Porous Nb2O5 Nanofibers Prepared via Reactive Needle-Less Electrospinning for Application in Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Results and Discussion
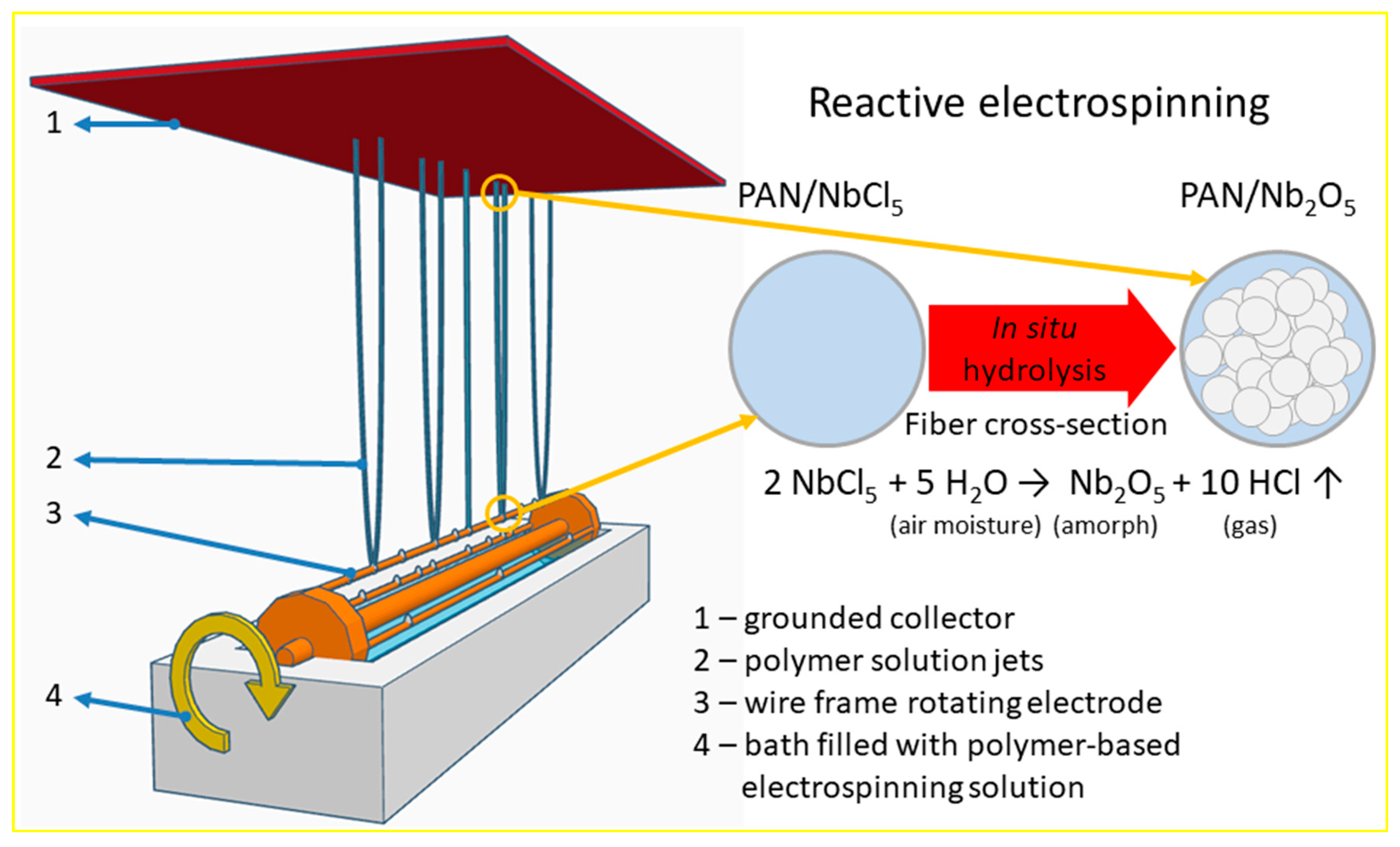
- (I)
- The blending of the precursor solution containing the mandatory support polymer and the ceramic precursor.
- (II)
- Electrospinning itself. The precursor fibers were electrospun by the needleless reactive approach in which the ceramic component is synthesized in situ during the fibers formation and collection. Here, reactive electrospinning includes the initial transformation reaction of the ceramic precursor—hydrolysis of NbCl5 by the air moisture to amorphous hydrated Nb2O5 trapped inside the PAN polymer fibers, thus resulting in amorphous pre-ceramic/polymer composite precursor fibers marked as PAN/Nb2O5:
- (III)
- The last one is the preparation of the ceramic nanofibers by heat treatment of the composite precursor PAN/Nb2O5 fibers, resulting in the final crystalline Nb2O5 nanofibers (Nb2O5 NFs).
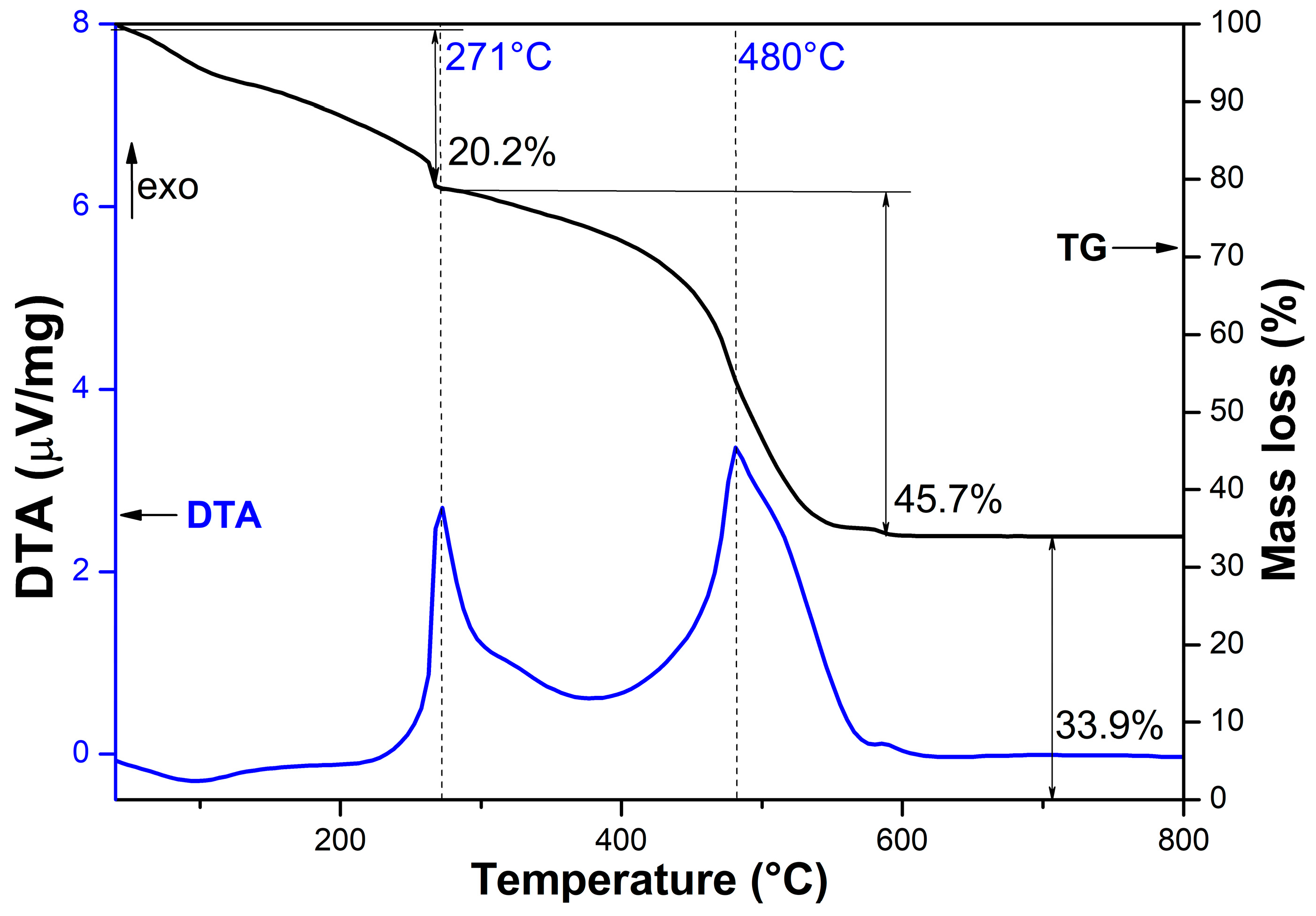
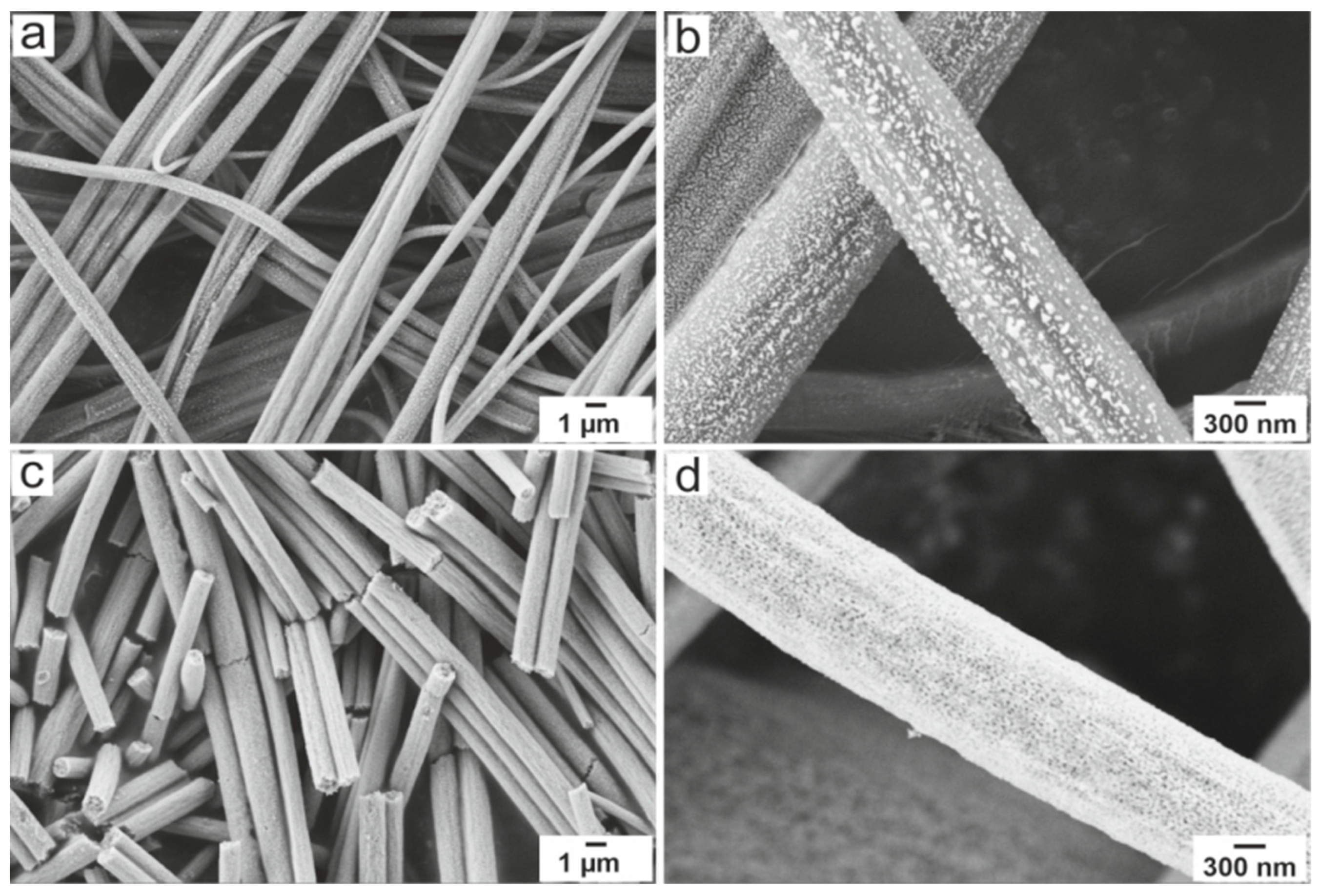
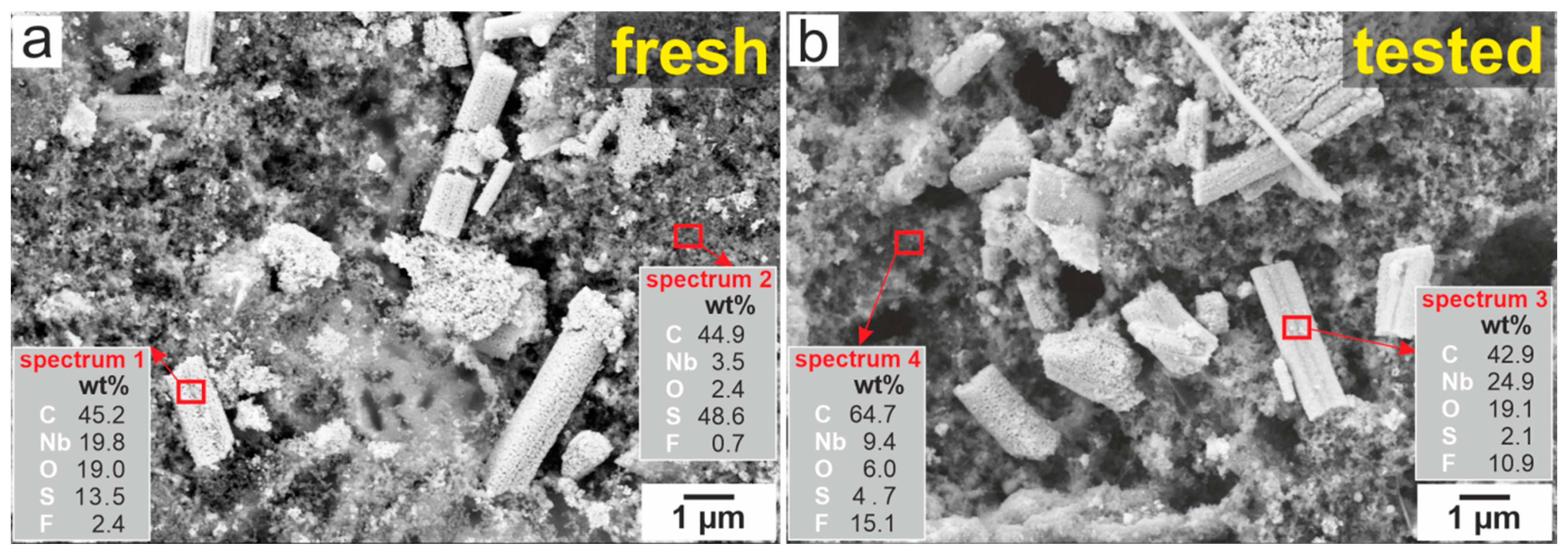
2.1. Structural Characterization
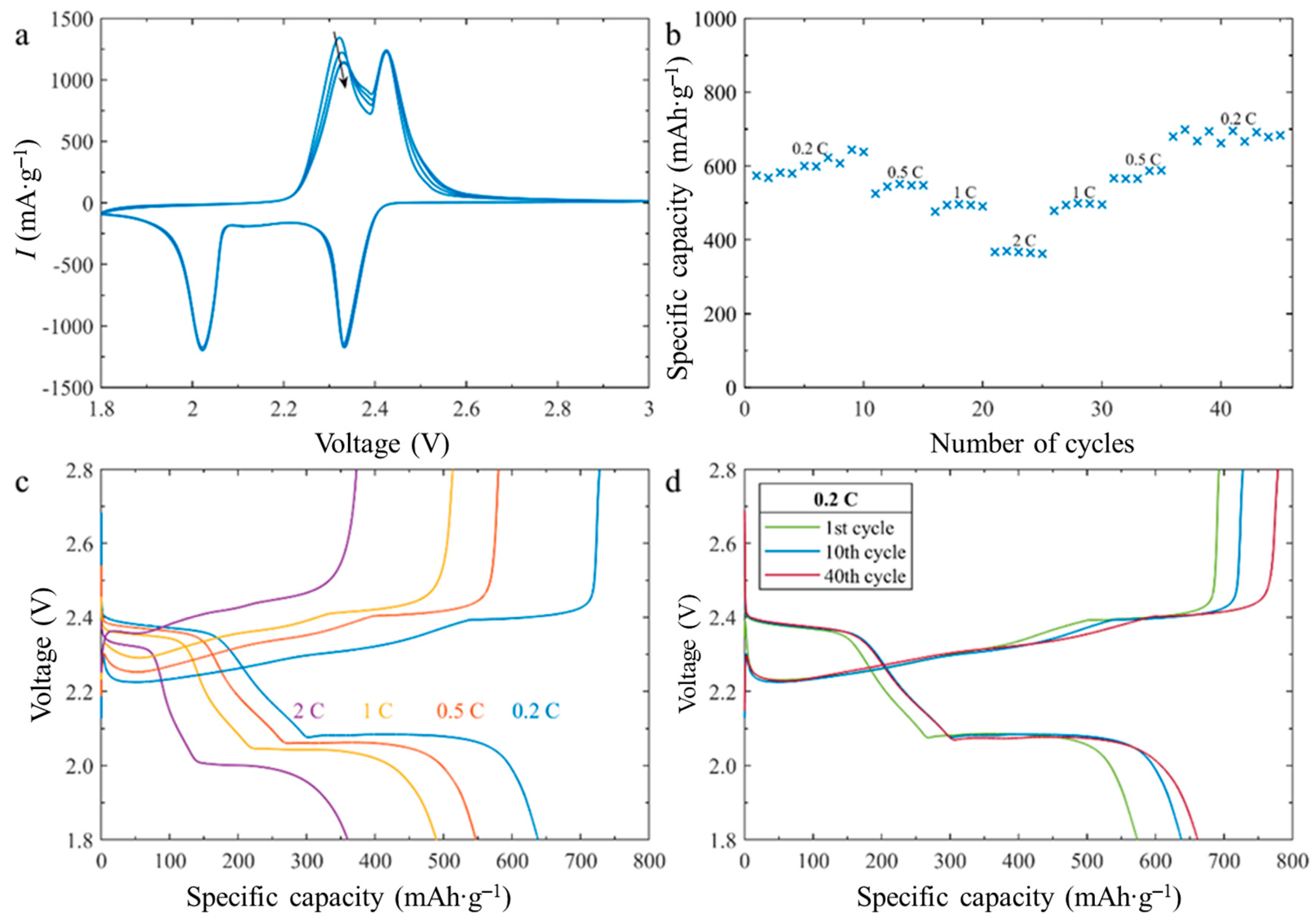
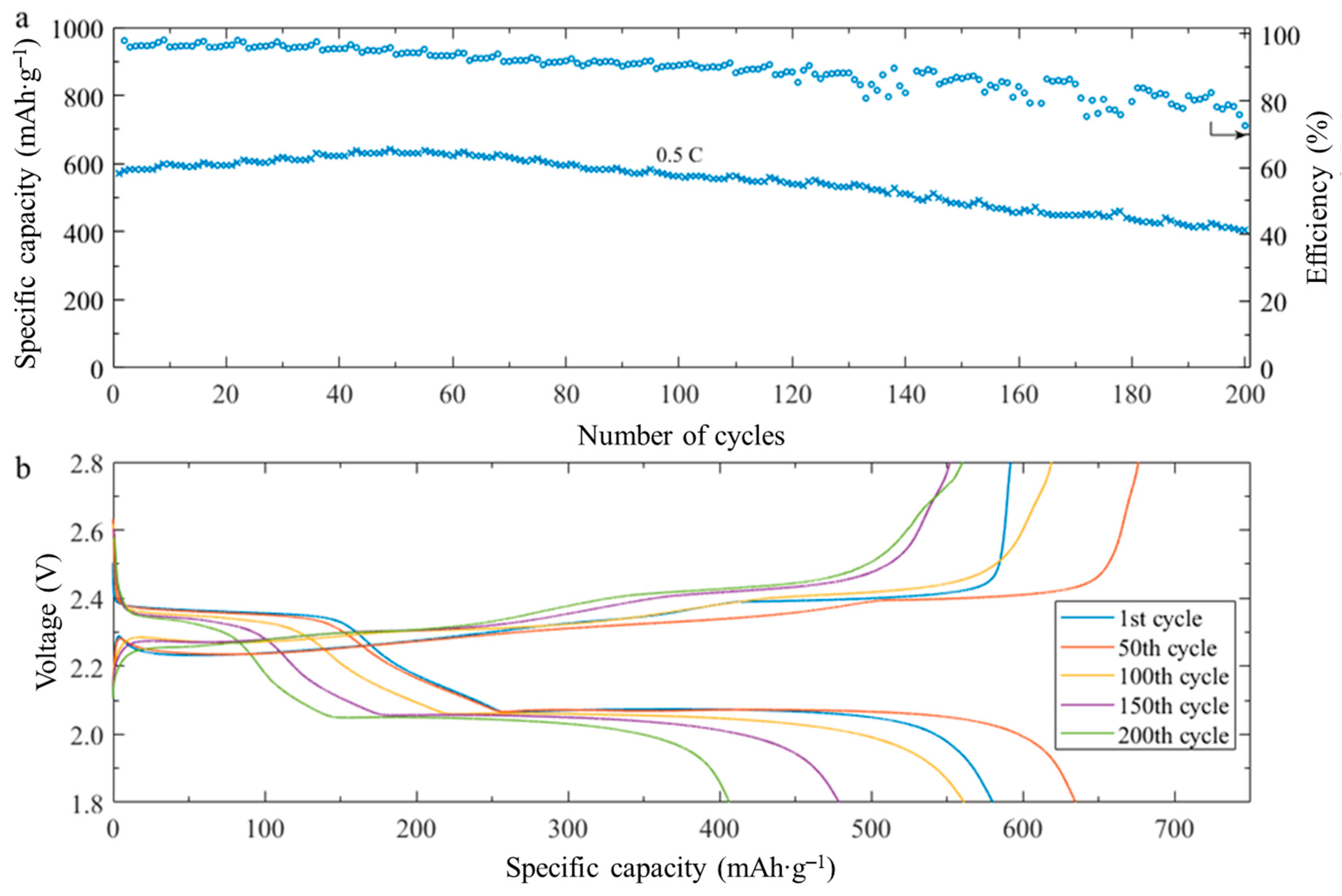
2.2. Electrochemical Characterization
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, X.; Xiang, K.; Zhu, Y.; Xiao, L.; Liao, H.; Chen, X.; Chen, H. Nb2O5-Decorated Nitrogen-Doped Carbon Nanotube Microspheres for Highly Efficient Sulfur Confinement in Lithium-Sulfur Batteries. Ind. Eng. Chem. Res. 2019, 58, 8724–8733. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium-Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, J.; Ying, Z.; Cai, Q.; Zheng, G.; Gan, Y.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.; et al. Strong Sulfur Binding with Conducting Magnéli-Phase TinO2n-1 Nanomaterials for Improving Lithium-Sulfur Batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Capkova, D.; Almasi, M.; Macko, J.; Kiraly, N.; Cech, O.; Cudek, P.; Strakova Fedorkova, A.; Knap, V.; Kazda, T. Activated and Carbonized Metal-Organic Frameworks for Improved Cycle Performance of Cathode Material in Lithium-Sulphur Batteries. J. Phys. Conf. Ser. 2022, 2382, 012010. [Google Scholar] [CrossRef]
- Choudhury, S. Hybrid Cathode Materials for Lithium-Sulfur Batteries. Curr. Opin. Electrochem. 2020, 21, 303–310. [Google Scholar] [CrossRef]
- Dong, C.; Gao, W.; Jin, B.; Jiang, Q. Advances in Cathode Materials for High-Performance Lithium-Sulfur Batteries. iScience 2018, 6, 151–198. [Google Scholar] [CrossRef]
- Wang, X.; Gao, T.; Fan, X.; Han, F.; Wu, Y.; Zhang, Z.; Li, J.; Wang, C. Tailoring Surface Acidity of Metal Oxide for Better Polysulfide Entrapment in Li-S Batteries. Adv. Funct. Mater. 2016, 26, 7164–7169. [Google Scholar] [CrossRef]
- Fan, X.; Sun, W.; Meng, F.; Xing, A.; Liu, J. Advanced Chemical Strategies for Lithium–Sulfur Batteries: A Review. Green Energy Environ. 2018, 3, 2–19. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.P.; Shao, Z. Intercalation Pseudocapacitance in Electrochemical Energy Storage: Recent Advances in Fundamental Understanding and Materials Development. Mater. Today Adv. 2020, 7, 100072. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Almeida, S.R.; Moreira, E.C.; Ferrer, M.M.; Jardim, P.L.G.; Moreira, M.L.; Raubach, C.W.; Cava, S. Photocatalytic Degradation of Rhodamine B Using Nb2O5 Synthesized with Different Niobium Precursors: Factorial Design of Experiments. Ceram. Int. 2021, 47, 20570–20578. [Google Scholar] [CrossRef]
- Nunes, B.N.; Lopes, O.F.; Patrocinio, A.O.T.; Bahnemann, D.W. Recent Advances in Niobium-Based Materials for Photocatalytic Solar Fuel Production. Catalysts 2020, 10, 126. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Y.; Li, B.; Xing, B.; Huang, G.; Zhao, H.; Jiang, Z.; Zhang, C.; Hong, S.W.; Cao, Y. Nanostructured T-Nb2O5-Based Composite with Reduced Graphene Oxide for Improved Performance Lithium-Ion Battery Anode. J. Mater. Sci. 2020, 55, 13062–13074. [Google Scholar] [CrossRef]
- Yuan, J.; Li, X.X.; Liu, J.; Zuo, S.; Li, X.X.; Li, F.; Gan, Y.; He, H.; Xu, X.; Zhang, X.; et al. Pomegranate-like Structured Nb2O5/Carbon@N-Doped Carbon Composites as Ultrastable Anode for Advanced Sodium/Potassium-Ion Batteries. J. Colloid Interface Sci. 2022, 613, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.; Zuo, Y.; Dixon, K.; Hou, D.; Lee, S.; Ma, Z.; Connell, J.G.; Zhou, H.; Deng, C.; Smith, K.; et al. Electrochemically Induced Amorphous-to-Rock-Salt Phase Transformation in Niobium Oxide Electrode for Li-Ion Batteries. Nat. Mater. 2022, 21, 795–803. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Le Viet, A.; Reddy, M.V.; Jose, R.; Chowdari, B.V.R. Nanostructured Nb2O5 Polymorphs by Electrospinning for Rechargeable Lithium Batteries. J. Phys. Chem. C 2010, 114, 664–671. [Google Scholar] [CrossRef]
- Deka, S. Nanostructured Mixed Transition Metal Oxide Spinels for Supercapacitor Applications. Dalton Trans. 2022, 52, 839–856. [Google Scholar] [CrossRef]
- Xiong, L.; Fu, Y.; Luo, Y.; Wei, Y.; Zhang, Z.; Wu, C.; Luo, S.; Wang, G.; Sawtell, D.; Xie, K.; et al. Prospective Applications of Transition Metal-Based Nanomaterials. J. Mater. Res. 2022, 37, 2109–2123. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Z.; Li, G.; Cheng, S.; Li, S.; Li, J.; Gao, R.; Li, M.; Sy, S.; Deng, Y.P.; et al. Revealing the Rapid Electrocatalytic Behavior of Ultrafine Amorphous Defective Nb2O5-x Nanocluster toward Superior Li-S Performance. ACS Nano 2020, 14, 4849–4860. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-Enhanced Redox Chemistry of Polysulphides on a Metallic and Polar Host for Lithium-Sulphur Batteries. Nat. Commun. 2014, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Ziolek, M. Niobium Compounds: Preparation, Characterization, and Application in Heterogeneous Catalysis. Chem. Rev. 1999, 99, 3603–3624. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.A.; Zoolfakar, A.S.; O’Mullane, A.P.; Austin, M.W.; Kalantar-Zadeh, K. Thin Films and Nanostructures of Niobium Pentoxide: Fundamental Properties, Synthesis Methods and Applications. J. Mater. Chem. A 2014, 2, 15683–15703. [Google Scholar] [CrossRef]
- Hu, Z.; He, Q.; Liu, Z.; Liu, X.; Qin, M.; Wen, B.; Shi, W.; Zhao, Y.; Li, Q.; Mai, L. Facile Formation of Tetragonal-Nb2O5 Microspheres for High-Rate and Stable Lithium Storage with High Areal Capacity. Sci. Bull. 2020, 65, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Song, Z.; Zhang, H.; Li, X. Niobium-Based Oxide Anodes toward Fast and Safe Energy Storage: A Review. Mater. Today Nano 2020, 11, 100082. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, X.; Liao, F.; Cheng, Q.; Mao, L.; Fa, H.; Chen, L. Recent Progress and Applications of Niobium-Based Nanomaterials and Their Composites for Supercapacitors and Hybrid Ion Capacitors. Sustain. Energy Fuels 2021, 5, 3039–3083. [Google Scholar] [CrossRef]
- Shan, Y.; Zheng, Z.; Liu, J.; Yang, Y.; Li, Z.; Huang, Z.; Jiang, D. Niobium Pentoxide: A Promising Surface-Enhanced Raman Scattering Active Semiconductor Substrate. npj Comput. Mater. 2017, 3, 11. [Google Scholar] [CrossRef]
- Prado, A.C.F.; Malafatti, J.O.D.; Oliveira, J.A.; Ribeiro, C.; Joya, M.R.; Luz, A.P.; Paris, E.C. Preparation and Application of Nb2O5 Nanofibers in CO2 Photoconversion. Nanomaterials 2021, 11, 3268. [Google Scholar] [CrossRef]
- Silva, M.K.; Marques, R.G.; Machado, N.R.C.F.; Santos, O.A.A. Evaluation of Nb2O5 and Ag/Nb2O5 in the Photocatalytic Degradation of Dyes from Textile Industries. Braz. J. Chem. Eng. 2002, 19, 359–363. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Youn, D.Y.; Kim, C.; Jung, J.W.; Ogata, A.F.; Bae, J.G.; Kim, I.D. Ag-Coated One-Dimensional Orthorhombic Nb2O5 Fibers as High Performance Electrodes for Lithium Storage. Electrochim. Acta 2018, 269, 388–396. [Google Scholar] [CrossRef]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The Thermal Degradation of Polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Storck, J.L.; Grothe, T.; Tuvshinbayar, K.; Diestelhorst, E.; Wehlage, D.; Brockhagen, B.; Wortmann, M.; Frese, N.; Ehrmann, A. Stabilization and Incipient Carbonization of Electrospun Polyacrylonitrile Nanofibers Fixated on Aluminum Substrates. Fibers 2020, 8, 55. [Google Scholar] [CrossRef]
- Samimi-Sohrforozani, E.; Azimi, S.; Abolhasani, A.; Malekian, S.; Arbab, S.; Zendehdel, M.; Abolhasani, M.M.; Yaghoobi Nia, N. Development of Porous Polyacrylonitrile Composite Fibers: New Precursor Fibers with High Thermal Stability. Electron. Mater. 2021, 2, 454–465. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. Thermal Analysis and Crystal Structure of Poly(Acrylonitrile-Co-Itaconic Acid) Copolymers Synthesized in Water. Polymers 2020, 12, 221. [Google Scholar] [CrossRef]
- Ren, Y.; Huo, T.; Qin, Y.; Liu, X. Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly. Materials 2018, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Mudra, E.; Streckova, M.; Pavlinak, D.; Medvecka, V.; Kovacik, D.; Kovalcikova, A.; Zubko, P.; Girman, V.; Dankova, Z.; Koval, V.; et al. Development of Al2O3 electrospun Fibers Prepared by Conventional Sintering Method or Plasma Assisted Surface Calcination. Appl. Surf. Sci. 2017, 415, 90–98. [Google Scholar] [CrossRef]
- Martin, S.C.; Liggat, J.J.; Snape, C.E. In Situ NMR Investigation into the Thermal Degradation and Stabilisation of PAN. Polym. Degrad. Stab. 2001, 74, 407–412. [Google Scholar] [CrossRef]
- Liu, J.; Xue, D.; Li, K. Single-Crystalline Nanoporous Nb2O5 Nanotubes. Nanoscale Res. Lett. 2011, 6, 138. [Google Scholar] [CrossRef]
- de MGomes, G.H.; de Andrade, R.R.; Mohallem, N.D.S. Investigation of Phase Transition Employing Strain Mapping in TT- and T-Nb2O5 Obtained by HRTEM Micrographs. Micron 2021, 148, 103112. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.S.; Roh, K.C.; et al. Facile Synthesis of Nb2O5@Carbon Core-Shell Nanocrystals with Controlled Crystalline Structure for High-Power Anodes in Hybrid Supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Shepa, I.; Mudra, E.; Vojtko, M.; Milkovic, O.; Dankova, Z.; Antal, V.; Annušová, A.; Majková, E.; Dusza, J. Influence of the Polymer Precursor Blend Composition on the Morphology of the Electrospun Oxide Ceramic Fibers. Results Phys. 2019, 13, 102243. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Knibbe, R.; Luo, B.; Ahad, S.A.; Sun, D.; Wang, L. Cyclic Voltammetry in Lithium–Sulfur Batteries—Challenges and Opportunities. Energy Technol. 2019, 7, 1801001. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Y.; Guo, X.; Wu, Z.; Chen, Y.; Li, J.; Li, L.; Zhong, B. A Novel Binder-Sulfonated Polystyrene for the Sulfur Cathode of Li-S Batteries. Ionics 2017, 23, 2251–2258. [Google Scholar] [CrossRef]


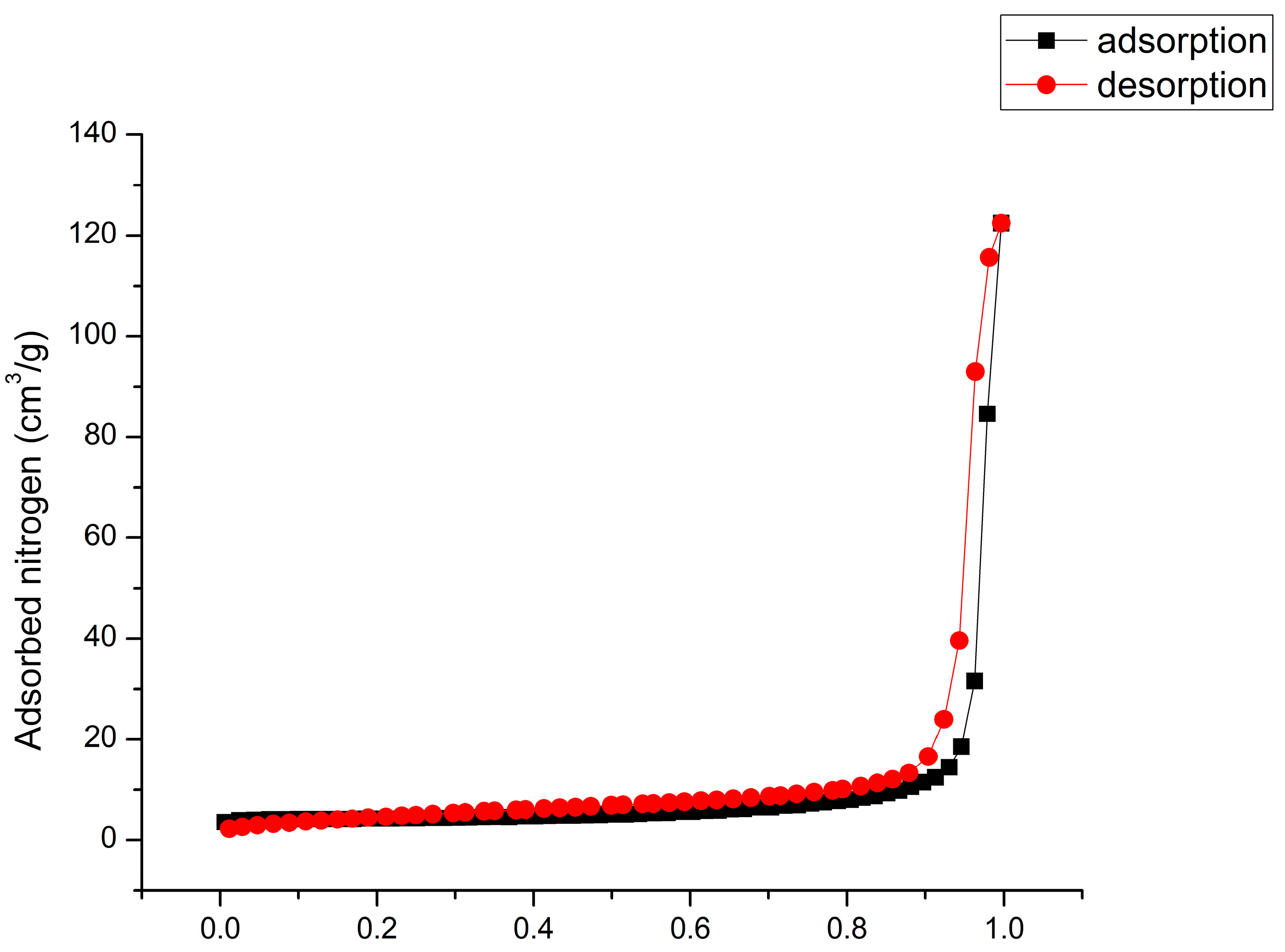
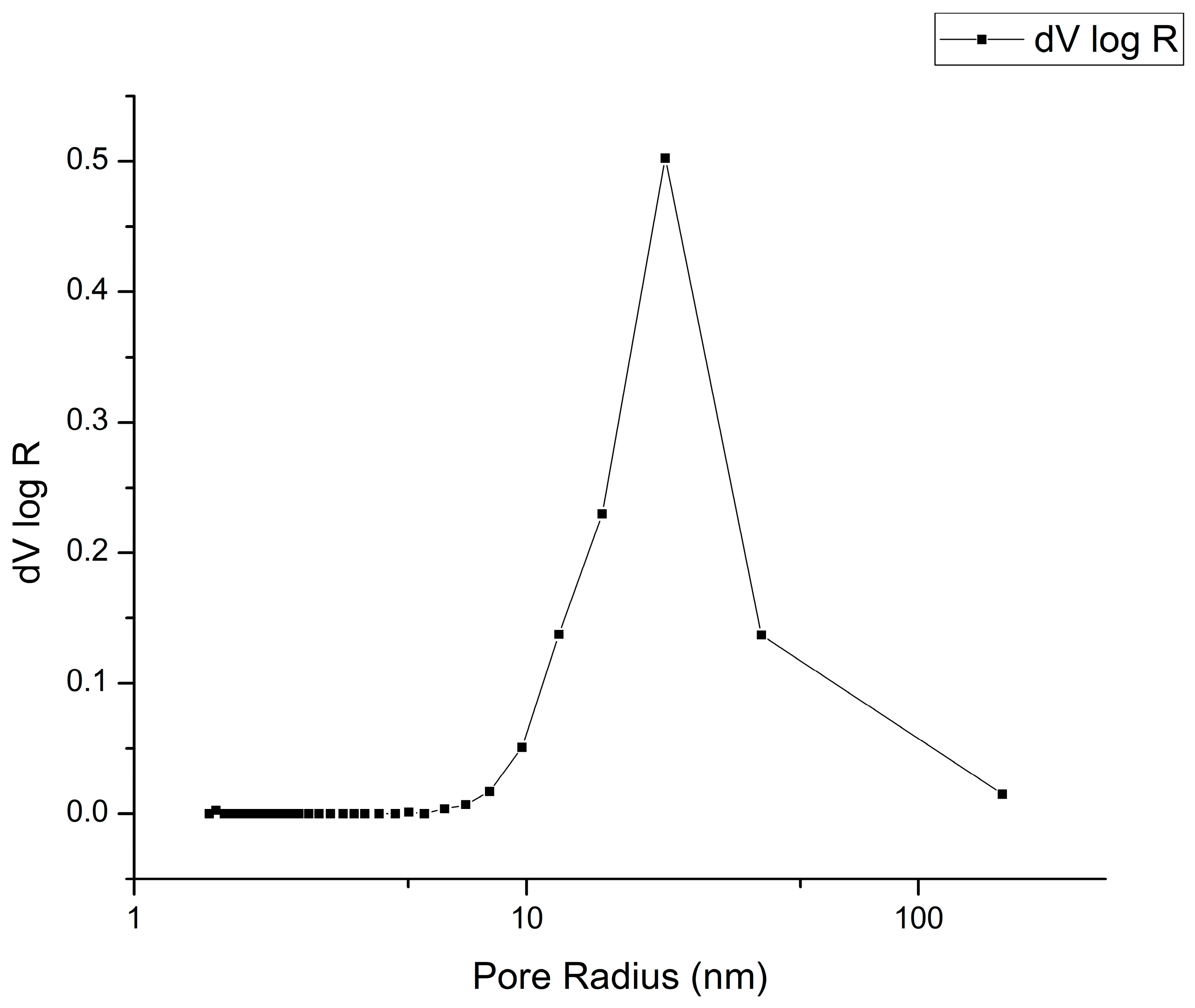

| Sample | SBET, m2/g | t-Plot Analysis | |||
|---|---|---|---|---|---|
| Vmicro, m3/g | S micro, m2/g | Sext, m2/g | Mean Pore Size, nm | ||
| Nb2O5@600 °C | 12.8 | 0.003 | 6.989 | 7.472 | 22.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepa, I.; Mudra, E.; Capkova, D.; Kovalcikova, A.; Petrus, O.; Kromka, F.; Milkovic, O.; Antal, V.; Balaz, M.; Lisnichuk, M.; et al. Porous Nb2O5 Nanofibers Prepared via Reactive Needle-Less Electrospinning for Application in Lithium–Sulfur Batteries. Inorganics 2023, 11, 456. https://doi.org/10.3390/inorganics11120456
Shepa I, Mudra E, Capkova D, Kovalcikova A, Petrus O, Kromka F, Milkovic O, Antal V, Balaz M, Lisnichuk M, et al. Porous Nb2O5 Nanofibers Prepared via Reactive Needle-Less Electrospinning for Application in Lithium–Sulfur Batteries. Inorganics. 2023; 11(12):456. https://doi.org/10.3390/inorganics11120456
Chicago/Turabian StyleShepa, Ivan, Erika Mudra, Dominika Capkova, Alexandra Kovalcikova, Ondrej Petrus, Frantisek Kromka, Ondrej Milkovic, Vitaliy Antal, Matej Balaz, Maksym Lisnichuk, and et al. 2023. "Porous Nb2O5 Nanofibers Prepared via Reactive Needle-Less Electrospinning for Application in Lithium–Sulfur Batteries" Inorganics 11, no. 12: 456. https://doi.org/10.3390/inorganics11120456






