Silicon Anode: A Perspective on Fast Charging Lithium-Ion Battery
Abstract
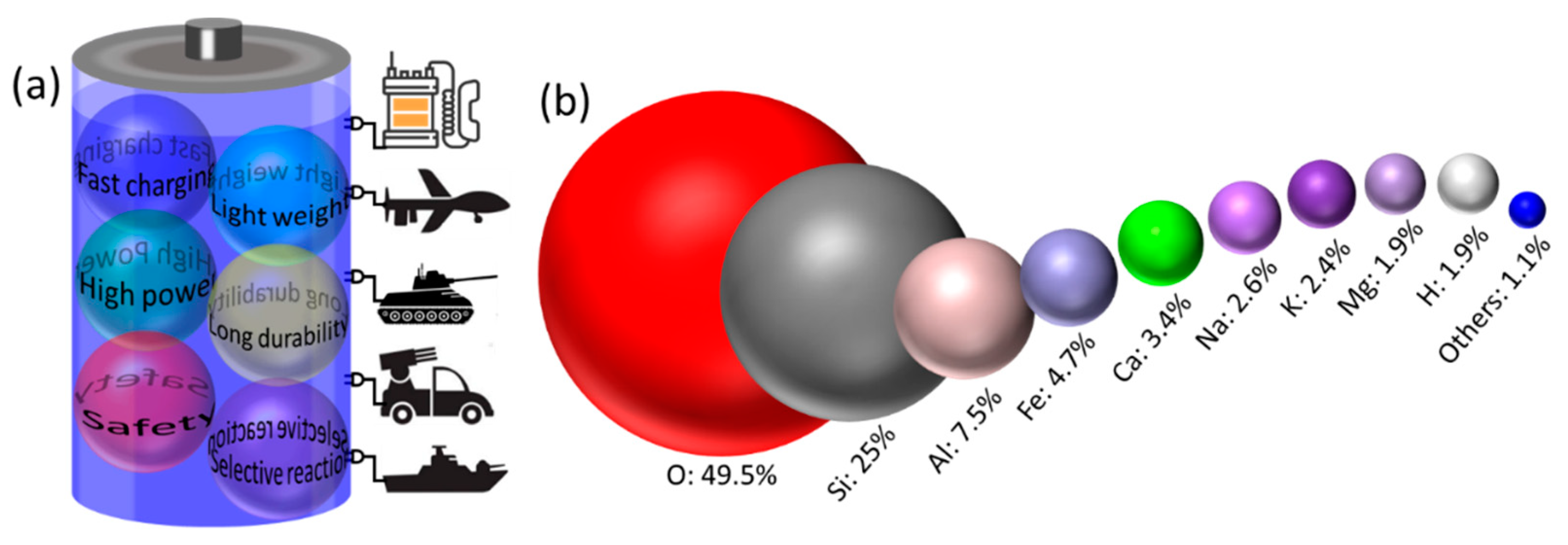
:1. Introduction
2. Key Considerations to Achieve Fast Charging
2.1. Conductivity of Materials
2.2. Ion Diffusivity of Materials
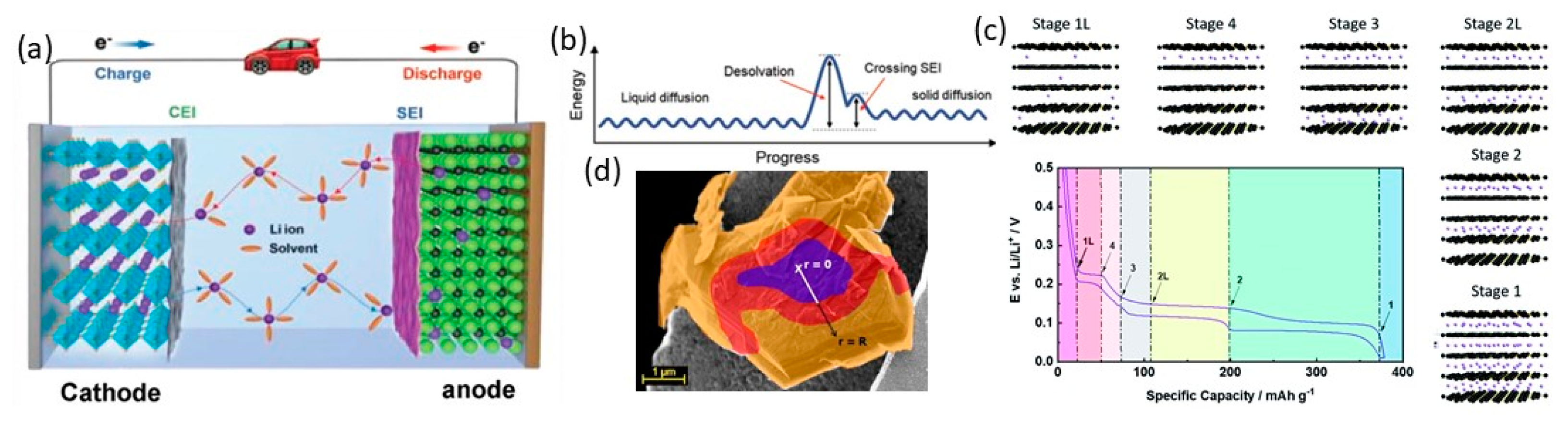
2.3. Stable SEI Formation
2.4. Electrolyte Modification
3. Graphite Anode
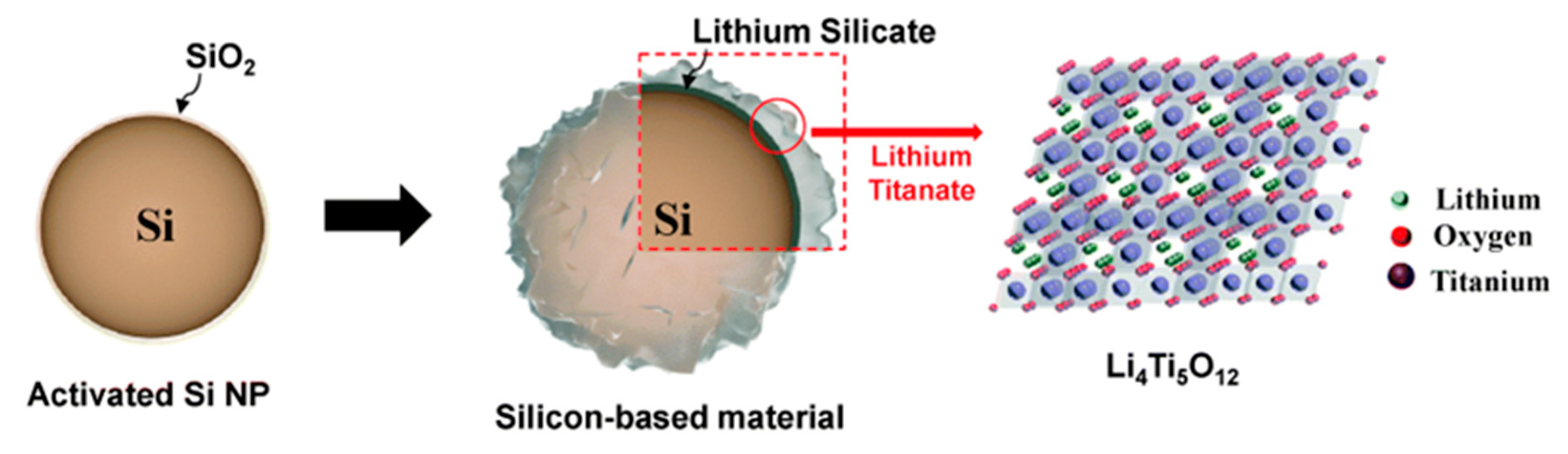
4. Titanium-Based Materials: Lithium Titanate (LTO)
5. Silicon Anode
5.1. Use of Silicon Carbon Composite for High-Energy and Fast-Charging LIBs
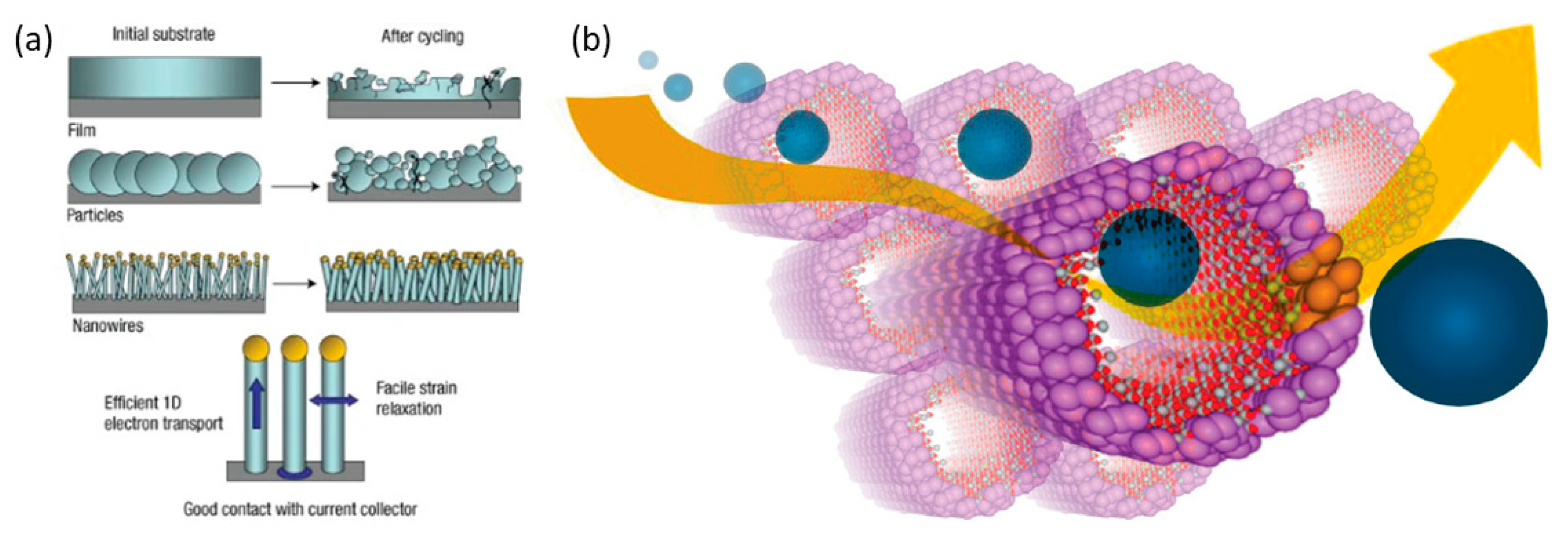
5.2. Nanosized Silicon Anode
5.3. Porous Silicon Anode
5.4. Silicon-Derivative Materials
5.4.1. SiOx
5.4.2. SiNx
6. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building Safe Lithium-Ion Batteries for Electric Vehicles: A Review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The Significance of Li-Ion Batteries in Electric Vehicle Life-Cycle Energy and Emissions and Recycling’s Role in Its Reduction. Energy Environ. Sci. 2014, 8, 158–168. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Global EV Outlook 2021—Analysis. Available online: https://www.iea.org/reports/global-ev-outlook-2021 (accessed on 9 February 2023).
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-Temperature Stationary Sodium-Ion Batteries for Large-Scale Electric Energy Storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhang, G.; Li, S.; Xu, Y.; Zhang, X.; Zhang, X.; Zheng, S.; Sun, X.; Ma, Y. Fast Charging Anode Materials for Lithium-Ion Batteries: Current Status and Perspectives. Adv. Funct. Mater. 2022, 32, 2200796. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Lee, S.; Agostini, M.; Jeong, M.-G.; Jung, H.-G.; Ming, J.; Sun, Y.-K.; Kim, J.; Hwang, J.-Y. Multiscale Understanding of Covalently Fixed Sulfur–Polyacrylonitrile Composite as Advanced Cathode for Metal–Sulfur Batteries. Adv. Sci. 2021, 8, 2101123. [Google Scholar] [CrossRef]
- Irle, R. Global EV Sales for 2022. Available online: https://www.ev-volumes.com (accessed on 9 February 2023).
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable Lithium Batteries with Aqueous Electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Tran, M.; Banister, D.; Bishop, J.D.K.; McCulloch, M.D. Realizing the Electric-Vehicle Revolution. Nat. Clim. Chang. 2012, 2, 328–333. [Google Scholar] [CrossRef]
- Thakur, A.K.; Ahmed, M.S.; Oh, G.; Kang, H.; Jeong, Y.; Prabakaran, R.; Vikram, M.P.; Sharshir, S.W.; Kim, J.; Hwang, J.-Y. Advancement in Graphene-Based Nanocomposites as High Capacity Anode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2021, 9, 2628–2661. [Google Scholar] [CrossRef]
- Thakur, A.K.; Ahmed, M.S.; Park, J.; Prabakaran, R.; Sidney, S.; Sathyamurthy, R.; Kim, S.C.; Periasamy, S.; Kim, J.; Hwang, J.-Y. A Review on Carbon Nanomaterials for K-Ion Battery Anode: Progress and Perspectives. Int. J. Energy Res. 2022, 46, 4033–4070. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K.T. Overview of Batteries and Battery Management for Electric Vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Gohier, A.; Laïk, B.; Kim, K.-H.; Maurice, J.-L.; Pereira-Ramos, J.-P.; Cojocaru, C.S.; Van, P.T. High-Rate Capability Silicon Decorated Vertically Aligned Carbon Nanotubes for Li-Ion Batteries. Adv. Mater. 2012, 24, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Mu, L.; Xu, Z.; Wang, J.; Wei, C.; Liu, L.; Pianetta, P.; Zhao, K.; Yu, X.; Lin, F.; et al. Chemomechanical Interplay of Layered Cathode Materials Undergoing Fast Charging in Lithium Batteries. Nano Energy 2018, 53, 753–762. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, J.; Gu, M.; Xiao, J.; Zhang, J.-G.; Wang, C.-M. Intragranular Cracking as a Critical Barrier for High-Voltage Usage of Layer-Structured Cathode for Lithium-Ion Batteries. Nat. Commun. 2017, 8, 14101. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2327. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li Plating as Unwanted Side Reaction in Commercial Li-Ion Cells—A Review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Hall, D.S.; Eldesoky, A.; Logan, E.R.; Tonita, E.M.; Ma, X.; Dahn, J.R. Exploring Classes of Co-Solvents for Fast-Charging Lithium-Ion Cells. J. Electrochem. Soc. 2018, 165, A2365. [Google Scholar] [CrossRef]
- Nazari, A.; Farhad, S. Heat Generation in Lithium-Ion Batteries with Different Nominal Capacities and Chemistries. Appl. Therm. Eng. 2017, 125, 1501–1517. [Google Scholar] [CrossRef]
- Ge, M.; Fang, X.; Rong, J.; Zhou, C. Review of Porous Silicon Preparation and Its Application for Lithium-Ion Battery Anodes. Nanotechnology 2013, 24, 422001. [Google Scholar] [CrossRef]
- Salah, M.; Murphy, P.; Hall, C.; Francis, C.; Kerr, R.; Fabretto, M. Pure Silicon Thin-Film Anodes for Lithium-Ion Batteries: A Review. J. Power Sources 2019, 414, 48–67. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material—Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zhang, R.; Du, Y.; Li, D.; Shen, D.; Yang, J.; Guo, Z.; Liu, H.K.; Elzatahry, A.A.; Zhao, D. Highly Reversible and Large Lithium Storage in Mesoporous Si/C Nanocomposite Anodes with Silicon Nanoparticles Embedded in a Carbon Framework. Adv. Mater. 2014, 26, 6749–6755. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M.; Pleskov, Y.V.; Terukov, E.I.; Kon’kov, O.I. Lithium Insertion into Amorphous Silicon Thin-Film Electrodes. J. Electroanal. Chem. 2007, 600, 217–225. [Google Scholar] [CrossRef]
- Ko, M.; Chae, S.; Cho, J. Challenges in Accommodating Volume Change of Si Anodes for Li-Ion Batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar] [CrossRef]
- Wu, J.; Cao, Y.; Zhao, H.; Mao, J.; Guo, Z. The Critical Role of Carbon in Marrying Silicon and Graphite Anodes for High-Energy Lithium-Ion Batteries. Carbon Energy 2019, 1, 57–76. [Google Scholar] [CrossRef]
- Duan, H.; Xu, H.; Wu, Q.; Zhu, L.; Zhang, Y.; Yin, B.; He, H. Silicon/Graphite/Amorphous Carbon as Anode Materials for Lithium Secondary Batteries. Molecules 2023, 28, 464. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Lu, X.; Qi, F.; Liang, Y.; Trukhanov, A.; Wu, Y.; Sun, Z.; Lu, X. Fully Active Bimetallic Phosphide Zn0.5Ge0.5P: A Novel High-Performance Anode for Na-Ion Batteries Coupled with Diglyme-Based Electrolyte. ACS Appl. Mater. Interfaces 2022, 14, 31803–31813. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-S.; Hsiao, K.-C.; Sireesha, P.; Huang, J.-Y. Lithium Silicates in Anode Materials for Li-Ion and Li Metal Batteries. Batteries 2022, 8, 2. [Google Scholar] [CrossRef]
- Mei, Y.; He, Y.; Zhu, H.; Ma, Z.; Pu, Y.; Chen, Z.; Li, P.; He, L.; Wang, W.; Tang, H. Recent Advances in the Structural Design of Silicon/Carbon Anodes for Lithium Ion Batteries: A Review. Coatings 2023, 13, 436. [Google Scholar] [CrossRef]
- Saager, S.; Decker, L.; Kopte, T.; Scheffel, B.; Zimmermann, B. High-Performance Anodes Made of Metallic Lithium Layers and Lithiated Silicon Layers Prepared by Vacuum Technologies. Batteries 2023, 9, 75. [Google Scholar] [CrossRef]
- Heß, M.; Novák, P. Shrinking Annuli Mechanism and Stage-Dependent Rate Capability of Thin-Layer Graphite Electrodes for Lithium-Ion Batteries. Electrochim. Acta 2013, 106, 149–158. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the Respective Effects of Heteroatom-Doped Carbon Materials in Batteries, Supercapacitors and the ORR to Design High Performance Materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on Modeling of the Anode Solid Electrolyte Interphase (SEI) for Lithium-Ion Batteries. npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Zheng, L.; Guo, F.; Kang, T.; Fan, Y.; Gu, W.; Mao, Y.; Liu, Y.; Huang, R.; Li, Z.; Shen, Y.; et al. Stable Lithium-Carbon Composite Enabled by Dual-Salt Additives. Nano-Micro Lett. 2021, 13, 111. [Google Scholar] [CrossRef]
- Han, B.; Liao, C.; Dogan, F.; Trask, S.E.; Lapidus, S.H.; Vaughey, J.T.; Key, B. Using Mixed Salt Electrolytes to Stabilize Silicon Anodes for Lithium-Ion Batteries via in Situ Formation of Li–M–Si Ternaries (M = Mg, Zn, Al, Ca). ACS Appl. Mater. Interfaces 2019, 11, 29780–29790. [Google Scholar] [CrossRef]
- Yazami, R.; Touzain, P. A Reversible Graphite-Lithium Negative Electrode for Electrochemical Generators. J. Power Sources 1983, 9, 365–371. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef]
- Sandhya, C.P.; John, B.; Gouri, C. Lithium Titanate as Anode Material for Lithium-Ion Cells: A Review. Ionics 2014, 20, 601–620. [Google Scholar] [CrossRef]
- Selinis, P.; Farmakis, F. Review—A Review on the Anode and Cathode Materials for Lithium-Ion Batteries with Improved Subzero Temperature Performance. J. Electrochem. Soc. 2022, 169, 010526. [Google Scholar] [CrossRef]
- Lee, J.-I.; Ko, Y.; Shin, M.; Song, H.-K.; Choi, N.-S.; Gyu Kim, M.; Park, S. High-Performance Silicon-Based Multicomponent Battery Anodes Produced via Synergistic Coupling of Multifunctional Coating Layers. Energy Environ. Sci. 2015, 8, 2075–2084. [Google Scholar] [CrossRef]
- Xia, Q.; Xu, A.; Du, L.; Yan, Y.; Wu, S. High-Rate, Long-Term Performance of LTO-Pillared Silicon/Carbon Composites for Lithium-Ion Batteries Anode under High Temperature. J. Alloys Compd. 2019, 800, 50–57. [Google Scholar] [CrossRef]
- Maibach, J.; Lindgren, F.; Eriksson, H.; Edström, K.; Hahlin, M. Electric Potential Gradient at the Buried Interface between Lithium-Ion Battery Electrodes and the SEI Observed Using Photoelectron Spectroscopy. J. Phys. Chem. Lett. 2016, 7, 1775–1780. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-Based Anodes for Lithium-Ion Batteries: Effectiveness of Materials Synthesis and Electrode Preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Huang, G.; Han, J.; Lu, Z.; Wei, D.; Kashani, H.; Watanabe, K.; Chen, M. Ultrastable Silicon Anode by Three-Dimensional Nanoarchitecture Design. ACS Nano 2020, 14, 4374–4382. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Yuan, S.; Lu, C. Research Progress of Silicon/Carbon Anode Materials for Lithium-Ion Batteries: Structure Design and Synthesis Method. ChemElectroChem 2020, 7, 4289–4302. [Google Scholar] [CrossRef]
- Jung, C.-H.; Choi, J.; Kim, W.-S.; Hong, S.-H. A Nanopore-Embedded Graphitic Carbon Shell on Silicon Anode for High Performance Lithium Ion Batteries. J. Mater. Chem. A 2018, 6, 8013–8020. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, S.; Zhu, B.; Tan, Y.; Hu, X.; Zong, L.; Zhu, J. Simultaneous Purification and Perforation of Low-Grade Si Sources for Lithium-Ion Battery Anode. Nano Lett. 2015, 15, 7742–7747. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Chen, S.; Zou, M.; Li, Z.; Cao, A.; Yuan, Q. Rational Design of Hierarchical Carbon/Mesoporous Silicon Composite Sponges as High-Performance Flexible Energy Storage Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 22819–22825. [Google Scholar] [CrossRef]
- Zhao, X.; Lehto, V.-P. Challenges and Prospects of Nanosized Silicon Anodes in Lithium-Ion Batteries. Nanotechnology 2020, 32, 042002. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-Performance Lithium Battery Anodes Using Silicon Nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-G.; Wang, C.; Wu, Q.-H.; Liu, X.; Yang, Y.; He, L.; Zhang, W. A silicon nanowire-reduced graphene oxide composite as a high-performance lithium ion battery anode material. Nanoscale 2014, 6, 3353–3360. [Google Scholar] [CrossRef]
- Park, M.-H.; Kim, M.G.; Joo, J.; Kim, K.; Kim, J.; Ahn, S.; Cui, Y.; Cho, J. Silicon Nanotube Battery Anodes. Nano Lett. 2009, 9, 3844–3847. [Google Scholar] [CrossRef]
- Ge, M.; Lu, Y.; Ercius, P.; Rong, J.; Fang, X.; Mecklenburg, M.; Zhou, C. Large-Scale Fabrication, 3D Tomography, and Lithium-Ion Battery Application of Porous Silicon. Nano Lett. 2014, 14, 261–268. [Google Scholar] [CrossRef]
- Esmanski, A.; Ozin, G.A. Silicon Inverse-Opal-Based Macroporous Materials as Negative Electrodes for Lithium Ion Batteries. Adv. Funct. Mater. 2009, 19, 1999–2010. [Google Scholar] [CrossRef]
- Pan, K.; Zou, F.; Canova, M.; Zhu, Y.; Kim, J.-H. Systematic Electrochemical Characterizations of Si and SiO Anodes for High-Capacity Li-Ion Batteries. J. Power Sources 2019, 413, 20–28. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon Oxides: A Promising Family of Anode Materials for Lithium-Ion Batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Jang, B.Y.; Lee, J.S.; Kim, J.S.; Nahm, S. Microstructures and Electrochemical Performances of Nano-Sized SiOx (1.18 ≤ x ≤ 1.83) as an Anode Material for a Lithium(Li)-Ion Battery. J. Power Sources 2013, 244, 115–121. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of High-Energy Li-Ion Batteries Comprising Silicon-Containing Anodes and Insertion-Type Cathodes. Nat. Commun. 2021, 12, 5459. [Google Scholar] [CrossRef] [PubMed]
- Ulvestad, A.; Mæhlen, J.P.; Kirkengen, M. Silicon Nitride as Anode Material for Li-Ion Batteries: Understanding the SiNx Conversion Reaction. J. Power Sources 2018, 399, 414–421. [Google Scholar] [CrossRef]
- Ulvestad, A.; Andersen, H.F.; Jensen, I.J.T.; Mongstad, T.T.; Mæhlen, J.P.; Prytz, Ø.; Kirkengen, M. Substoichiometric Silicon Nitride—An Anode Material for Li-Ion Batteries Promising High Stability and High Capacity. Sci. Rep. 2018, 8, 8634. [Google Scholar] [CrossRef] [PubMed]
- de Guzman, R.C.; Yang, J.; Cheng, M.M.-C.; Salley, S.O.; Ng, K.Y.S. High Capacity Silicon Nitride-Based Composite Anodes for Lithium Ion Batteries. J. Mater. Chem. A 2014, 2, 14577–14584. [Google Scholar] [CrossRef]
- Chae, S.; Park, S.; Ahn, K.; Nam, G.; Lee, T.; Sung, J.; Kim, N.; Cho, J. Gas Phase Synthesis of Amorphous Silicon Nitride Nanoparticles for High-Energy LIBs. Energy Environ. Sci. 2020, 13, 1212–1221. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Xu, Z.; Liu, Z.; Yang, J.; Chen, J. High-Safety and Long-Life Silicon-Based Lithium-Ion Batteries via a Multifunctional Binder. ACS Appl. Mater. Interfaces 2020, 12, 54842–54850. [Google Scholar] [CrossRef]
- Zhang, Q.; Xi, B.; Chen, W.; Feng, J.; Qian, Y.; Xiong, S. Synthesis of Carbon Nanotubes-Supported Porous Silicon Microparticles in Low-Temperature Molten Salt for High-Performance Li-Ion Battery Anodes. Nano Res. 2022, 15, 6184–6191. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Oh, G.; Jung, H.-Y.; Hwang, J.-Y. Silicon Anode: A Perspective on Fast Charging Lithium-Ion Battery. Inorganics 2023, 11, 182. https://doi.org/10.3390/inorganics11050182
Lee J, Oh G, Jung H-Y, Hwang J-Y. Silicon Anode: A Perspective on Fast Charging Lithium-Ion Battery. Inorganics. 2023; 11(5):182. https://doi.org/10.3390/inorganics11050182
Chicago/Turabian StyleLee, Jun, Gwangeon Oh, Ho-Young Jung, and Jang-Yeon Hwang. 2023. "Silicon Anode: A Perspective on Fast Charging Lithium-Ion Battery" Inorganics 11, no. 5: 182. https://doi.org/10.3390/inorganics11050182




