Dependency of Crystal Violet Dye Removal Behaviors onto Mesoporous V2O5-g-C3N4 Constructed by Simplistic Ultrasonic Method
Abstract
:1. Introduction
2. Results and Discussions
2.1. The V2O5-g-C3N4 Nanosorbent Characteristics
2.2. Dyes Adsorption onto the V2O5-g-C3N4 Nanocomposite
2.2.1. Adsorption Capability of the V2O5-g-C3N4 Nanocomposite towards Organic Dyes
2.2.2. Impact of Initial pH and Concentration on CV Dyes Elimination
2.2.3. Adsorption Isotherms Modeling
2.2.4. Contact Time and Adsorption Kinetics Modeling
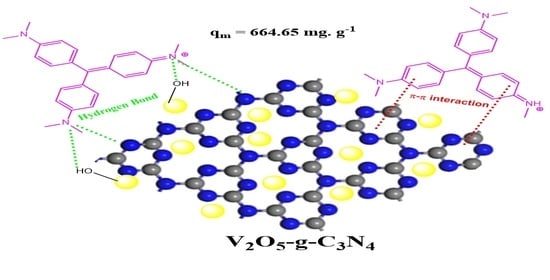
2.2.5. Adsorption Mechanism
2.2.6. Reusability Test
3. Materials and Methods
3.1. Chemicals
3.2. Nanomaterials Synthesis
3.3. The V2O5-g-C3N4 Nanocomposite Characterization
3.4. CV Dye Adsorption Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aissa, B.; Khezami, L.; Taha, K.; Elamin, N.; Mustafa, B.; Al-Ayed, A.; Modwi, A. Yttrium oxide-doped ZnO for effective adsorption of basic fuchsin dye: Equilibrium, kinetics, and mechanism studies. Int. J. Environ. Sci. Technol. 2021, 19, 9901–9914. [Google Scholar] [CrossRef]
- Rahali, S.; Ben Aissa, M.A.; Khezami, L.; Elamin, N.; Seydou, M.; Modwi, A. Adsorption behavior of Congo red onto barium-doped ZnO nanoparticles: Correlation between experimental results and DFT calculations. Langmuir 2021, 37, 7285–7294. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.A.; Ghoniem, M.; Diawara, M.; Rahali, S.; Abdulkhair, B.Y.; Elamin, M.; Aissa, M.A.B.; Seydou, M. Enhanced adsorptive removal of indigo carmine dye by bismuth oxide doped MgO based adsorbents from aqueous solution: Equilibrium, kinetic and computational studies. RSC Adv. 2022, 12, 24786–24803. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiong, Y.; Wan, H.; Chen, J.; Fang, S.; Song, X.; Li, R.; Duan, M.; Hu, R. In-situ investigation of dye pollutant adsorption performance on graphitic carbon nitride surface: ATR spectroscopy experiment and MD simulation insight. J. Hazard. Mater. 2021, 418, 126297. [Google Scholar] [CrossRef]
- Largo, F.; Haounati, R.; Ouachtak, H.; Hafid, N.; Jada, A.; Addi, A.A. Design of organically modified sepiolite and its use as adsorbent for hazardous Malachite Green dye removal from water. Water Air Soil Pollut. 2023, 234, 183. [Google Scholar] [CrossRef]
- Ouachtak, H.; Akhouairi, S.; Addi, A.A.; Akbour, R.A.; Jada, A.; Douch, J.; Hamdani, M. Mobility and retention of phenolic acids through a goethite-coated quartz sand column. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 9–19. [Google Scholar] [CrossRef]
- Alizadeh, N.; Shariati, S.; Besharati, N. Adsorption of crystal violet and methylene blue on azolla and fig leaves modified with magnetite iron oxide nanoparticles. Int. J. Environ. Res. 2017, 11, 197–206. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Guerdaoui, A.; El Haouti, R.; Haounati, R.; Ighnih, H.; Toubi, Y.; Alakhras, F.; Rehman, R.; Hafid, N.; Addi, A.A. Combined molecular dynamics simulations and experimental studies of the removal of cationic dyes on the eco-friendly adsorbent of activated carbon decorated montmorillonite Mt@AC. RSC Adv. 2023, 13, 5027–5044. [Google Scholar] [CrossRef]
- Pathak, A.; Khandegar, V.; Kumar, A. Statistical Investigation in Conjunction with a Box–Behnken Design for the Removal of Dyes Using Electrocoagulation. J. Hazard. Toxic Radioact. Waste 2022, 26, 04022001. [Google Scholar] [CrossRef]
- Kyi, P.P.; Quansah, J.O.; Lee, C.-G.; Moon, J.-K.; Park, S.-J. The removal of crystal violet from textile wastewater using palm kernel shell-derived biochar. Appl. Sci. 2020, 10, 2251. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Juang, R.-S. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013, 219, 109–117. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Thekkedath, A.; Sugaraj, S.; Sridharan, K. Nanomaterials in Advanced Oxidation Processes (AOPs) in Anionic Dye Removal. In Advanced Oxidation Processes in Dye-Containing Wastewater; Springer: Berlin/Heidelberg, Germany, 2022; pp. 129–165. [Google Scholar]
- Varjani, S.; Rakholiya, P.; Shindhal, T.; Shah, A.V.; Ngo, H.H. Trends in dye industry effluent treatment and recovery of value added products. J. Water Process Eng. 2021, 39, 101734. [Google Scholar] [CrossRef]
- Ali, H.; Muhammad, S.K. Biosorption of crystal violet from water on leaf biomass of Calotropis procera. J. Environ. Sci. Technol 2008, 1, 143–150. [Google Scholar] [CrossRef]
- Lin, J.; Pan, Z.; Wang, X. Photochemical reduction of CO2 by graphitic carbon nitride polymers. ACS Sustain. Chem. Eng. 2014, 2, 353–358. [Google Scholar] [CrossRef]
- Yang, W.; Godin, R.; Kasap, H.; Moss, B.; Dong, Y.; Hillman, S.A.; Steier, L.; Reisner, E.; Durrant, J.R. Electron accumulation induces efficiency bottleneck for hydrogen production in carbon nitride photocatalysts. J. Am. Chem. Soc. 2019, 141, 11219–11229. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Fang, J.; Li, L.; Deng, J.; Chen, F. Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: A review. J. Alloys Compd. 2022, 901, 163589. [Google Scholar] [CrossRef]
- Toghan, A.; Modwi, A. Boosting unprecedented indigo carmine dye photodegradation via mesoporous MgO@g-C3N4 nanocomposite. J. Photochem. Photobiol. A Chem. 2021, 419, 113467. [Google Scholar] [CrossRef]
- Shvalagin, V.V.; Korzhak, G.V.; Kuchmiy, S.Y.; Skoryk, M.A.; Selyshchev, O.V.; Zahn, D.R. Facile preparation and high photocatalytic activity of crystalline graphitic carbon nitride in hydrogen evolution from electron donor solutions under visible light. J. Photochem. Photobiol. A Chem. 2020, 390, 112295. [Google Scholar] [CrossRef]
- Yan, L.; Gao, H.; Chen, Y. Na-Doped Graphitic Carbon Nitride for Removal of Aqueous Contaminants via Adsorption and Photodegradation. ACS Appl. Nano Mater. 2021, 4, 7746–7757. [Google Scholar] [CrossRef]
- Ding, P.; Ji, H.; Li, P.; Liu, Q.; Wu, Y.; Guo, M.; Zhou, Z.; Gao, S.; Xu, W.; Liu, W. Visible-light degradation of antibiotics catalyzed by titania/zirconia/graphitic carbon nitride ternary nanocomposites: A combined experimental and theoretical study. Appl. Catal. B Environ. 2022, 300, 120633. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, W.; Zhao, Y.; Mu, X.; Zhou, X.; Chu, F.; Wang, B.; Hu, Y. Graphite-like Carbon Nitride/Polyphosphoramide Nanohybrids for Enhancement on Thermal Stability and Flame Retardancy of Thermoplastic Polyurethane Elastomers. ACS Appl. Polym. Mater. 2022, 4, 121–128. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Jiang, L.; Yu, H.; Zhao, Y.; Chen, H.; Yuan, X.; Liang, J.; Li, H.; Wu, Z. Defective polymeric carbon nitride: Fabrications, photocatalytic applications and perspectives. Chem. Eng. J. 2022, 427, 130991. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Khezami, L.; Aissa, M.A.B.; Modwi, A.; Ismail, M.; Guesmi, A.; Algethami, F.K.; Ticha, M.B.; Assadi, A.A.; Nguyen-Tri, P. Harmonizing the photocatalytic activity of g-C3N4 nanosheets by ZrO2 stuffing: From fabrication to experimental study for the wastewater treatment. Biochem. Eng. J. 2022, 182, 108411. [Google Scholar] [CrossRef]
- Fernandes, E.; Drosopoulou, S.; Mazierski, P.; Miodyńska, M.; Gołaszewska, D.; Zaleska-Medynska, A.; Martins, R.C.; Gomes, J. Carbon nitride photoactivation evaluation and degradation of a mixture of parabens by ozone assistance. J. Water Process Eng. 2022, 49, 103018. [Google Scholar] [CrossRef]
- Falletta, E.; Longhi, M.; Di Michele, A.; Boffito, D.C.; Bianchi, C.L. Floatable graphitic carbon nitride/alginate beads for the photodegradation of organic pollutants under solar light irradiation. J. Clean. Prod. 2022, 371, 133641. [Google Scholar] [CrossRef]
- Wang, T.; Huang, M.; Liu, X.; Zhang, Z.; Liu, Y.; Tang, W.; Bao, S.; Fang, T. Facile one-step hydrothermal synthesis of α-Fe2O3/gC3N4 composites for the synergistic adsorption and photodegradation of dyes. RSC Adv. 2019, 9, 29109–29119. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, X.; Gondal, M.; Liu, Y.; Shen, K.; Xu, Q. Comparative study of pure g-C3N4 and sulfur-doped g-C3N4 catalyst performance in photo-degradation of persistent pollutant under visible light. J. Nanosci. Nanotechnol. 2018, 18, 4142–4154. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and LEDs. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.-N. g-C3N4: Properties, Pore Modifications, and Photocatalytic Applications. Nanomaterials 2021, 12, 121. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Ghoniem, M.; Nguyen-Tri, P.; Baaloudj, O.; Guesmi, A.; AlGethami, F.; Amer, M.; Assadi, A. Superior removal of dyes by mesoporous MgO/g-C3N4 fabricated through ultrasound method: Adsorption mechanism and process modeling. Environ. Res. 2022, 205, 112543. [Google Scholar] [CrossRef]
- Toghan, A.; Abd El-Lateef, H.M.; Taha, K.K.; Modwi, A. Mesoporous TiO2@g-C3N4 composite: Construction, characterization, and boosting indigo carmine dye destruction. Diam. Relat. Mater. 2021, 118, 108491. [Google Scholar] [CrossRef]
- Modwi, A.; Ismail, M.; Idriss, H.; Aissa, M.; Khezami, L.; Bououdina, M. Efficient Removal of Cd (II) from Aquatic Media by Heteronanostructure MgO@TiO2@g-C3N4. J. Nanomater. 2022, 2022, 1458442. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Shamspur, T.; Mostafavi, A. Novel adsorbent g-C3N4/ZnV2O4 for efficient removal of crystal violet dye: Removal process optimization, adsorption isotherms, and kinetic modeling. Appl. Organomet. Chem. 2022, 36, e6867. [Google Scholar] [CrossRef]
- Wei, S.; Kamali, A.R. Waste plastic derived Co3Fe7/CoFe2O4@carbon magnetic nanostructures for efficient dye adsorption. J. Alloys Compd. 2021, 886, 161201. [Google Scholar] [CrossRef]
- Wei, S.; Kamali, A.R. Trifunctional mesoporous magnetic adsorbent-photocatalyst nanocomposite for efficient removal of potassium ethyl xanthate from mining wastewater. J. Water Process Eng. 2022, 49, 103067. [Google Scholar] [CrossRef]
- Modwi, A.; Idriss, H.; Khezami, L.; Albadri, A.; Ismail, M.; Assadi, A.A.; Nguyen-Tri, P. Ba2+ removal from aquatic medium via TiY2O5@g-C3N4 nanocomposites. Diam. Relat. Mater. 2023, 135, 109830. [Google Scholar] [CrossRef]
- Zang, Y.-N.; Yang, S.-S.; Ding, J.; Zhao, S.-Y.; Chen, C.-X.; He, L.; Ren, N.-Q. A biochar-promoted V2O5/gC3N4 Z-Scheme heterostructure for enhanced simulated solar light-driven photocatalytic activity. RSC Adv. 2021, 11, 15106–15117. [Google Scholar] [CrossRef]
- Sajid, M.M.; Shad, N.A.; Javed, Y.; Khan, S.B.; Zhang, Z.; Amin, N.; Zhai, H. Preparation and characterization of Vanadium pentoxide (V2O5) for photocatalytic degradation of monoazo and diazo dyes. Surf. Interfaces 2020, 19, 100502. [Google Scholar] [CrossRef]
- Ali, H.; Ismail, A. Honeycomb-like V2O5 based films: Synthesis, structural, thermal, and optical properties for environmental applications. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3012–3029. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Meng, J.; Yang, Z.; Wu, J.; Rong, Y.; Deng, L.; Shi, Y. Synergistic effects in V3O7/V2O5 composite material for high capacity and long cycling life aqueous rechargeable zinc ion batteries. J. Power Sources 2020, 474, 228569. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Lyu, L.; Zeng, Q.; Xing, X.; Hu, C. Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation. Environ. Sci. Technol. 2018, 52, 14371–14380. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Chen, X.; Leng, L.; Li, H. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl. Catal. B Environ. 2015, 174, 445–454. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, X.; Duan, P.; Xia, R.; Zhang, N.; Cheng, B.; Wang, Z.; Zhang, Y. V2O5/Pg-C3N4 Z-scheme enhanced heterogeneous photocatalytic removal of methyl orange from water under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125580. [Google Scholar] [CrossRef]
- Khezami, L.; Aissa, M.A.B.; Modwi, A.; Guesmi, A.; Algethami, F.K.; Bououdina, M. Efficient removal of organic dyes by Cr-doped ZnO nanoparticles. Biomass Convers. Biorefinery 2022, 1–14. [Google Scholar] [CrossRef]
- Ghoniem, M.G.; Ali, F.A.M.; Abdulkhair, B.Y.; Elamin, M.R.A.; Alqahtani, A.M.; Rahali, S.; Ben Aissa, M.A. Highly selective removal of cationic dyes from wastewater by MgO nanorods. Nanomaterials 2022, 12, 1023. [Google Scholar] [CrossRef]
- Wathukarage, A.; Herath, I.; Iqbal, M.; Vithanage, M. Mechanistic understanding of crystal violet dye sorption by woody biochar: Implications for wastewater treatment. Environ. Geochem. Health 2019, 41, 1647–1661. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant adsorption isotherms: A review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Razavi, M.M. Water reuse: Brackish water desalination using Prosopis juliflora. Environ. Technol. Innov. 2020, 17, 100614. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and removal of crystal violet dye from aqueous solution by modified rice husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bagotia, N.; Yadav, S.; Sharma, N.; Sharma, A.K.; Kumar, S. Environmental application of Saccharum munja biomass-derived hybrid composite for the simultaneous removal of cationic and anionic dyes and remediation of dye polluted water: A step towards pilot-scale studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129539. [Google Scholar] [CrossRef]
- Quansah, J.O.; Hlaing, T.; Lyonga, F.N.; Kyi, P.P.; Hong, S.-H.; Lee, C.-G.; Park, S.-J. Nascent rice husk as an adsorbent for removing cationic dyes from textile wastewater. Appl. Sci. 2020, 10, 3437. [Google Scholar] [CrossRef]
- El Naeem, G.A.; Abd-Elhamid, A.; Farahat, O.O.; El-Bardan, A.A.; Soliman, H.M.; Nayl, A. Adsorption of crystal violet and methylene blue dyes using a cellulose-based adsorbent from sugercane bagasse: Characterization, kinetic and isotherm studies. J. Mater. Res. Technol. 2022, 19, 3241–3254. [Google Scholar]
- Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Nocardiopsis sp. for the removal of triphenylmethane dyes: Decolorization and optimization studies. Water Air Soil Pollut. 2021, 232, 414. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Facile synthesis of polypyrrole decorated chitosan-based magsorbent: Characterizations, performance, and applications in removing cationic and anionic dyes from aqueous medium. Int. J. Biol. Macromol. 2020, 161, 88–100. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Nabil, G.M.; Abouelanwar, M.E. Multifunctionalized graphene oxide@nanopolyaniline@zirconium silicate nanocomposite for rapid microwable removal of dyes. J. Nanostruct. Chem. 2021, 11, 645–662. [Google Scholar] [CrossRef]
- Nasiri, J.; Motamedi, E.; Naghavi, M.R.; Ghafoori, M. Removal of crystal violet from water using β-cyclodextrin functionalized biogenic zero-valent iron nanoadsorbents synthesized via aqueous root extracts of Ferula persica. J. Hazard. Mater. 2019, 367, 325–338. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Chien, S.; Clayton, W. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Mäkilä, E.; Repo, E.; Heinonen, M.; Salonen, J.; Kukk, E.; Sillanpää, M.; Titirici, M.-M. Meso-and microporous soft templated hydrothermal carbons for dye removal from water. Green Chem. 2016, 18, 1137–1146. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Highly efficient removal of methylene blue dye from an aqueous solution using cellulose acetate nanofibrous membranes modified by polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, W.; Zhou, L.; Wang, L.; Zhang, J.; Liu, Y.; Lei, J. Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem. Eng. J. 2022, 427, 131503. [Google Scholar] [CrossRef]
- Sun, W.; Liu, T.; Xia, K.; Zhou, J.; Liu, X.; Zhang, X. Preparation of Adsorbent Based on Polyacrylate Latex Solid Waste and Its Application in the Treatment of Dye Wastewater. ACS Omega 2022, 7, 13243–13253. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Reduced graphene oxide as an effective adsorbent for removal of malachite green dye: Plausible adsorption pathways. J. Colloid Interface Sci. 2017, 501, 11–21. [Google Scholar] [CrossRef]
- Ali, M.; Modwi, A.; Idriss, H.; Aldaghri, O.; Ismail, M.; Ibnaouf, K. Detoxification of Pb (II) from aquatic media via CaMgO2@g-C3N4 nanocomposite. Mater. Lett. 2022, 322, 132501. [Google Scholar] [CrossRef]







| Equilibrium Model | Non-Linear Form | Parameters | Values |
|---|---|---|---|
| Langmuir [51] | qm (mg g−1) | 664.65 | |
| KL (mg g−1) | 0.058 | ||
| RL (L. mg−1) | 0.08 | ||
| R2 | 0.995 | ||
| Freundlich [52] | n | 2.33 | |
| KF (L. mg−1) | 73.25 | ||
| R2 | 0.974 |
| Adsorbents | qe (mg g−1) | pH | References |
|---|---|---|---|
| Xanthated Rice husks | 90.02 | 10 | [53] |
| Saccharum munja biomass-functionalized carbon nanotubes | 180.51 | 7 | [54] |
| Nascent Rice Husk | 24.47 | 10 | [55] |
| Cellulose based on sugarcane | 107.50 | 8–13 | [56] |
| Nocardiopsis sp | 15.90 | 7 | [57] |
| MChs-Ppy | 11.84 | 8 | [58] |
| GO@NPANI@ZrSiO4 | 15.81 | 7 | [59] |
| Fe noparticles/βCD | 100 | 9 | [60] |
| V2O5-g-C3N4 nanocomposite | 664.65 | 7 | This paper |
| Kinetics Models | Equations | Parameters | Values |
|---|---|---|---|
| PFO [61] | qe (mg g−1) | 242.71 | |
| k1 (min−1) | 0.95 | ||
| R2 | 0.606 | ||
| PSO [61] | qe(calculated) (mg g−1) | 244.69 | |
| qe(experimental) (mg g−1) | |||
| k2 (g mg−1·min−1) | 0.018 | ||
| R2 | 0.932 | ||
| IPD [62] | C1 (mg g−1) | 223.53 | |
| Kdif1 (mg g−1 min1/2) | 3.77 | ||
| R2 | 0.983 | ||
| C2 (mg g−1) | 244.35 | ||
| Kdif2 (mg g−1 min1/2) | 0.02 | ||
| R2 | 0.908 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Aissa, M.A.; Modwi, A.; Albadri, A.E.A.E.; Saleh, S.M. Dependency of Crystal Violet Dye Removal Behaviors onto Mesoporous V2O5-g-C3N4 Constructed by Simplistic Ultrasonic Method. Inorganics 2023, 11, 146. https://doi.org/10.3390/inorganics11040146
Ben Aissa MA, Modwi A, Albadri AEAE, Saleh SM. Dependency of Crystal Violet Dye Removal Behaviors onto Mesoporous V2O5-g-C3N4 Constructed by Simplistic Ultrasonic Method. Inorganics. 2023; 11(4):146. https://doi.org/10.3390/inorganics11040146
Chicago/Turabian StyleBen Aissa, Mohamed Ali, Abueliz Modwi, Abuzar E. A. E. Albadri, and Sayed M. Saleh. 2023. "Dependency of Crystal Violet Dye Removal Behaviors onto Mesoporous V2O5-g-C3N4 Constructed by Simplistic Ultrasonic Method" Inorganics 11, no. 4: 146. https://doi.org/10.3390/inorganics11040146