Synthesis and Characterization of a Novel Non-Isolated-Pentagon-Rule Isomer of Th@C76:Th@C1(17418)-C76
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Isolation of Th@C76
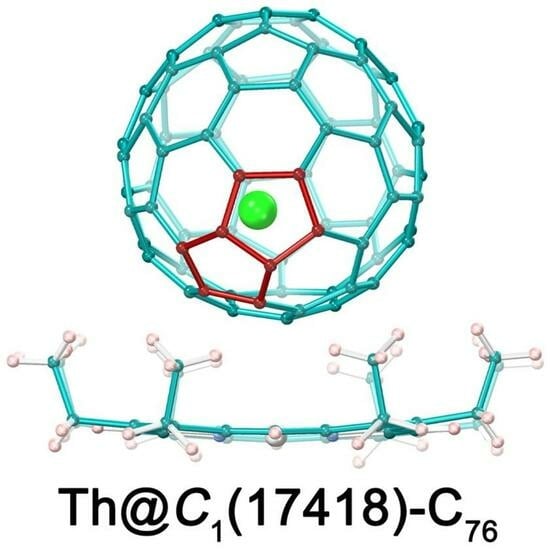
2.2. Molecular Structure of Th@C1(17418)-C76
2.3. Spectroscopic Characterizations
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef]
- Bao, L.; Peng, P.; Lu, X. Bonding inside and outside Fullerene Cages. Acc. Chem. Res. 2018, 51, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Guo, T.; Jin, C.M.; Haufler, R.E.; Chibante, L.P.F.; Fure, J.; Wang, L.H.; Alford, J.M.; Smalley, R.E. Fullerenes with Metals Inside. J. Phys. Chem. 1991, 95, 7564–7568. [Google Scholar] [CrossRef]
- Slanina, Z.; Nagase, S. Sc3N@C80: Computations on the Two-Isomer Equilibrium at High Temperatures. Chem. Phys. Chem. 2005, 6, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-S.; Feng, L.; Wu, J.-Y.; Xu, W.; Xiang, J.-F.; Tan, K.; Ma, Y.-H.; Zheng, J.-P.; Jiang, L.; Lu, X.; et al. Planar Quinary Cluster inside a Fullerene Cage: Synthesis and Structural Characterizations of Sc3NC@C80-Ih. J. Am. Chem. Soc. 2010, 132, 16362–16364. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Fujii, R.; Miyake, Y.; Sakaguchi, K.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Achiba, Y. Structural study of four Ca@C82 isomers by 13C NMR spectroscopy. Chem. Phys. Lett. 2003, 377, 197–200. [Google Scholar] [CrossRef]
- Xu, Z.; Nakane, T.; Shinohara, H. Production and Isolation of Ca@C82 (I–IV) and Ca@C84 (I,II) Metallofullerenes. J. Am. Chem. Soc. 1996, 118, 11309–11310. [Google Scholar] [CrossRef]
- Xu, W.; Feng, L.; Calvaresi, M.; Liu, J.; Liu, Y.; Niu, B.; Shi, Z.; Lian, Y.; Zerbetto, F. An experimentally observed trimetallofullerene Sm3@Ih-C80: Encapsulation of three metal atoms in a cage without a nonmetallic mediator. J. Am. Chem. Soc. 2013, 135, 4187–4190. [Google Scholar] [CrossRef]
- Kurihara, H.; Lu, X.; Iiduka, Y.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Akasaka, T.; Nagase, S. Sc2C2@C80 Rather than Sc2@C82: Templated Formation of Unexpected C2v(5)-C80 and Temperature-Dependent Dynamic Motion of Internal Sc2C2 Cluster. J. Am. Chem. Soc. 2011, 133, 2382–2385. [Google Scholar] [CrossRef]
- Hu, S.; Liu, T.; Shen, W.; Slanina, Z.; Akasaka, T.; Xie, Y.; Uhlik, F.; Huang, W.; Lu, X. Isolation and Structural Characterization of Er@C2v(9)-C82 and Er@Cs(6)-C82: Regioselective Dimerization of a Pristine Endohedral Metallofullerene Induced by Cage Symmetry. Inorg. Chem. 2019, 58, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Bao, L.; Hu, S.; Yang, L.; Jin, P.; Xie, Y.; Akasaka, T.; Lu, X. Crystallographic characterization of Lu2C2n (2n = 76–90): Cluster selection by cage size. Chem. Sci. 2019, 10, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Bao, L.; Wu, Y.; Pan, C.; Zhao, S.; Fang, H.; Xie, Y.; Jin, P.; Peng, P.; Li, F.-F.; et al. Lu2@C2n (2n = 82, 84, 86): Crystallographic Evidence of Direct Lu–Lu Bonding between Two Divalent Lutetium Ions Inside Fullerene Cages. J. Am. Chem. Soc. 2017, 139, 9979–9984. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.B.; Cardona, C.M.; Guldi, D.M.; Sankaranarayanan, S.G.; Reese, M.O.; Kopidakis, N.; Peet, J.; Walker, B.; Bazan, G.C.; Van Keuren, E.; et al. Endohedral fullerenes for organic photovoltaic devices. Nat. Mater. 2009, 8, 208–212. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Qian, P.; Lu, X.; Sun, B.; Zhang, X.; Wang, L.; Gao, X.; Li, H.; Chen, Z.; et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat. Commun. 2015, 6, 5988. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, C.; Zhang, M.; Bai, Z.; Xie, F.F.; Tan, Y.Z.; Guo, Y.; Hu, K.J.; Cao, L.; Zhang, S.; et al. A Gd@C82 single-molecule electret. Nat. Nanotech. 2020, 15, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Yoshida, K.; Sakata, S.; Hirakawa, K. Electron transport in endohedral metallofullerene Ce@C82 single-molecule transistors. Appl. Phys. Lett. 2015, 106, 043108. [Google Scholar] [CrossRef]
- Wakahara, T.; Kobayashi, J.; Yamada, M.; Maeda, Y.; Tsuchiya, T.; Okamura, M.; Akasaka, T.; Waelchli, M.; Kobayashi, K.; Nagase, S.; et al. Characterization of Ce@C82 and its anion. J. Am. Chem. Soc. 2004, 126, 4883–4887. [Google Scholar] [CrossRef]
- Akasaka, T.; Okubo, S.; Kondo, M.; Maeda, Y.; Wakahara, T.; Kato, T.; Suzuki, T.; Yamamoto, K.; Kobayashi, K.; Nagase, S. Isolation and characterization of two Pr@C82 isomers. Chem. Phys. Lett. 2000, 319, 153–156. [Google Scholar] [CrossRef]
- Ding, J.; Lin, N.; Weng, L.-T.; Cue, N.; Yang, S. Isolation and characterization of a new metallofullerene Nd@C82. Chem. Phys. Lett. 1996, 261, 92–97. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, Y.; Slanina, Z.; Gu, Z.; Shi, Z.; Uhlík, F.; Zhao, Y.; Feng, L. Popular C82 Fullerene Cage Encapsulating a Divalent Metal Ion Sm2+: Structure and Electrochemistry. Inorg. Chem. 2015, 54, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Y.; Sugai, T.; Nishibori, E.; Iwata, K.; Sakata, M.; Takata, M.; Shinohara, H. An Anomalous Endohedral Structure of Eu@C82 Metallofullerenes. Angew. Chem. Int. Ed. 2005, 44, 4568–4571. [Google Scholar] [CrossRef] [PubMed]
- Senapati, L.; Schrier, J.; Whaley, K.B. Electronic Transport, Structure, and Energetics of Endohedral Gd@C82 Metallofullerenes. Nano Lett. 2004, 4, 2073–2078. [Google Scholar] [CrossRef]
- Iwasaki, K.; Wanita, N.; Hino, S.; Yoshimura, D.; Okazaki, T.; Shinohara, H. Ultraviolet photoelectron spectra of Tb@C82. Chem. Phys. Lett. 2004, 398, 389–392. [Google Scholar] [CrossRef]
- Iida, S.; Kubozono, Y.; Slovokhotov, Y.; Takabayashi, Y.; Kanbara, T.; Fukunaga, T.; Fujiki, S.; Emura, S.; Kashino, S. Structure and electronic properties of Dy@C82 studied by UV–VIS absorption, X-ray powder diffraction and XAFS. Chem. Phys. Lett. 2001, 338, 21–28. [Google Scholar] [CrossRef]
- Huang, H.J.; Yang, S.H.; Zhang, X.X. Magnetic Behavior of Pure Endohedral Metallofullerene Ho@C82: A Comparison with Gd@C82. J. Phys. Chem. B 1999, 103, 5928–5932. [Google Scholar] [CrossRef]
- Sanakis, Y.; Tagmatarchis, N.; Aslanis, E.; Ioannidis, N.; Petrouleas, V.; Shinohara, H.; Prassides, K. Dual-Mode X-Band EPR Study of Two Isomers of the Endohedral Metallofullerene Er@C82. J. Am. Chem. Soc. 2001, 123, 9924–9925. [Google Scholar] [CrossRef]
- Suzuki, M.; Slanina, Z.; Mizorogi, N.; Lu, X.; Nagase, S.; Olmstead, M.M.; Balch, A.L.; Akasaka, T. Single-Crystal X-ray Diffraction Study of Three Yb@C82 Isomers Cocrystallized with NiII(octaethylporphyrin). J. Am. Chem. Soc. 2012, 134, 18772–18778. [Google Scholar] [CrossRef]
- Iwamoto, M.; Ogawa, D.; Yasutake, Y.; Azuma, Y.; Umemoto, H.; Ohashi, K.; Izumi, N.; Shinohara, H.; Majima, Y. Molecular Orientation of Individual Lu@C82 Molecules Demonstrated by Scanning Tunneling Microscopy. J. Phys. Chem. C 2010, 114, 14704–14709. [Google Scholar] [CrossRef]
- Wang, Y.F.; Morales-Martinez, R.; Zhang, X.X.; Yang, W.; Wang, Y.X.; Rodriguez-Fortea, A.; Poblet, J.M.; Feng, L.; Wang, S.; Chen, N. Unique Four-Electron Metal-to-Cage Charge Transfer of Th to a C82 Fullerene Cage: Complete Structural Characterization of Th@C3v(8)-C82. J. Am. Chem. Soc. 2017, 139, 5110–5116. [Google Scholar] [CrossRef]
- Jin, M.; Zhuang, J.; Wang, Y.; Yang, W.; Liu, X.; Chen, N. Th@Td(19151)-C76: A Highly Symmetric Fullerene Cage Stabilized by a Tetravalent Actinide Metal Ion. Inorg. Chem. 2019, 58, 16722–16726. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Morales-Martinez, R.; Zhuang, J.; Yao, Y.R.; Li, X.; Poblet, J.M.; Rodriguez-Fortea, A.; Chen, N. Th@D5h(6)-C80: A highly symmetric fullerene cage stabilized by a single metal ion. Chem. Commun. 2021, 57, 6624–6627. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Morales-Martinez, R.; Zhuang, J.; Yao, Y.R.; Wang, Y.; Feng, L.; Poblet, J.M.; Rodriguez-Fortea, A.; Chen, N. Synthesis and Characterization of Two Isomers of Th@C82: Th@C2v(9)-C82 and Th@C2(5)-C82. Inorg. Chem. 2021, 60, 11496–11502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Morales-Martinez, R.; Cai, W.; Zhuang, J.; Yang, W.; Echegoyen, L.; Poblet, J.M.; Rodriguez-Fortea, A.; Chen, N. Th@C1(11)-C86: An actinide encapsulated in an unexpected C86 fullerene cage. Chem. Commun. 2019, 55, 9271–9274. [Google Scholar] [CrossRef]
- Cai, W.; Morales-Martínez, R.; Zhang, X.; Najera, D.; Romero, E.L.; Metta-Magaña, A.; Rodríguez-Fortea, A.; Fortier, S.; Chen, N.; Poblet, J.M.; et al. Single crystal structures and theoretical calculations of uranium endohedral metallofullerenes (U@C2n, 2n = 74, 82) show cage isomer dependent oxidation states for U. Chem. Sci. 2017, 8, 5282–5290. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Abella, L.; Zhuang, J.; Zhang, X.; Feng, L.; Wang, Y.; Morales-Martínez, R.; Esper, R.; Boero, M.; Metta-Magaña, A.; et al. Synthesis and Characterization of Non-Isolated-Pentagon-Rule Actinide Endohedral Metallofullerenes U@C1(17418)-C76, U@C1(28324)-C80, and Th@C1(28324)-C80: Low-Symmetry Cage Selection Directed by a Tetravalent Ion. J. Am. Chem. Soc. 2018, 140, 18039–18050. [Google Scholar] [CrossRef]
- Jin, P.; Liu, C.; Li, Y.; Li, L.; Zhao, Y. Th@C76. Computational characterization of larger actinide endohedral fullerenes. Int. J. Quantum. Chem. 2018, 118, e25501. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, X.; Ehara, M. Theoretical Insights into Monometallofullerene Th@C76: Strong Covalent Interaction between Thorium and the Carbon Cage. Inorg. Chem. 2018, 57, 2961–2964. [Google Scholar] [CrossRef]
- Bao, L.; Li, Y.; Yu, P.; Shen, W.; Jin, P.; Lu, X. Preferential Formation of Mono-Metallofullerenes Governed by the Encapsulation Energy of the Metal Elements: A Case Study on Eu@C2n (2n = 74–84) Revealing a General Rule. Angew. Chem. Int. Ed. 2020, 59, 5259–5262. [Google Scholar] [CrossRef]
- Hao, Y.; Feng, L.; Xu, W.; Gu, Z.; Hu, Z.; Shi, Z.; Slanina, Z.; Uhlik, F. Sm@C2v(19138)-C76: A Non-IPR Cage Stabilized by a Divalent Metal Ion. Inorg. Chem. 2015, 54, 4243–4248. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Shen, Y.; Yao, Y.-R.; Meng, Q.; Chen, N. Synthesis and Characterization of a Novel Non-Isolated-Pentagon-Rule Isomer of Th@C76:Th@C1(17418)-C76. Inorganics 2023, 11, 422. https://doi.org/10.3390/inorganics11110422
Xia Y, Shen Y, Yao Y-R, Meng Q, Chen N. Synthesis and Characterization of a Novel Non-Isolated-Pentagon-Rule Isomer of Th@C76:Th@C1(17418)-C76. Inorganics. 2023; 11(11):422. https://doi.org/10.3390/inorganics11110422
Chicago/Turabian StyleXia, Yunpeng, Yi Shen, Yang-Rong Yao, Qingyu Meng, and Ning Chen. 2023. "Synthesis and Characterization of a Novel Non-Isolated-Pentagon-Rule Isomer of Th@C76:Th@C1(17418)-C76" Inorganics 11, no. 11: 422. https://doi.org/10.3390/inorganics11110422