Progress on Noble-Metal-Free Organic–Inorganic Hybrids for Electrochemical Water Oxidation
Abstract
:1. Introduction
2. Fundamental Principles of Water Oxidation
2.1. Mechanisms of Electrochemical Water Oxidation
2.2. Important Descriptors for the Evaluation of Electrochemical Performance
3. Organic–Inorganic Hybrid Materials for Electrochemical Water Oxidation
3.1. Metal–Organic Frameworks (MOFs) and Their Derivates
3.2. Derivates of Covalent Organic Frameworks (COFs)
3.3. Other Organic–Inorganic Hybrid Materials (OIHs) for Electrochemical Water Oxidation
4. Summary and Perspectives
- (1)
- Rational design and fabrication of OIHs. The chemical compositions including both organic molecules and transition metal-based inorganic compounds in the reported OIHs are limited, while they are critical to determine the OER activity of the hybrids. Consequently, developing a universal and controllable method to fabricate the targeted OIH hybrids with high quality and in large scale is required in further studies. What’s more, efforts should be made to expand the chemical composition of OIHs, enabling the synthesis of a wider range of organic molecules and transition metal-based inorganic compounds. This will facilitate the creation of highly tailored and functional materials.
- (2)
- Developing OIHs for efficient OER in low-pH conditions. In most OIHs, the active metal sites are immobilized through relatively weak chemical bonds, leading to their vulnerability in excessive acidic conditions. This is the reason why the majority of currently available OIHs function effectively only in alkaline environments. However, in terms of cost and safety, expanding the application of OIHs as OER catalysts that work well in acidic or neutral mediums is important [121]. To this end, the structure–performance relationship and reaction mechanism of OIHs towards OER should be identified to expand the application environments. The structure of the active center should be carefully determined, and the in situ techniques should be coupled with computational modelling to obtain the dynamic structural evolution of active centers in acidic or neutral electrolytes. This knowledge will guide the design of novel acidic-resistant OIHs with enhanced performance.
- (3)
- Understanding the low stability of OIHs. Although thousands of OIHs have been reported as OER electrocatalysts, most of them suffer from poor stability, especially under harsh reaction conditions. However, little in-depth research has been conducted on revealing the underlying reasons, leaving significant room for further interpretation. Several factors are suggested to be considered in future studies: (a) The effect of strong alkaline solutions. This consideration is important as the harsh environment may contribute to the collapse of the crystal structure and the coordination bonds in OIHs. (b) The potential applied during the OER process. The high positive potential to meet the high current density requirement would lead to the oxidation of organic components in OIHs that should be carefully determined. Note that measuring the Faradaic efficiency of O2 is a helpful method to identify the accurate current density from water oxidation and provides some hints to the stability of organic components in OIHs, which should be highlighted when comparing the catalytic performance of OIHs in future investigations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEM | adsorbates evolution mechanism |
| AFM | atomic force microscopy |
| BDC | benzenedicarboxylic acid |
| BPTC | biphenyl-3,4′,5-tricarboxylic acid |
| BTC | benzenedicarboxylic acid |
| CC | carbon cloth |
| CNT | carbon nanotubes |
| COF | covalent organic frameworks |
| CV | cyclic voltametric |
| Cdl | electrochemical double-layer capacitance |
| EPR | electron paramagnetic resonance |
| EQCM-D | electrochemical quartz crystal microbalance with dissipation |
| EXAFS | extended X-ray absorption fine structure |
| FE | Faradaic efficiency |
| GC | glass carbon |
| GO | graphene oxide |
| HER | hydrogen evolution reaction |
| HRTEM | high-resolution transmission electron microscopy |
| LOM | lattice-oxygen participated mechanism |
| MOF | metal organic frameworks |
| MPN | metal–polyphenolic network |
| NB | nanoboxes |
| NF | nickel foam |
| NFF | nickel iron foam |
| OER | oxygen evolution reaction |
| OIH | organic–inorganic hybrid materials |
| RHE | reversible hydrogen electrode |
| SECM | scanning electrochemical microscopy |
| SEM | scanning electron microscopy |
| SSS | stainless steel strip |
| TA | tannic acid |
| TM | transition metals |
| XAS | synchrotron X-ray absorption spectroscopy |
| ZIF | zeolitic imidazolate frameworks |
| overpotential | |
| b | Tafel slope |
References
- Ma, J.; Wang, J.; Li, J.; Tian, Y.; Zhang, T. A Green Synthesis Strategy for Cobalt Phosphide Deposited on N, P Co-Doped Graphene for Efficient Hydrogen Evolution. Materials 2023, 16, 6119. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, Z.P.; Evans, J.M.; Meier, M.C.; Papadantonakis, K.M.; Lewis, N.S. Decoupled electrochemical water-splitting systems: A review and perspective. Energy Environ. Sci. 2021, 14, 4740–4759. [Google Scholar] [CrossRef]
- Pleshivtseva, Y.; Derevyanov, M.; Pimenov, A.; Rapoport, A. Comparative analysis of global trends in low carbon hydrogen production towards the decarbonization pathway. Int. J. Hydrogen Energy 2023, 48, 32191–32240. [Google Scholar] [CrossRef]
- Yu, W.; Liu, H.; Zhao, Y.; Fu, Y.; Xiao, W.; Dong, B.; Wu, Z.; Chai, Y.; Wang, L. Amorphous NiOn coupled with trace PtOx toward superior electrocatalytic overall water splitting in alkaline seawater media. Nano Res. 2023, 16, 6517–6530. [Google Scholar] [CrossRef]
- Tan, Z.; Zhang, L.; Wu, T.; Zhou, B.; Long, X. Composition Tuned Electron Rearrangement of Transition-Metal-Based Compounds Promotes Hydrogen Generation. J. Phys. Chem. C 2023, 127, 15747–15756. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, H.; Hao, Z.; Yu, M.; Chen, X.; Chen, J. Electrodeposition of (hydro)oxides for an oxygen evolution electrode. Chem. Sci. 2020, 11, 10614–10625. [Google Scholar] [CrossRef]
- Long, X.; Ma, Z.; Yu, H.; Gao, X.; Pan, X.; Chen, X.; Yang, S.; Yi, Z. Porous FeNi oxide nanosheets as advanced electrochemical catalysts for sustained water oxidation. J. Mater. Chem. A 2016, 4, 14939–14943. [Google Scholar] [CrossRef]
- Long, X.; Lin, H.; Zhou, D.; An, Y.; Yang, S. Enhancing full water-splitting performance of transition metal bifunctional electrocatalysts in alkaline solutions by tailoring CeO2–transition metal oxides–Ni nanointerfaces. ACS Energy Lett. 2018, 3, 290–296. [Google Scholar] [CrossRef]
- Alobaid, A.; Wang, C.; Adomaitis, R.A. Mechanism and Kinetics of HER and OER on NiFe LDH Films in an Alkaline Electrolyte. J. Electrochem. Soc. 2018, 165, J3395. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.F.; Sun, X.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: Identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Chen, Z.; Zhu, H.; Cai, R.; Lin, Z.; Chen, Y.; Wang, Y.; Gao, J.; Long, X.; Yang, S. Fe (III) Docking-Activated Sites in Layered Birnessite for Efficient Water Oxidation. J. Am. Chem. Soc. 2023, 145, 11215–11226. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Batool, M.; Liu, Z.; Nadeem, M.A.; Jin, R. Layered Double Hydroxide-Derived Nanomaterials for Efficient Electrocatalytic Water Splitting: Recent Progress and Future Perspective. ACS Energy Lett. 2022, 7, 3311–3328. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, Q.; Huang, D.; Jiao, C.; Liu, Y.; Liu, Y.; Fang, J.; Sun, M.; Yu, L. Operando Reconstruction toward Dual-Cation-Defects Co-Containing NiFe Oxyhydroxide for Ultralow Energy Consumption Industrial Water Splitting Electrolyzer. Adv. Energy Mater. 2023, 13, 2203595. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S.M.; García de Arquer, F.P.; Dinh, C.T.; Li, J.; Wang, Z.; Zheng, X.; Zhang, L.; et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992. [Google Scholar] [CrossRef]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional Heterostructured Transition Metal Phosphides for Efficient Electrochemical Water Splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, Z.-Y. Transition metal–phosphorus-based materials for electrocatalytic energy conversion reactions. Catal. Sci. Technol. 2017, 7, 330–347. [Google Scholar] [CrossRef]
- Su, J.; Zhou, J.; Wang, L.; Liu, C.; Chen, Y. Synthesis and application of transition metal phosphides as electrocatalyst for water splitting. Sci. Bull. 2017, 62, 633–644. [Google Scholar] [CrossRef]
- Cai, R.; Ju, M.; Chen, J.; Ren, J.; Yu, J.; Long, X.; Yang, S. Recent advances in surface/interface engineering of noble-metal free catalysts for energy conversion reactions. Mater. Chem. Front. 2021, 5, 3576–3592. [Google Scholar] [CrossRef]
- Long, X.; Qiu, W.; Wang, Z.; Wang, Y.; Yang, S. Recent advances in transition metal–based catalysts with heterointerfaces for energy conversion and storage. Mater. Today Chem. 2019, 11, 16–28. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Zheng, W.; Ma, J.; Li, L.; Zheng, Y.; Sun, D.; Wen, Z.; Liu, Z.; Wang, Y.; et al. Self-Polarized Organic–Inorganic Hybrid Ferroelectric Cathode Coatings Assisted High Performance All-Solid-State Lithium Battery. Adv. Funct. Mater. 2023, 33, 2300791. [Google Scholar] [CrossRef]
- Ma, X.; Cao, X.; Yao, M.; Shan, L.; Shi, X.; Fang, G.; Pan, A.; Lu, B.; Zhou, J.; Liang, S. Organic–Inorganic Hybrid Cathode with Dual Energy-Storage Mechanism for Ultrahigh-Rate and Ultralong-Life Aqueous Zinc-Ion Batteries. Adv. Mater. 2022, 34, 2105452. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Tombesi, A. MOFs for Electrochemical Energy Conversion and Storage. Inorganics 2023, 11, 65. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, J.; Qi, D.; Sonar, P.; Liu, L.; Lou, Z.; Shen, G.; Wei, Z. Flexible Sensors Based on Organic–Inorganic Hybrid Materials. Adv. Mater. Technol. 2021, 6, 2000889. [Google Scholar] [CrossRef]
- Li, D.; Jia, Z.; Tang, Y.; Song, C.; Liang, K.; Ren, H.; Li, F.; Chen, Y.; Wang, Y.; Lu, X.; et al. Inorganic–Organic Hybrid Phototransistor Array with Enhanced Photogating Effect for Dynamic Near-Infrared Light Sensing and Image Preprocessing. Nano Lett. 2022, 22, 5434–5442. [Google Scholar] [CrossRef]
- Kladsomboon, S.; Thippakorn, C.; Seesaard, T. Development of Organic-Inorganic Hybrid Optical Gas Sensors for the Non-Invasive Monitoring of Pathogenic Bacteria. Sensors 2018, 18, 3189. [Google Scholar] [CrossRef]
- Goodman, E.D.; Zhou, C.; Cargnello, M. Design of Organic/Inorganic Hybrid Catalysts for Energy and Environmental Applications. ACS Cent. Sci. 2020, 6, 1916–1937. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Li, Y.; Hu, Y.; Li, H.; Xu, C.C.; Yang, S. Functionalized organic–inorganic hybrid porous coordination polymer-based catalysts for biodiesel production via trans/esterification. Green Chem. 2022, 24, 7763–7786. [Google Scholar] [CrossRef]
- Erigoni, A.; Diaz, U. Porous Silica-Based Organic-Inorganic Hybrid Catalysts: A Review. Catalysts 2021, 11, 79. [Google Scholar] [CrossRef]
- Lei, Z.; Cai, W.; Rao, Y.; Wang, K.; Jiang, Y.; Liu, Y.; Jin, X.; Li, J.; Lv, Z.; Jiao, S.; et al. Coordination modulation of iridium single-atom catalyst maximizing water oxidation activity. Nat. Commun. 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Metallic nanostructures with low dimensionality for electrochemical water splitting. Chem. Soc. Rev. 2020, 49, 3072–3106. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Gomez, R.; Fernandez-Vega, A.; Feliu, J.M.; Aldaz, A. Hydrogen evolution on platinum single crystal surfaces: Effects of irreversibly adsorbed bismuth and antimony on hydrogen adsorption and evolution on platinum (100). J. Phys. Chem. 1993, 97, 4769–4776. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Reier, T.; Nong, H.N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction in Acidic Environments—Reaction Mechanisms and Catalysts. Adv. Energy Mater. 2017, 7, 1601275. [Google Scholar] [CrossRef]
- Zhang, N.; Chai, Y. Lattice oxygen redox chemistry in solid-state electrocatalysts for water oxidation. Energy Environ. Sci. 2021, 14, 4647–4671. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Abild-Pedersen, F.; Greeley, J.; Studt, F.; Rossmeisl, J.; Munter, T.R.; Moses, P.G.; Skúlason, E.; Bligaard, T.; Nørskov, J.K. Scaling Properties of Adsorption Energies for Hydrogen-Containing Molecules on Transition-Metal Surfaces. Phys. Rev. Lett. 2007, 99, 016105. [Google Scholar] [CrossRef]
- Greeley, J. Theoretical Heterogeneous Catalysis: Scaling Relationships and Computational Catalyst Design. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 605–635. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Loffreda, D.; Koper, M.T.M.; Sautet, P. Introducing structural sensitivity into adsorption–energy scaling relations by means of coordination numbers. Nat. Chem. 2015, 7, 403–410. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Zhang, K.; Wu, Z.; Gao, F.; Du, Y. Heterogeneous interface engineering for boosting electron transfer induced by MOF-derived Yolk-shell trimetallic phosphide nanospindles for robust water oxidation electrocatalysis. Appl. Surf. Sci. 2022, 590, 153102. [Google Scholar] [CrossRef]
- Liu, X.; You, B.; Yu, X.-Y.; Chipman, J.; Sun, Y. Electrochemical oxidation to construct a nickel sulfide/oxide heterostructure with improvement of capacitance. J. Mater. Chem. A 2016, 4, 11611–11615. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Heitbaum, J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. Interfacial Electrochem. 1987, 237, 251–260. [Google Scholar] [CrossRef]
- Tyburski, R.; Liu, T.; Glover, S.D.; Hammarström, L. Proton-Coupled Electron Transfer Guidelines, Fair and Square. J. Am. Chem. Soc. 2021, 143, 560–576. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Anantharaj, S.; Sugime, H.; Noda, S. Why shouldn’t double-layer capacitance (Cdl) be always trusted to justify Faradaic electrocatalytic activity differences? J. Electroanal. Chem. 2021, 903, 115842. [Google Scholar] [CrossRef]
- Shin, S.-J.; Kim, D.H.; Bae, G.; Ringe, S.; Choi, H.; Lim, H.-K.; Choi, C.H.; Kim, H. On the importance of the electric double layer structure in aqueous electrocatalysis. Nat. Commun. 2022, 13, 174. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; He, Y.; Zhu, H. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 2021, 9, 5320–5363. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Xu, S.-M.; Lu, L.; Xu, M.; Ji, K.; Ge, R.; Yan, Y.; Ma, L.; Kong, X.; et al. Selectively Upgrading Lignin Derivatives to Carboxylates through Electrochemical Oxidative C(OH)−C Bond Cleavage by a Mn-Doped Cobalt Oxyhydroxide Catalyst. Angew. Chem. Int. Ed. 2021, 60, 8976–8982. [Google Scholar] [CrossRef]
- Kempler, P.A.; Nielander, A.C. Reliable reporting of Faradaic efficiencies for electrocatalysis research. Nat. Commun. 2023, 14, 1158. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, X.; Kang, Z.; Liu, X.; Sun, D. Isoreticular chemistry within metal-organic frameworks for gas storage and separation. Coord. Chem. Rev. 2021, 443, 213968. [Google Scholar] [CrossRef]
- Jia, T.; Gu, Y.; Li, F. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: A review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Denny, M.S., Jr.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal-organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016, 1, 16078. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Sanati, S.; Morsali, A.; Garcia, H. First-row transition metal-based materials derived from bimetallic metal-organic frameworks as highly efficient electrocatalysts for electrochemical water splitting. Energy Environ. Sci. 2022, 15, 3119–3151. [Google Scholar] [CrossRef]
- Carson, F.; Agrawal, S.; Gustafsson, M.; Bartoszewicz, A.; Moraga, F.; Zou, X.; Martin-Matute, B. Ruthenium Complexation in an Aluminium Metal-Organic Framework and Its Application in Alcohol Oxidation Catalysis. Chem.-A Eur. J. 2012, 18, 15337–15344. [Google Scholar] [CrossRef]
- Khan, I.S.; Garzon-Tovar, L.; Mateo, D.; Gascon, J. Metal-Organic-Frameworks and Their Derived Materials in Photo-Thermal Catalysis. Eur. J. Inorg. Chem. 2022, 2022, e202200316. [Google Scholar] [CrossRef]
- Xuan, X.; Chen, S.; Zhao, S.; Yoon, J.Y.; Boczkaj, G.; Sun, X. Carbon Nanomaterials From Metal-Organic Frameworks: A New Material Horizon for CO2 Reduction. Front. Chem. 2020, 8, 573797. [Google Scholar] [CrossRef]
- Han, A.; Wang, B.; Kumar, A.; Qin, Y.; Jin, J.; Wang, X.; Yang, C.; Dong, B.; Jia, Y.; Liu, J.; et al. Recent Advances for MOF-Derived Carbon-Supported Single-Atom Catalysts. Small Methods 2019, 3, 1800471. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Peng, Y.; Sanati, S.; Morsali, A.; Garcia, H. Metal-Organic Frameworks as Electrocatalysts. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214707. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Ge, K.; Sun, S.; Zhao, Y.; Yang, K.; Wang, S.; Zhang, Z.; Cao, J.; Yang, Y.; Zhang, Y.; Pan, M.; et al. Facile Synthesis of Two-Dimensional Iron/Cobalt Metal–Organic Framework for Efficient Oxygen Evolution Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 12097–12102. [Google Scholar] [CrossRef]
- Cao, C.; Ma, D.D.; Xu, Q.; Wu, X.T.; Zhu, Q.L. Semisacrificial Template Growth of Self-Supporting MOF Nanocomposite Electrode for Efficient Electrocatalytic Water Oxidation. Adv. Funct. Mater. 2018, 29, 1807418. [Google Scholar] [CrossRef]
- Li, W.; Fang, W.; Wu, C.; Dinh, K.N.; Ren, H.; Zhao, L.; Liu, C.; Yan, Q. Bimetal–MOF nanosheets as efficient bifunctional electrocatalysts for oxygen evolution and nitrogen reduction reaction. J. Mater. Chem. A 2020, 8, 3658–3666. [Google Scholar] [CrossRef]
- Wang, C.-P.; Feng, Y.; Sun, H.; Wang, Y.; Yin, J.; Yao, Z.; Bu, X.-H.; Zhu, J. Self-Optimized Metal–Organic Framework Electrocatalysts with Structural Stability and High Current Tolerance for Water Oxidation. ACS Catal. 2021, 11, 7132–7143. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, C.; He, C.-T.; An, P.; Xie, F.; Jiang, S.; Zhu, Y.; Wu, K.-H.; Zhang, B.; Li, H.; et al. Structural transformation of highly active metal–organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 2020, 5, 881–890. [Google Scholar] [CrossRef]
- Wang, X.-L.; Dong, L.-Z.; Qiao, M.; Tang, Y.-J.; Liu, J.; Li, Y.; Li, S.-L.; Su, J.-X.; Lan, Y.-Q. Exploring the Performance Improvement of the Oxygen Evolution Reaction in a Stable Bimetal–Organic Framework System. Angew. Chem. Int. Ed. 2018, 57, 9660–9664. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, D.-D.; Wu, Y.-P.; Zhao, J.; Wu, T.; Zhang, J.; Li, D.-S.; Sun, C.; Feng, P.; Bu, X. Stable Hierarchical Bimetal–Organic Nanostructures as HighPerformance Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 4227–4231. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Y.; Chen, P.; Zhang, S.; Jiang, H.; Yang, L.; Wang, Y.; Zhang, L.; Shen, J.; Zhao, X.; et al. A Freestanding 3D Heterostructure Film Stitched by MOF-Derived Carbon Nanotube Microsphere Superstructure and Reduced Graphene Oxide Sheets: A Superior Multifunctional Electrode for Overall Water Splitting and Zn-Air Batteries. Adv. Mater. 2020, 32, e2003313. [Google Scholar] [CrossRef]
- Jia, X.; Wu, J.; Lu, K.; Li, Y.; Qiao, X.; Kaelin, J.; Lu, S.; Cheng, Y.; Wu, X.; Qin, W. Organic–inorganic hybrids of Fe–Co polyphenolic network wrapped Fe3O4 nanocatalysts for significantly enhanced oxygen evolution. J. Mater. Chem. A 2019, 7, 14302–14308. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.L.; Lu, X.F.; Wu, Z.-P.; Luan, D.; Lou, X.W. Trimetallic Spinel NiCo2−xFexO4 Nanoboxes for Highly Efficient Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2021, 60, 11841–11846. [Google Scholar] [CrossRef]
- Mesbah, A.; Rabu, P.; Sibille, R.; Lebègue, S.; Mazet, T.; Malaman, B.; François, M. From Hydrated Ni3(OH)2(C8H4O4)2(H2O)4 to Anhydrous Ni2(OH)2(C8H4O4): Impact of Structural Transformations on Magnetic Properties. Inorg. Chem. 2014, 53, 872–881. [Google Scholar] [CrossRef]
- Carton, A.; Mesbah, A.; Mazet, T.; Porcher, F.; François, M. Ab initio crystal structure of nickel(II) hydroxy-terephthalate by synchrotron powder diffraction and magnetic study. Solid State Sci. 2007, 9, 465–471. [Google Scholar] [CrossRef]
- Jin, S. How to Effectively Utilize MOFs for Electrocatalysis. ACS Energy Lett. 2019, 4, 1443–1445. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mullangi, D.; Chandran, C.; Vaidhyanathan, R. Nanopores of a Covalent Organic Framework: A Customizable Vessel for Organocatalysis. ACS Omega 2022, 7, 15275–15295. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki–Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef]
- Mullangi, D.; Dhavale, V.; Shalini, S.; Nandi, S.; Collins, S.; Woo, T.; Kurungot, S.; Vaidhyanathan, R. Low-Overpotential Electrocatalytic Water Splitting with Noble-Metal-Free Nanoparticles Supported in a sp3 N-Rich Flexible COF. Adv. Energy Mater. 2016, 6, 1600110. [Google Scholar] [CrossRef]
- Nandi, S.; Singh, S.K.; Mullangi, D.; Illathvalappil, R.; George, L.; Vinod, C.P.; Kurungot, S.; Vaidhyanathan, R. Low Band Gap Benzimidazole COF Supported Ni3N as Highly Active OER Catalyst. Adv. Energy Mater. 2016, 6, 1601189. [Google Scholar] [CrossRef]
- Lyu, H.; Diercks, C.S.; Zhu, C.; Yaghi, O.M. Porous Crystalline Olefin-Linked Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 6848–6852. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Z.; Liu, W.; Zhang, F.; Yan, H. Novel Bifunctional Nitrogen Doped MoS2/COF-C4N Vertical Heterostructures for Electrocatalytic HER and OER. Catalysts 2023, 13, 90. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, W.; Zhang, F.-M.; Yang, Z.-D.; Zhang, G.; Zeng, X.C. COF-C4N Nanosheets with uniformly anchored single metal sites for electrocatalytic OER: From theoretical screening to target synthesis. Appl. Catal. B Environ. 2023, 325, 122366. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.; Huang, Y.; He, X.; Su, X.; Xiao, L.; Yu, Y.; Huang, X.; Luo, F. Flexible and robust bimetallic covalent organic frameworks for the reversible switching of electrocatalytic oxygen evolution activity. J. Mater. Chem. A 2020, 8, 5907–5912. [Google Scholar] [CrossRef]
- Feng, X.; Gao, Z.; Xiao, L.; Lai, Z.; Luo, F. A Ni/Fe complex incorporated into a covalent organic framework as a single-site heterogeneous catalyst for efficient oxygen evolution reaction. Inorg. Chem. Front. 2020, 7, 3925–3931. [Google Scholar] [CrossRef]
- Zong, H.; Liu, W.; Li, M.; Gong, S.; Yu, K.; Zhu, Z. Oxygen-Terminated Nb2CO2 MXene with Interfacial Self-Assembled COF as a Bifunctional Catalyst for Durable Zinc-Air Batteries. ACS Appl. Mater. Interfaces 2022, 14, 10738–10746. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective. Mater. Chem. Front. 2023, 7, 3298–3331. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, Z.; Gao, Y.; Yuan, D.; Kang, Z.; Qian, Y.; Yan, N.; Zhao, D. Synthesis of a Sulfonated Two-Dimensional Covalent Organic Framework as an Efficient Solid Acid Catalyst for Biobased Chemical Conversion. ChemSusChem 2015, 8, 3208–3212. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.-D.; Dong, H.; Sun, N.; Lu, Y.; Zhang, F.-M.; Zhang, G. Theory-Driven Design and Targeting Synthesis of a Highly-Conjugated Basal-Plane 2D Covalent Organic Framework for Metal-Free Electrocatalytic OER. ACS Energy Lett. 2019, 4, 2251–2258. [Google Scholar] [CrossRef]
- Ju, M.; Cai, R.; Ren, J.; Chen, J.; Qi, L.; Long, X.; Yang, S. Conductive Polymer Intercalation Tunes Charge Transfer and Sorption–Desorption Properties of LDH Enabling Efficient Alkaline Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 37063–37070. [Google Scholar] [CrossRef]
- Aman, S.; Gouadria, S.; Manzoor, S.; Abdullah, M.; Khosa, R.Y.; Ashiq, M.N.; Ansari, M.Z.; Alfantazi, A. Facile synthesis of CuZrO3@PPY nanohybrid balls embedded 3-dimensional network with synergistic effect for efficient oxygen evolution reaction. Surf. Interfaces 2023, 36, 102607. [Google Scholar] [CrossRef]
- Lyu, S.; Guo, C.; Wang, J.; Li, Z.; Yang, B.; Lei, L.; Wang, L.; Xiao, J.; Zhang, T.; Hou, Y. Exceptional catalytic activity of oxygen evolution reaction via two-dimensional graphene multilayer confined metal-organic frameworks. Nat. Commun. 2022, 13, 6171. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Lei, H.; Zhou, G.; Zhang, W.; Cao, R. Convenient Immobilization of Cobalt Corroles on Carbon Nanotubes through Covalent Bonds for Electrocatalytic Hydrogen and Oxygen Evolution Reactions. ChemSusChem 2019, 12, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, X.-P.; Zhao, B.; Li, P.; Qi, J.; Guo, X.; Wang, B.; Lei, H.; Zhang, W.; Apfel, U.-P.; et al. Enzyme-Inspired Iron Porphyrins for Improved Electrocatalytic Oxygen Reduction and Evolution Reactions. Angew. Chem. 2021, 133, 7654–7659. [Google Scholar] [CrossRef]
- Moulson, A.J. Transition Metal Oxides. In Concise Encyclopedia of Advanced Ceramic Materials; Brook, R.J., Ed.; Pergamon: Oxford, UK, 1991; pp. 497–499. [Google Scholar]
- Walia, S.; Balendhran, S.; Nili, H.; Zhuiykov, S.; Rosengarten, G.; Wang, Q.H.; Bhaskaran, M.; Sriram, S.; Strano, M.S.; Kalantar-zadeh, K. Transition metal oxides—Thermoelectric properties. Prog. Mater Sci. 2013, 58, 1443–1489. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Li, Y.-H.; Zhang, F.; Qi, M.-Y.; Chen, X.; Tang, Z.-R.; Yamada, Y.M.A.; Anpo, M.; Conte, M.; Xu, Y.-J. Rationally designed transition metal hydroxide nanosheet arrays on graphene for artificial CO2 reduction. Nat. Commun. 2020, 11, 5181. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, P.; Xie, R.; Xiong, Y.; Jia, C.; Fu, Y.; Song, P.; Chen, L.; Zhang, Y.; Liao, T. High-Temperature Nitridation Induced Carbon Nanotubes@NiFe-Layered-Double-Hydroxide Nanosheets Taking as an Oxygen Evolution Reaction Electrocatalyst for CO2 Electroreduction. Adv. Mater. Interfaces 2021, 8, 2101165. [Google Scholar] [CrossRef]
- Duarte, M.F.P.; Rocha, I.M.; Figueiredo, J.L.; Freire, C.; Pereira, M.F.R. CoMn-LDH@carbon nanotube composites: Bifunctional electrocatalysts for oxygen reactions. Catal. Today 2018, 301, 17–24. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef]
- Long, X.; Li, J.; Xiao, S.; Yan, K.; Wang, Z.; Chen, H.; Yang, S. A Strongly Coupled Graphene and FeNi Double Hydroxide Hybrid as an Excellent Electrocatalyst for the Oxygen Evolution Reaction. Angew. Chem. 2014, 126, 7714–7718. [Google Scholar] [CrossRef]
- Xu, X.; Chu, H.; Zhang, Z.; Dong, P.; Baines, R.; Ajayan, P.M.; Shen, J.; Ye, M. Integrated Energy Aerogel of N,S-rGO/WSe2/NiFe-LDH for Both Energy Conversion and Storage. ACS Appl. Mater. Interfaces 2017, 9, 32756–32766. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2011, 133, 7296–7299. [Google Scholar] [CrossRef]
- Bai, X.; Cao, T.; Xia, T.; Wu, C.; Feng, M.; Li, X.; Mei, Z.; Gao, H.; Huo, D.; Ren, X.; et al. MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting. Nanomaterials 2023, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Long, X.; An, Y.; Zhou, D.; Yang, S. Three-Dimensional Decoupling Co-Catalyst from a Photoabsorbing Semiconductor as a New Strategy To Boost Photoelectrochemical Water Splitting. Nano Lett. 2019, 19, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, C.; Lu, X. Conducting polymers-derived fascinating electrocatalysts for advanced hydrogen and oxygen electrocatalysis. Coord. Chem. Rev. 2022, 464, 214555. [Google Scholar] [CrossRef]
- Alsultan, M.; Ranjbar, A.; Swiegers, G.F.; Wallace, G.G.; Balakrishnan, S.; Huang, J. Application of Conducting Polymers in Solar Water-Splitting Catalysis. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 223–251. [Google Scholar]
- Zhu, Y.; Liu, C.; Cui, S.; Lu, Z.; Ye, J.; Wen, Y.; Shi, W.; Huang, X.; Xue, L.; Bian, J.; et al. Multistep Dissolution of Lamellar Crystals Generates Superthin Amorphous Ni(OH)2 Catalyst for UOR. Adv. Mater. 2023, 35, e2301549. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Wolszczak, M.; Kroh, J.; Abdel-Hamid, M.M. Some aspects of the radiation processing of conducting polymers. Radiat. Phys. Chem. 1995, 45, 71–78. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, W.; Yuan, P.; Yang, G.; Mu, S.; Liang, J.; Xia, H.; Guo, K.; Liu, M.; Zhao, S.; et al. Boosting Nitrogen Reduction to Ammonia on FeN4 Sites by Atomic Spin Regulation. Adv. Sci. 2021, 8, 2102915. [Google Scholar] [CrossRef]
- Liang, Y.; Banjac, K.; Martin, K.; Zigon, N.; Lee, S.; Vanthuyne, N.; Garcés-Pineda, F.A.; Galán-Mascarós, J.R.; Hu, X.; Avarvari, N.; et al. Enhancement of electrocatalytic oxygen evolution by chiral molecular functionalization of hybrid 2D electrodes. Nat. Commun. 2022, 13, 3356. [Google Scholar] [CrossRef]
- Ma, Q.; Mu, S. Acidic oxygen evolution reaction: Mechanism, catalyst classification, and enhancement strategies. Interdiscip. Mater. 2023, 2, 53–90. [Google Scholar] [CrossRef]



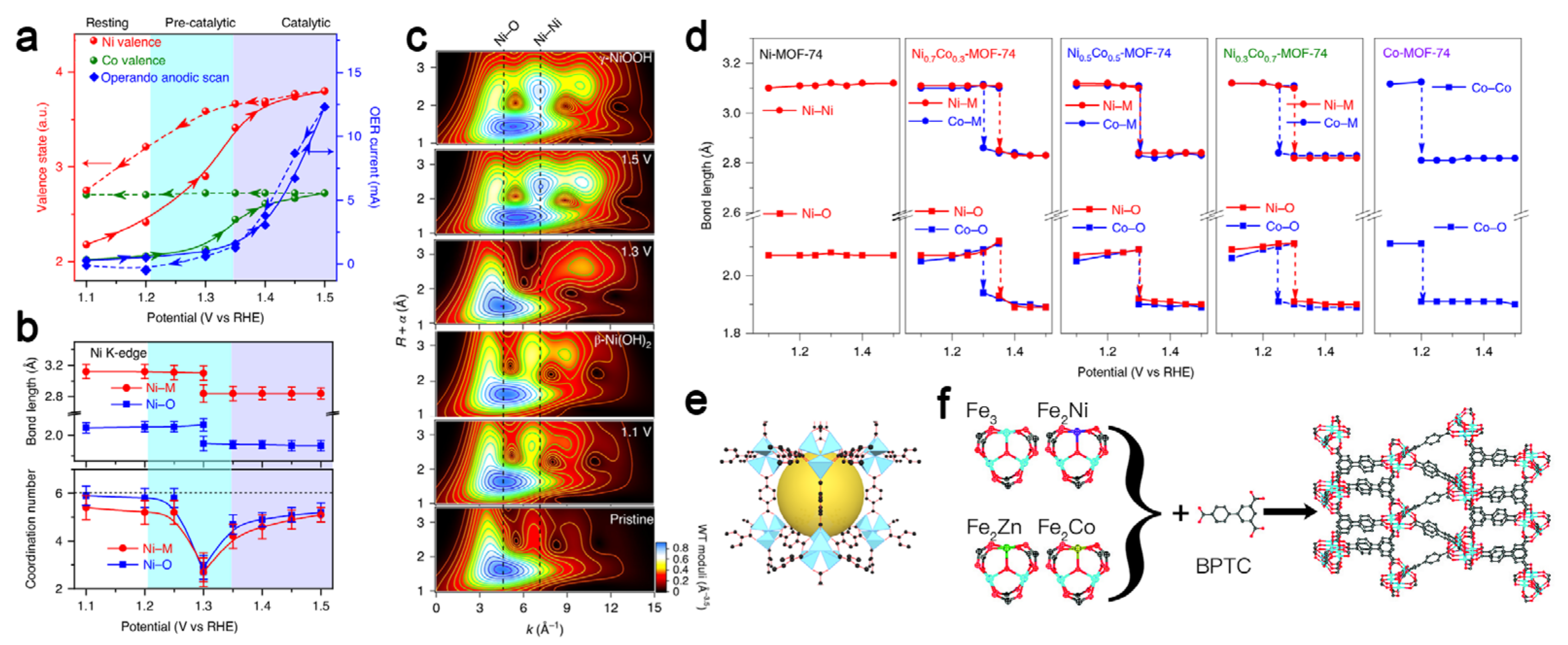


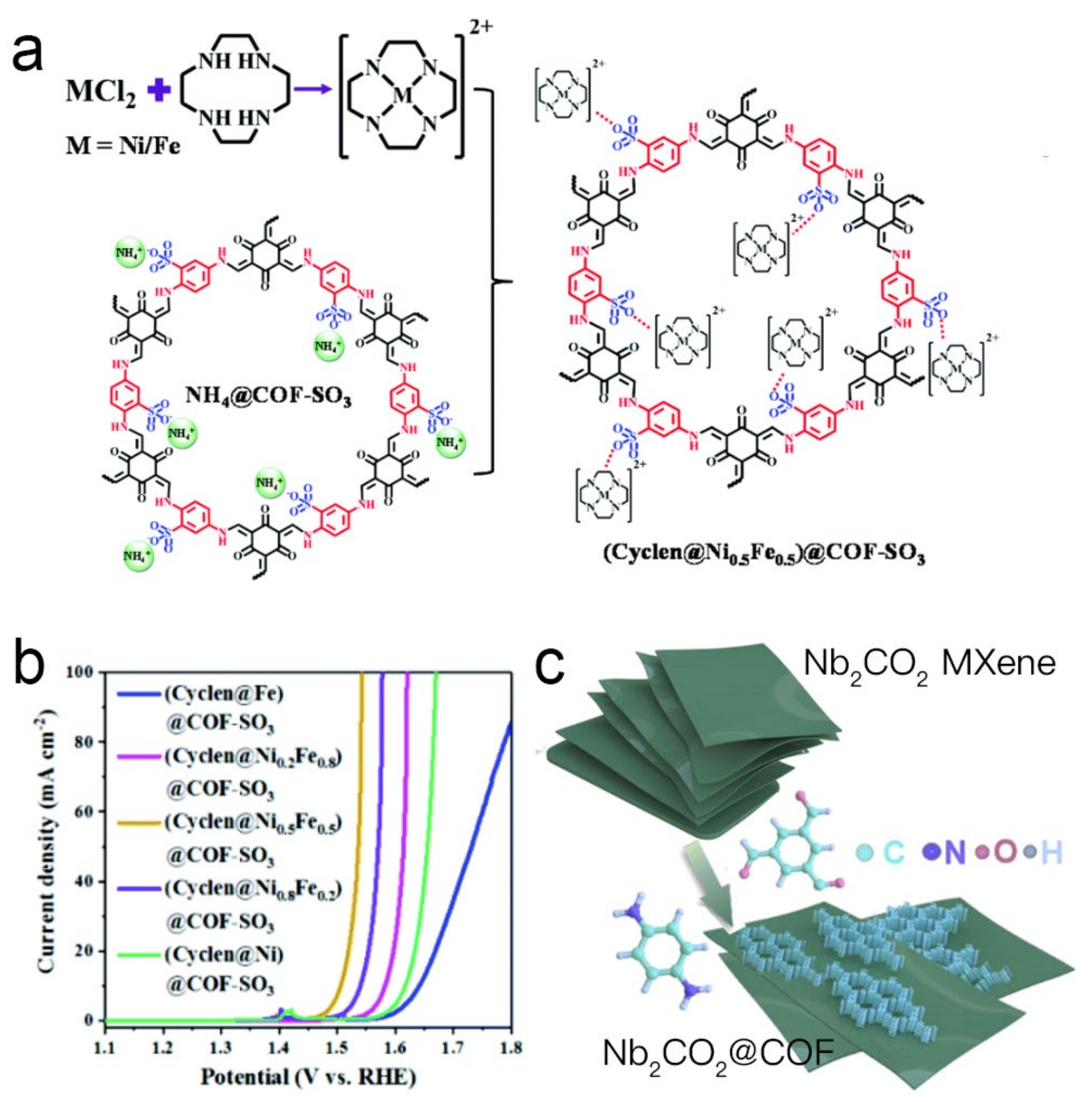

| Catalyst | Electrode Substrate | Electrolyte | Overpotential (V, at 10 mA cm−2) | Stability | Double-Layer Capacitance (mF cm−2) | Faradaic Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| NiCo-UMOFNs | GC 1 | 1.0 M KOH | 0.250 | 40,000 s | / | 99.3 | [69] |
| 2D MOF-Fe/Co(1:2) | GC disks | 1.0 M KOH | 0.238 | 50,000 s | 66.9 | / | [70] |
| NiFe-NFF | NFF 2 | 1.0 M KOH | 0.227 | 1000 cycles | 12.72 | 100 | [71] |
| Co3Fe-MOF | GC | 1.0 M KOH | 0.280 | 10 h | 17.74 | / | [72] |
| Fe2Ni-MOF | NF 3 | 1.0 M KOH | 0.213 | 1033 h 4 | 5.8 | / | [73] |
| Ni0.9Fe0.1-MOF-74 | GC | 1.0 M KOH | 0.198 | / | / | 99.6 | [74] |
| Fe2Ni-BPTC (NNU-23) | CC 5 | 0.1 M KOH | 0.365 | 2000 cycles | 5.10 | / | [75] |
| CTGU-10c2 | GC | 0.1 M KOH | 0.280 | 1000 cycles | 8.9 | / | [76] |
| Ni@N-HCGHF | GC | 1.0 M KOH | 0.260 | 2000 cycles | / | / | [77] |
| MPN@Fe3O4 | GC | 1.0 M KOH | 0.260 | 24 h | 25.8 | / | [78] |
| NiCo2−xFexO4 NBs | CP 6 | 1.0 M KOH | 0.274 | 25 h | 30.7 | / | [79] |
| Catalyst | Substrate | Electrolyte | Overpotential (V, at 10 mA cm−2) | Stability | Double-Layer Capacitance (mF cm−2) | Faradaic Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| Co4Ni12-COF | GC | 0.1 M KOH | 0.258 | 13 h | 0.398 | 90.0 | [87] |
| Ni3N-COF | GC | 1.0 M KOH | 0.230 | 20 h | 3.63 × 10−2 | 98.0 1 | [88] |
| COF-C4N | CC | 1.0 M KOH | 0.349 | 20 h | 5.01 | / | [97] |
| N-MoS2@COF-C4N1:1 | CC | 1.0 M KOH | 0.349 | 70,000 s | 4.19 | / | [90] |
| Co@COF-C4N1:1 | CC | 1.0 M KOH | 0.280 | 20 h | 42.04 | / | [91] |
| CoV@COF-SO3 | / | 1.0 M KOH | 0.318 | 1000 cycles | 3.38 | 99.0 | [92] |
| (cyclen@NiFe)@COF-SO3 | CP | 1.0 M KOH | 0.276 | 25 h | 0.462 | 99.0 | [93] |
| Nb2CO2@COF | GC | 1.0 M KOH | 0.373 | 10 h | 2.17 | / | [94] |
| Catalyst | Substrate | Electrolyte | Overpotential (V, at 10 mA cm−2) | Stability | Double-Layer Capacitance (mF cm−2) | Faradaic Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| LDH-ppy | NF | 1.0 M KOH | 0.248 | 10 h | / | 94.5 | [98] |
| CuZrO3@ppy | SSS 1 | 1.0 M KOH | 0.226 | 50 h | 0.96 | / | [99] |
| NiFe-BTC//G | graphite foil | 1.0 M KOH | 0.106 | 150 h 2 | 81.6 | 100 | [100] |
| CoCor-CNT | GC | 0.1 M pH 7 phosphate buffer | 0.470 | 100 cycles | / | / | [101] |
| FePor/CNT | GC | 0.1 M KOH | 0.500 | 8 h | 0.21 | 98.0 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Zhang, L.; Wu, T.; Zhan, Y.; Zhou, B.; Dong, Y.; Long, X. Progress on Noble-Metal-Free Organic–Inorganic Hybrids for Electrochemical Water Oxidation. Inorganics 2023, 11, 424. https://doi.org/10.3390/inorganics11110424
Tan Z, Zhang L, Wu T, Zhan Y, Zhou B, Dong Y, Long X. Progress on Noble-Metal-Free Organic–Inorganic Hybrids for Electrochemical Water Oxidation. Inorganics. 2023; 11(11):424. https://doi.org/10.3390/inorganics11110424
Chicago/Turabian StyleTan, Zheng, Lihua Zhang, Tong Wu, Yinbo Zhan, Bowei Zhou, Yilin Dong, and Xia Long. 2023. "Progress on Noble-Metal-Free Organic–Inorganic Hybrids for Electrochemical Water Oxidation" Inorganics 11, no. 11: 424. https://doi.org/10.3390/inorganics11110424







