Positive Influence of Oxalate and Cyanate on the Supercapacitance Performance of V/Co 2D-Nanolayered Structures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical and Elemental Analysis
2.2. X-ray Diffraction
2.3. Fourier Transform Infrared Spectroscopy
2.4. Thermal Analyses
2.5. Scanning Electron Microscopy
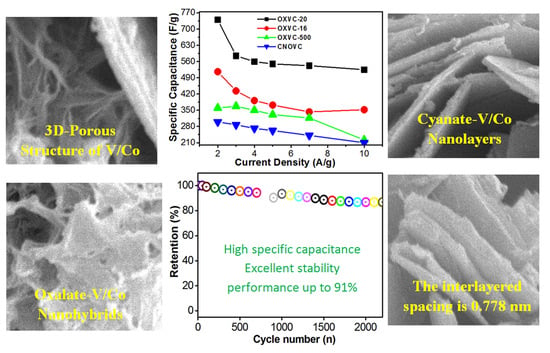
2.6. Electrochemical Studies
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.M.-J.; Chen, C.; Ayvalı, T.; Suo, H.; Zheng, J.; Teixeira, I.F.; Ye, L.; Zou, H.; O’Hare, D.; Tsang, S.C.E. CO2 hydrogenation to methanol over catalysts derived from single cationic layer CuZnGa LDH precursors. ACS Catal. 2018, 8, 4390–4401. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpaa, M. Synthesis and application of LDH intercalated cellulose nanocomposite for separation of rare earth elements (REEs). Chem. Eng. J. 2017, 309, 130–139. [Google Scholar] [CrossRef]
- Cao, Z.; Adnan, N.N.M.; Wang, G.; Rawal, A.; Shi, B.; Liu, R.; Liang, K.; Zhao, L.; Gooding, J.J.; Boyer, C. Enhanced colloidal stability and protein resistance of layered double hydroxide nanoparticles with phosphonic acid-terminated PEG coating for drug delivery. J. Colloid Interface Sci. 2018, 521, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sanati, S.; Rezvani, Z. g-C3N4 nanosheet @ CoAl-layered double hydroxide composites for electrochemical energy storage in supercapacitors. Chem. Eng. J. 2019, 362, 743–757. [Google Scholar] [CrossRef]
- Liu, N.; Lin, J.; Wu, J.; Huang, M.; Fan, L.; Song, Z.; Geng, C.; Zhu, T.; Pan, W. Application of CoV-LDH nano-flower in asymmetric supercapacitors with high electrochemical properties. Electrochim. Acta 2020, 336, 135550. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater. Today 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Hou, Y.; Lohe, M.R.; Zhang, J.; Liu, S.; Zhuang, X.; Feng, X. Vertically oriented cobalt selenide/NiFe layered-double-hydroxide nanosheets supported on exfoliated graphene foil: An efficient 3D electrode for overall water splitting. Energy Environ. Sci. 2016, 9, 478–483. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Wu, F.; Hu, B.; Meng, F.; Niu, K.; Lin, F.; Zheng, H. An investigation of ultrathin nickel-iron layered double hydroxide nanosheets grown on nickel foam for high-performance supercapacitor electrodes. J. Alloys Compd. 2017, 714, 63–70. [Google Scholar] [CrossRef]
- Zai, J.; Liu, Y.; Li, X.; Ma, Z.-F.; Qi, R.; Qian, X. 3D hierarchical Co–Al layered double hydroxides with long-term stabilities and high rate performances in supercapacitors. Nano-Micro Lett. 2017, 9, 21. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.; Wang, J.; Liu, F.; Zhang, X. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim. Acta 2016, 206, 108–115. [Google Scholar] [CrossRef]
- Tang, J.; Shen, Y.; Miao, X.; Qin, H.; Song, D.; Li, Y.; Qu, Y.; Yin, Z.; Ren, J.; Wang, L. Template-directed growth of hierarchically structured MOF-derived LDH cage hybrid arrays for supercapacitor electrode. J. Electroanal. Chem. 2019, 840, 174–181. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Chu, W.; Wang, N. High-stable α-phase NiCo double hydroxide microspheres via microwave synthesis for supercapacitor electrode materials. Chem. Eng. J. 2017, 316, 277–287. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Yang, Y.; Luo, B.; Zhang, X.; Fong, E.; Chu, W.; Huang, K. Unique 3D flower-on-sheet nanostructure of NiCo LDHs: Controllable microwave-assisted synthesis and its application for advanced supercapacitors. J. Alloys Compd. 2019, 788, 1029–1036. [Google Scholar] [CrossRef]
- Jing, M.; Hou, H.; Banks, C.E.; Yang, Y.; Zhang, Y.; Ji, X. Alternating voltage introduced NiCo double hydroxide layered nanoflakes for an asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 22741–22744. [Google Scholar] [CrossRef]
- Hou, L.; Du, Q.; Su, L.; Di, S.; Ma, Z.; Chen, L.; Shao, G. Ni-Co layered double hydroxide with self-assembled urchin like morphology for asymmetric supercapacitors. Mater. Lett. 2019, 237, 262–265. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Z.; Qin, Z.; Sun, H.; Jiao, X.; Chen, D. LDH nanocages synthesized with MOF templates and their high performance as supercapacitors. Nanoscale 2013, 5, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Joshi, M.C.; Agarwal, K.; Balasubramaniam, B.; Gupta, R.K. Three-dimensional nickel vanadium layered double hydroxide nanostructures grown on carbon cloth for high-performance flexible supercapacitor applications. Nanoscale Adv. 2019, 1, 2400–2407. [Google Scholar] [CrossRef]
- Sahoo, R.; Lee, T.H.; Pham, D.T.; Luu, T.H.T.; Lee, Y.H. Fast-Charging High-Energy Battery–Supercapacitor Hybrid: Anodic Reduced Graphene Oxide–Vanadium(IV) Oxide Sheet-on-Sheet Heterostructure. ACS Nano 2019, 13, 10776–10786. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z. Design Strategies for Vanadium-based Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 16358–16367. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, Y.; Chen, Y.; Liu, Q.; Ge, T.; Xu, L.; Mai, L. Vanadium-based nanowires for sodium-ion batteries. Nanotechnology 2019, 30, 192001. [Google Scholar] [CrossRef]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries. Adv. Funct. Mater. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Shao, M.; Deng, J.; Zhong, F.; Cao, Y.; Ai, X.; Qian, J.; Yang, H. An all-vanadium aqueous lithium ion battery with high energy density and long lifespan. Energy Storage Mater. 2019, 18, 92–99. [Google Scholar] [CrossRef]
- Zand, Z.; Salimi, P.; Mohammadi, M.R.; Bagheri, R.; Chernev, P.; Song, Z.; Dau, H.; Gorlin, M.; Najafpour, M.M. Nickel-Vanadium Layered Double Hydroxide under Water-Oxidation Reaction: New Findings and Challenges. ACS Sustain. Chem. Eng. 2019, 7, 17252–17262. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, C.; Liu, Y.; Wang, Z.; Wang, P.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H.; Huang, B. Sulfuration of NiV-layered double hydroxide towards novel supercapacitor electrode with enhanced performance. Chem. Eng. J. 2018, 351, 119–126. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Martins, P.R.; Araki, K.; Angnes, L. Recent progress in water splitting and hybrid supercapacitors based on nickel-vanadium layered double hydroxides. J. Energy Chem. 2021, 57, 496–515. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; da Silva, M.I.; Toma, H.E.; Angnes, L.; Martins, P.R.; Araki, K. Trimetallic oxides/hydroxides as hybrid supercapacitor electrode materials: A review. J. Mater. Chem. 2020, 8, 10534–10570. [Google Scholar] [CrossRef]
- Wu, Z.; Khalafallah, D.; Teng, C.; Wang, X.; Zou, Q.; Chen, J.; Zhi, M.; Hong, Z. Vanadium doped hierarchical porous nickel-cobalt layered double hydroxides nanosheet arrays for high-performance supercapacitor. J. Alloys Compd. 2020, 838, 155604. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, M.; Park, J.H.; Kim, E.S.; Liu, S.; Chung, K.Y.; Jun, S.C. An unexpected phase-transformation of cobalt–vanadium layered double hydroxides toward high energy density hybrid supercapacitor. J. Power Source 2021, 486, 229341. [Google Scholar] [CrossRef]
- Qu, C.; Jiao, Y.; Zhao, B.; Chen, D.; Zou, R.; Walton, K.S.; Liu, M. Nickel-based pillared MOFs for high-performance supercapacitors: Design, synthesis and stability study. Nano Energy 2016, 26, 66–73. [Google Scholar] [CrossRef]
- Liu, X.; Shi, C.; Zhai, C.; Cheng, M.; Liu, Q.; Wang, G. Cobalt-based layered metal–organic framework as an ultrahigh capacity supercapacitor electrode material. ACS Appl. Mater. Interfaces 2016, 8, 4585–4591. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of hierarchical porous nickel based metal-organic framework (Ni-MOF) constructed with nanosheets as novel pseudo-capacitive material for asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef]
- Fu, H.-R.; Xu, Z.-X.; Zhang, J. Water-stable metal–organic frameworks for fast and high dichromate trapping via single-crystal-to-single-crystal ion exchange. Chem. Mater. 2015, 27, 205–210. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.; Deng, L.; Cao, F.; Han, J.; Wang, L. Cu (II) coordination polymers constructed by tetrafluoro terephthalic acid and varied imidazole-containing ligands: Syntheses, structures and properties. J. Solid State Chem. 2018, 258, 24–31. [Google Scholar] [CrossRef]
- Du, M.; Li, C.-P.; Liu, C.-S.; Fang, S.-M. Design and construction of coordination polymers with mixed-ligand synthetic strategy, Coord. Chem. Rev. 2013, 257, 1282–1305. [Google Scholar]
- D’Amato, R.; Donnadio, A.; Carta, M.; Sangregorio, C.; Tiana, D.; Vivani, R.; Taddei, M.; Costantino, F. Water-based synthesis and enhanced CO2 capture performance of perfluorinated cerium-based metal-organic frameworks with UiO-66 and MIL-140 topology. ACS Sustain. Chem. Eng. 2018, 7, 394–402. [Google Scholar] [CrossRef]
- Yadav, B.; Trivedi, M.; Yadav, A.K.; Singh, G.; Kumar, A.; Kumar, G.; Husain, A.; Rath, N. Synthetic, spectral, structural and catalytic activity of infinite 3-D and 2-D copper (ii) coordination polymers for substrate size-dependent catalysis for CO2 conversion. Dalton Trans. 2019, 48, 10078–10088. [Google Scholar]
- Lou, X.; Hu, X.; Li, C.; Ning, Y.; Chen, Q.; Shen, M.; Hu, B. Room-temperature synthesis of a cobalt 2,3,5,6-tetra fluoroterephthalic coordination polymer with enhanced capacity and cycling stability for lithium batteries. New J. Chem. 2017, 41, 1813–1819. [Google Scholar] [CrossRef]
- Saber, O.; Aljaafari, A.; Asiri, S.; Batoo, K.M. Designing Magnetic Layered Double Hydroxides and Two-Dimensional Magnetic Nano-Nets of Cobalt Ferrite through a Novel Approach. Appl. Sci. 2018, 8, 2099. [Google Scholar] [CrossRef]
- Saber, O.; Aljaafari, A.; Osama, A.; Alshoaibi, A. Optimization Conditions for Crystal Growth of Novel Nanolayers, Nanohybrids and Nanocomposites Based on Cobalt, Zirconium, Titanium and Silicon. ChemistrySelect 2019, 4, 580–588. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Y.; Li, C.; Chen, W.; Zhu, W.; Wang, L. Staggered nickel–vanadium layered double hydroxide nanosheets on reduced graphene oxide via in-situ growth for enhanced supercapacitor performance. J. Alloys Compd. 2023, 935, 168048. [Google Scholar] [CrossRef]
- Amutha, R.; Akilandeswari, S.; Muruganandham, M.; Sillanpää, M.; Ahmmad, B.; Ohkubo, T. Template-free synthesis of self-assembly Co3O4 micro/nanocrystals. J. Nanosci. Nanotechnol. 2011, 11, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Saber, O.; Osama, M.; Alshoaibi, A.; Shaalan, N.M.; Osama, D. Designing inorganic–magnetic–organic nanohybrids for producing effective photocatalysts for the purification of water. RSC Adv. 2022, 12, 18282–18295. [Google Scholar] [CrossRef]
- Miyata, S. The syntheses of hydrotalcitelike compounds and their structures and physicochemical properties. Clays Clay Miner. 1995, 23, 369–375. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, H.C. Decomposition Pathways of Hydrotalcite-like Compounds Mg1−xAlx(OH)2(NO3)X·nH2O as a Continuous Function of Nitrate Anions. Chem. Mater. 2001, 13, 4564–4572. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T.J. Basic Properties of Mg2+ 1−xAl3+ x Layered Double Hydroxides Intercalated by Carbonate, Hydroxide, Chloride, and Sulfate Anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Saber, O.; Ansari, S.A.; Alshoaibi, A. Development of Ti/Ni Nanolayered Structures to Be a New Candidate for Energy Storage Applications. Appl. Sci. 2020, 10, 6935. [Google Scholar] [CrossRef]
- Saber, O.; Aljaafari, A.; Alomair, H.A.; Alshoaibi, A. Novel Strategy for Producing Nanoplatelets to be Used as Building Blocks for Shaping Nanofibers through Layered Double Hydroxides and Poly Vinyl Alcohol. ChemistrySelect 2019, 4, 4293–4300. [Google Scholar] [CrossRef]
- Benito, P.; Guinea, I.; Labajos, F.M.; Rives, V. Microwave-hydrothermally aged Zn, Al hydrotalcite-like compounds: Influence of the composition and the irradiation conditions. Microporous Mesoporous Mater. 2008, 110, 292–302. [Google Scholar] [CrossRef]
- Benito, P.; Herrero, M.; Barriga, C.; Labajos, F.M.; Rives, V. Microwave-assisted homogeneous precipitation of hydrotalcites by urea hydrolysis. Inorg. Chem. 2008, 47, 5453–5463. [Google Scholar] [CrossRef]
- Chang, P.-H.; Chang, Y.-P.; Chen, S.-Y.; Yu, C.-T.; Chyou, Y.-P. Ca-rich Ca-Al-oxide, high-temperature-stable sorbents prepared from hydrotalcite precursors: Synthesis, characterization, and CO2 capture capacity. Chem. Sus. Chem. 2011, 4, 1844–1851. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Ansari, S.A.; Parveen, N.; Shaalan, N.M.; Osama, A.; Osama, M. Positive Influence of Oxalate and Cyanate on the Supercapacitance Performance of V/Co 2D-Nanolayered Structures. Inorganics 2023, 11, 458. https://doi.org/10.3390/inorganics11120458
Saber O, Ansari SA, Parveen N, Shaalan NM, Osama A, Osama M. Positive Influence of Oxalate and Cyanate on the Supercapacitance Performance of V/Co 2D-Nanolayered Structures. Inorganics. 2023; 11(12):458. https://doi.org/10.3390/inorganics11120458
Chicago/Turabian StyleSaber, Osama, Sajid Ali Ansari, Nazish Parveen, Nagih M. Shaalan, Aya Osama, and Mostafa Osama. 2023. "Positive Influence of Oxalate and Cyanate on the Supercapacitance Performance of V/Co 2D-Nanolayered Structures" Inorganics 11, no. 12: 458. https://doi.org/10.3390/inorganics11120458