22 | 1.95 | 3142 |
| FAPbCl3 | 5.76 [57] | 191.10 | 2.10 [33] | 3116 |
| MAPbCl3 (cm−1) | MA0.87FA0.13PbCl3 (cm−1) | MA0.77FA0.23PbCl3 (cm−1) | Mode Assignment |
|---|---|---|---|
| 36 | 36 | 36 | PbCl6 motion [62,63] |
| 63 | 62 | 62 | PbCl6 motion [63] |
| 90 | 89 | 88 | δs (Cl–Pb–Cl) [59] |
| 122 | 123 | 123 | δas (Cl–Pb–Cl) [59] |
| 182 | 177 | 174 | νas (Pb–Cl) [59,61] |
| 239 | 232 | 228 | R of MA+ cation [63] |
| 484 | 486 | 485 | τ (MA) [64] |
| 522 | 522 | δ (FA) [11,61] | |
| 922 | 924 | 923 | ρ (MA) [61] |
| 962 | 962 | 961 | |
| 976 | 978 | 978 | ν (C−N) [59] |
| 1117 | 1116 | νs (CN) [11,61] | |
| 1246 | 1247 | 1247 | ρ (MA) [59,62] |
| 1396 | 1395 | ρ (NH2+) [11,60,61] | |
| 1427 | 1428 | 1428 | δs (CH3) [59] |
| 1455 | 1458 | 1458 | δas (CH3) [59] |
| 1485 | 1486 | 1486 | δs (NH3) [59] |
| 1597 | 1600 | 1600 | δas (NH3) [59] |
| 1715 | 1716 | νas (CN) [60,61] | |
| 2830 | 2831 | 2831 | Combination modes [65] |
| 2897 | 2898 | 2898 | νs (C–H) [62] |
| 2947 | 2949 | 2948 | sym. CH3 stretch [65] |
| 2972 | 2973 | 2973 | νas (C–H) [59,62,65] |
| 3042 | 3042 | 3041 | νs (CH3) [59,62,65] |
| 3120 | 3120 | 3118 | νs (NH3) [62] |
| 3189 | 3190 | 3190 | νs (NH3) [62] |
| 3221 | 3229 | 3228 | ν (NH2) [60,61,62] |
| 3336 | 3336 | ν (NH2) [60,61] | |
| 3438 | 3436 | sym. NH3+ stretch [65] |
| Composition | VLA (m/s) | VTA (m/s) | C11 (GPa) | C44 (Gpa) |
|---|---|---|---|---|
| MAPbCl3 (Brillouin) | 3574 | 1087 | 40.22 | 3.72 |
| MA0.87FA0.13PbCl3 (Brillouin) | 3507 | 990 | 38.71 | 3.08 |
| MA0.77FA0.23PbCl3 (Brillouin) | 3471 | 961 | 37.85 | 2.90 |
| FAPbCl3 (DFT calculation) | - | - | 14.64 [34] | 3.59 [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junaid, S.B.; Naqvi, F.H.; Ko, J.-H. The Effect of Cation Incorporation on the Elastic and Vibrational Properties of Mixed Lead Chloride Perovskite Single Crystals. Inorganics 2023, 11, 416. https://doi.org/10.3390/inorganics11100416
Junaid SB, Naqvi FH, Ko J-H. The Effect of Cation Incorporation on the Elastic and Vibrational Properties of Mixed Lead Chloride Perovskite Single Crystals. Inorganics. 2023; 11(10):416. https://doi.org/10.3390/inorganics11100416
Chicago/Turabian StyleJunaid, Syed Bilal, Furqanul Hassan Naqvi, and Jae-Hyeon Ko. 2023. "The Effect of Cation Incorporation on the Elastic and Vibrational Properties of Mixed Lead Chloride Perovskite Single Crystals" Inorganics 11, no. 10: 416. https://doi.org/10.3390/inorganics11100416