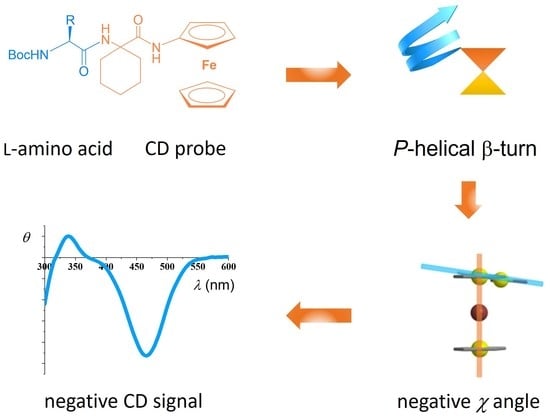
Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore
Abstract
:1. Introduction
2. Results and Discussion
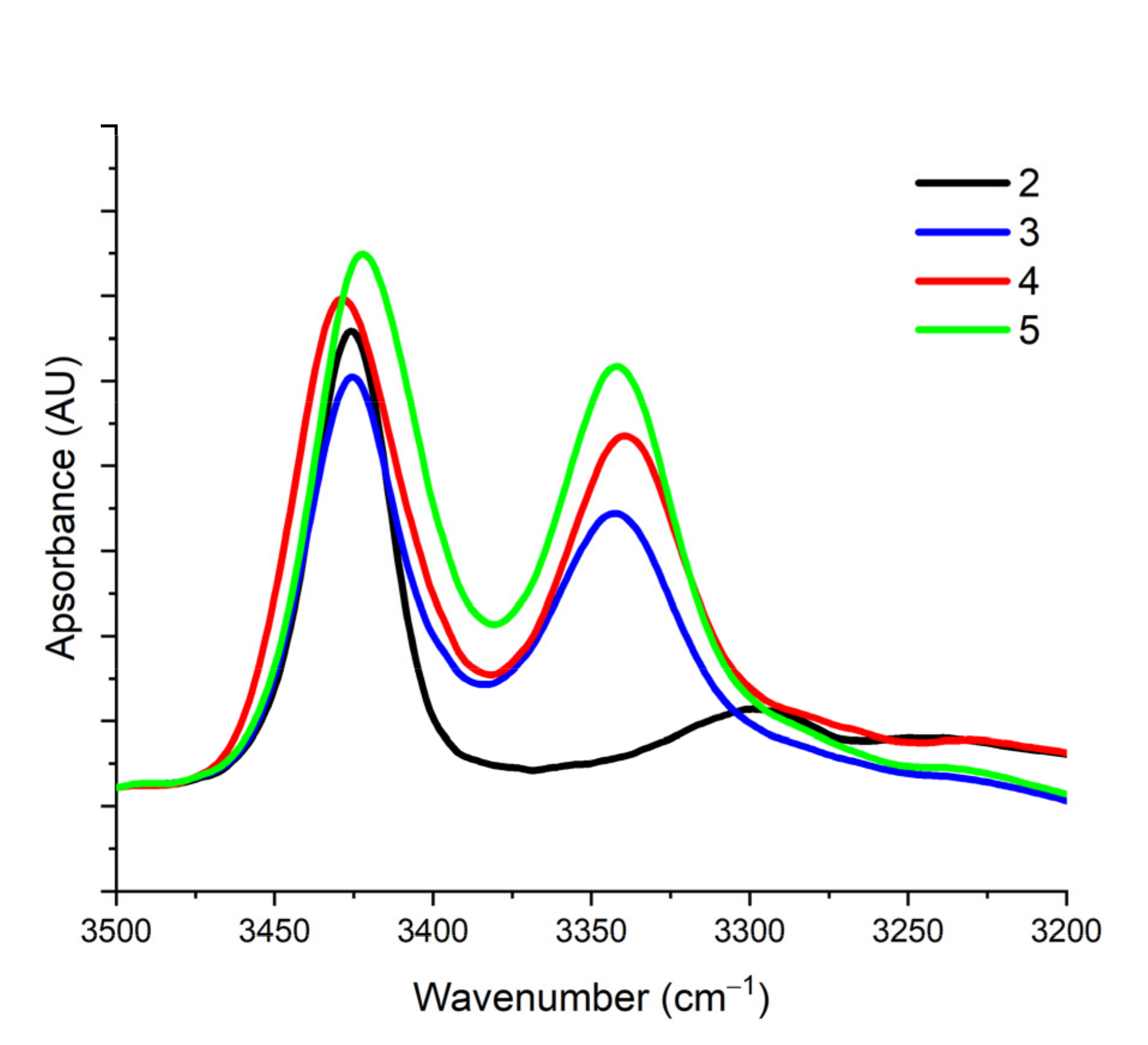
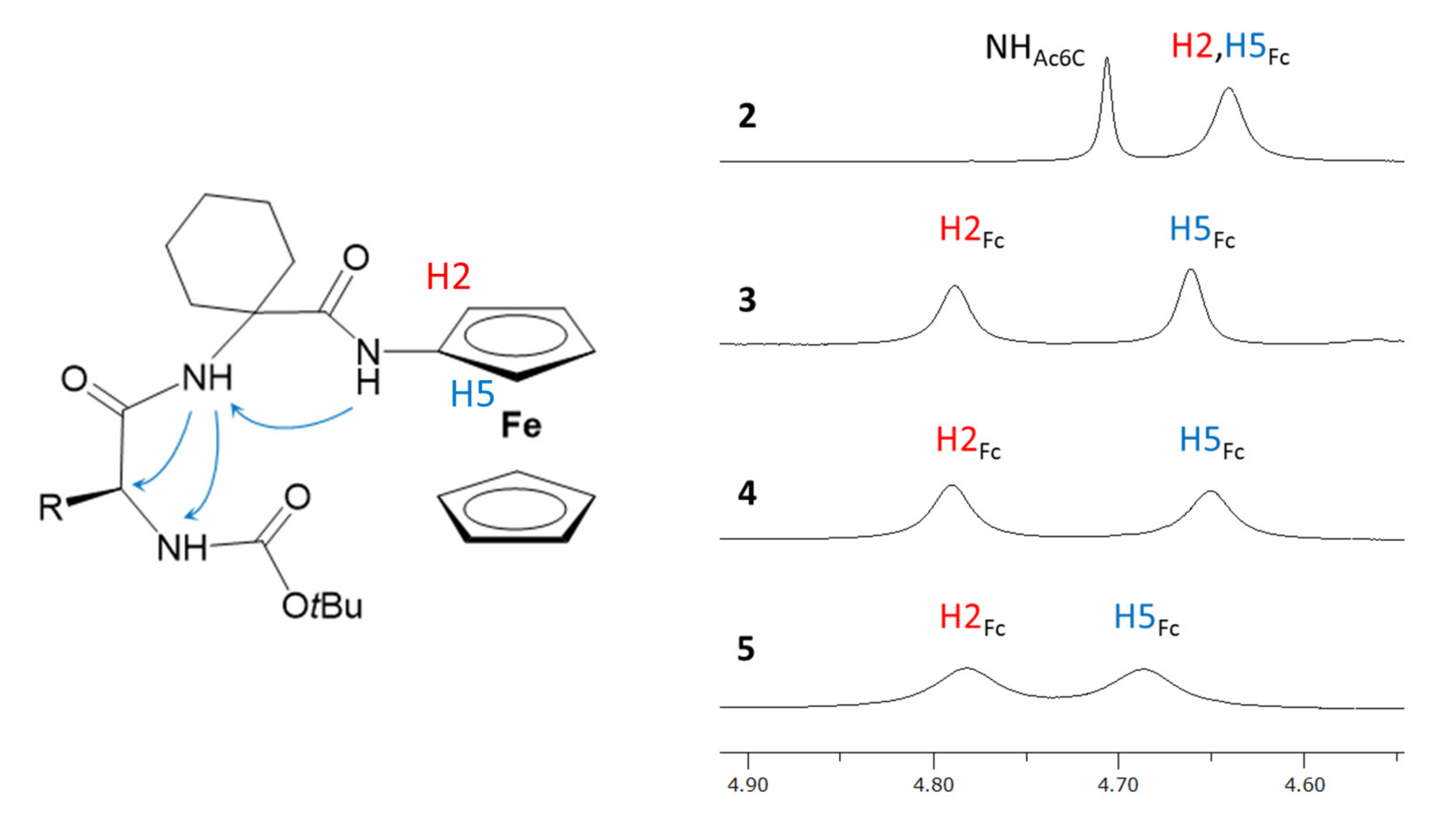
2.1. Infrared (IR) and NMR Studies
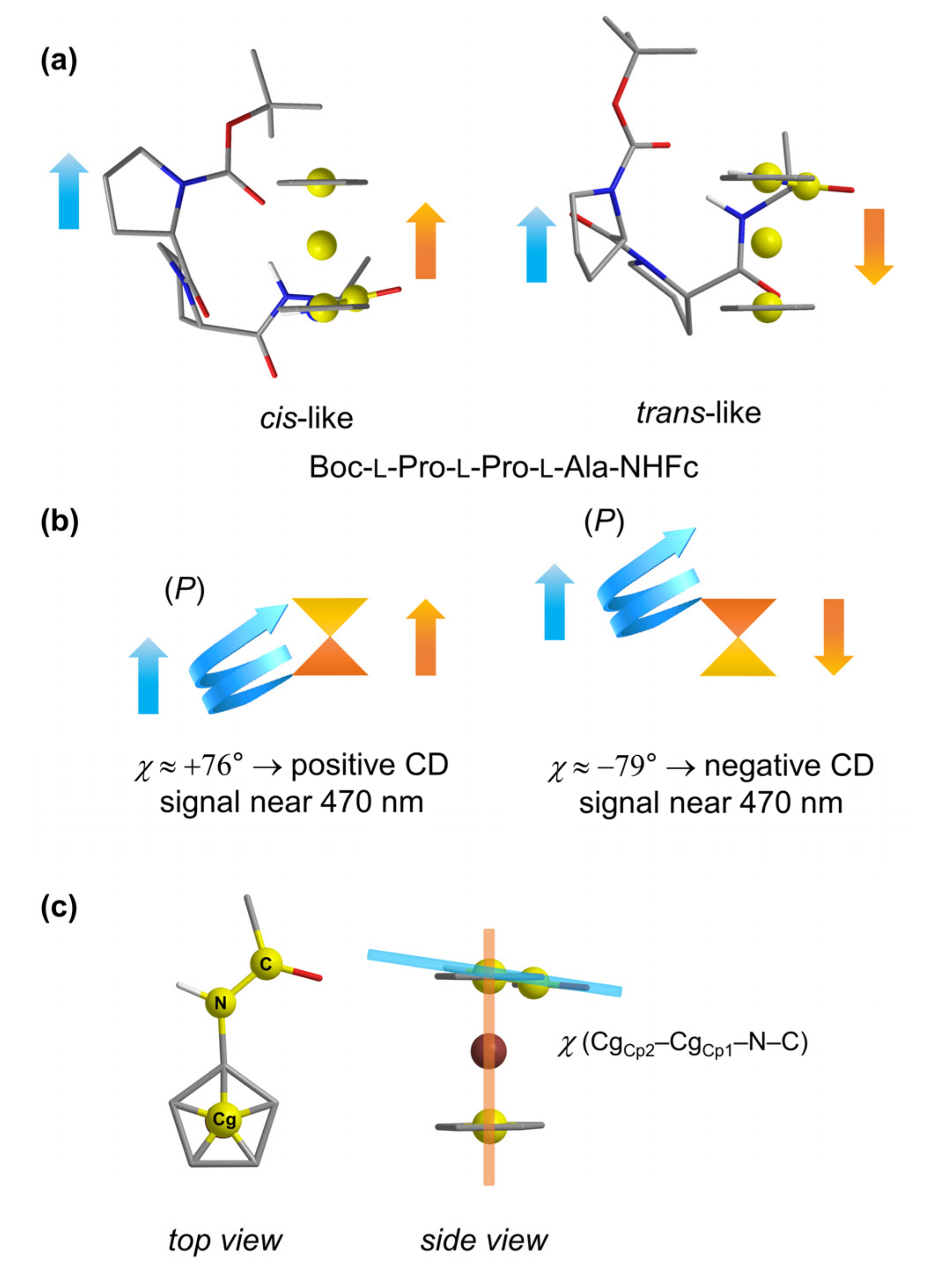
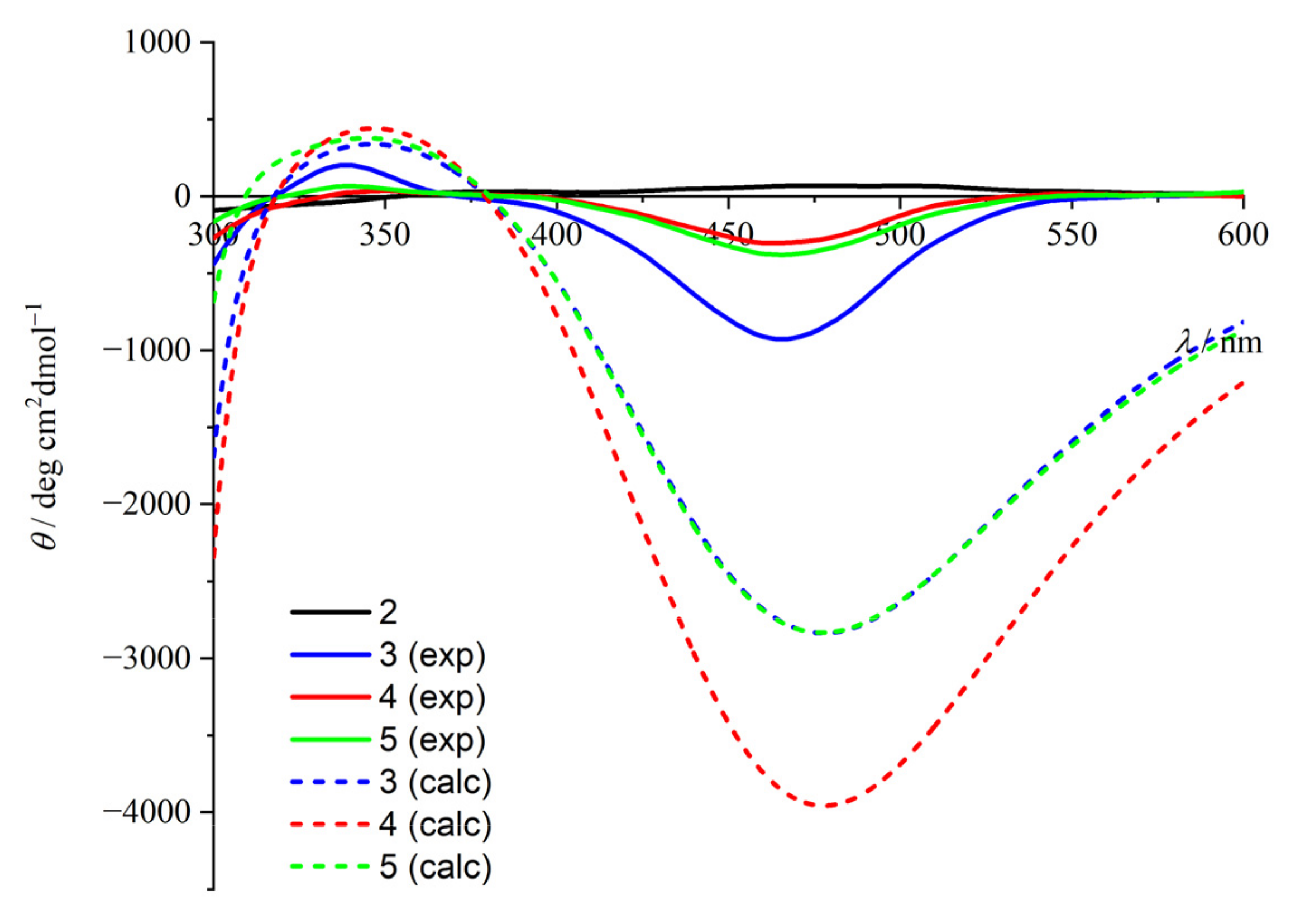
2.2. CD Studies
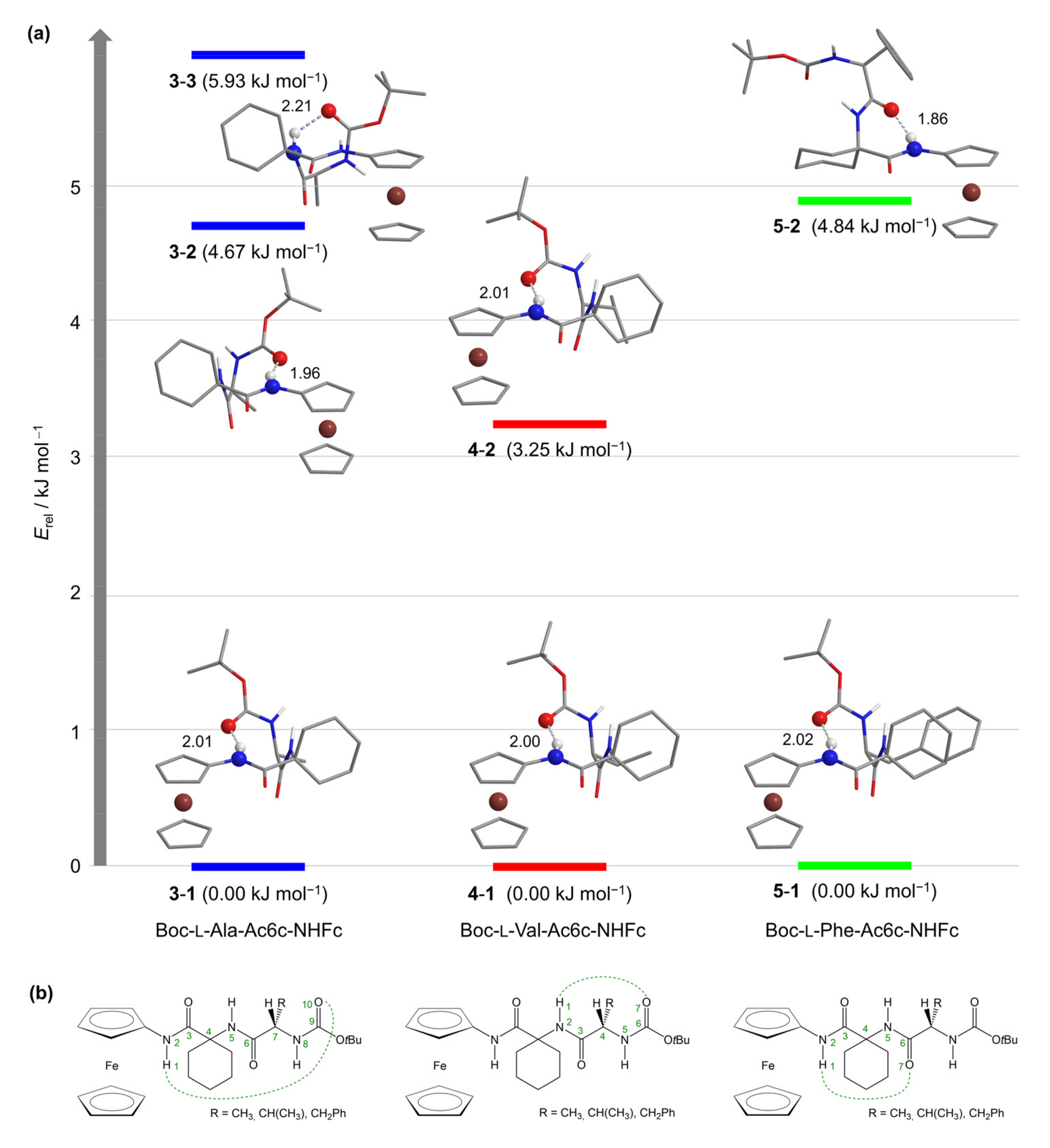
2.3. Computational Studies
3. Materials and Methods
3.1. General
3.2. Synthesis of Boc−Ac6c−NH−Fc (2) and Boc−AA−Ac6c−NH−Fc (3–5)
3.2.1. Boc–Ac6c–NH–Fc (2)
3.2.2. Boc–L–Ala–Ac6c–NH–Fc (3)
3.2.3. Boc–L–Val–Ac6c–NH–Fc (4)
3.2.4. Boc–L–Phe–Ac6c–NH–Fc (5)
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozcelik, A.; Pereira-Cameselle, R.; Poklar Ulrih, N.; Petrovic, A.G.; Alonso-Gómez, J.L. Chiroptical Sensing: A Conceptual Introduction. Sensors 2020, 20, 974. [Google Scholar] [CrossRef] [Green Version]
- Saito, F.; Schreiner, P.R. Determination of the Absolute Configurations of Chiral Alkanes—An Analysis of the Available Tools. Eur. J. Org. Chem. 2020, 2020, 6328–6339. [Google Scholar] [CrossRef]
- Herrera, B.T.; Pilicer, S.L.; Anslyn, E.V.; Joyce, L.A.; Wolf, C. Optical Analysis of Reaction Yield and Enantiomeric Excess: A New Paradigm Ready for Prime Time. J. Am. Chem. Soc. 2018, 140, 10385–10401. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luoa, L.; Wei, W. Simultaneous determination of the concentration and enantiomeric excess of amino acids with a coumarin-derived achiral probe. Anal. Methods 2021, 13, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zha, D.; Ye, H.; Hai, Y.; Zhou, Y.; Anslyn, E.V.; You, L. Dynamic covalent chemistry within biphenyl scaffolds: Reversible covalent bonding, control of selectivity, and chirality sensing with a single system. Angew. Chem. Int. Ed. 2018, 57, 1300–1305. [Google Scholar] [CrossRef]
- Zong, Z.; Cao, Z.; Hao, A.; Xing, P. Dynamic axial chirality of ferrocene diamino acids: Hydration effects and chiroptical applications. J. Mater. Chem. C 2021, 9, 12191–12200. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Sripada, A.; Wolf, C. Quantitative Chiroptical Sensing of Free Amino Acids, Biothiols, Amines, and Amino Alcohols with an Aryl Fluoride Probe. J. Am. Chem. Soc. 2019, 141, 16382–16387. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Zandia, L.S.; Wolf, C. Chiroptical sensing of homocysteine. Org. Biomol. Chem. 2020, 18, 8629–8632. [Google Scholar] [CrossRef]
- Thanzeel, F.Y.; Balaraman, K.; Wolf, C. Quantitative Chirality and Concentration Sensing of Alcohols, Diols, Hydroxy Acids, Amines and Amino Alcohols using Chlorophosphite Sensors in a Relay Assay. Angew. Chem. Int. Ed. 2020, 59, 21382–21386. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Wolf, C. Optical Terpene and Terpenoid Sensing: Chiral Recognition, Determination of Enantiomeric Composition and Total Concentration Analysis with Late Transition Metal Complexes. J. Am. Chem. Soc. 2020, 142, 4121–4125. [Google Scholar] [CrossRef]
- Hassan, D.S.; Thanzeel, F.Y.; Wolf, C. Stereochemical analysis of chiral amines, diamines, and amino alcohols: Practical chiroptical sensing based on dynamic covalent chemistry. Chirality 2020, 32, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.C.; De los Santos, Z.A.; Wolf, C. Chiroptical sensing of unprotected amino acids, hydroxy acids, amino alcohols, amines and carboxylic acids with metal salts. Chem. Commun. 2019, 55, 6297–6300. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Lynch, C.C.; Wolf, C. Dynamic Covalent Optical Chirality Sensing with a Sterically Encumbered Aminoborane. Chem. Eur. J. 2022, 28, e202202028. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.S.; De los Santos, Z.A.; Brady, K.G.; Murkli, S.; Isaacs, L.; Wolf, C. Chiroptical sensing of amino acids, amines, amino alcohols, alcohols and terpenes with π-extended acyclic cucurbiturils. Org. Biomol. Chem. 2021, 19, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Formen, J.S.S.K.; Wolf, C. Chiroptical Switching and Quantitative Chirality Sensing with (Pseudo)halogenated Quinones. Angew. Chem. Int. Ed. 2021, 60, 27031–27038. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Patti, A.; Pedotti, S.; Mazzeo, G.; Longhi, G.; Abbate, S.; Paoloni, L.; Bloino, J.; Rampino, S.; Barone, V. Ferrocenes with simple chiral substituents: An in-depth theoretical and experimental VCD and ECD study. Phys. Chem. Chem. Phys. 2019, 21, 9419–9432. [Google Scholar] [CrossRef]
- Brahma, S.; Ikbal, S.S.; Dhamija, A.; Prasad Rath, S. Highly Enhanced Bisignate Circular Dichroism of Ferrocene-Bridged Zn(II) Bisporphyrin Tweezer with Extended Chiral Substrates due to Well-Matched Host–Guest System. Inorg. Chem. 2014, 53, 2381–2395. [Google Scholar] [CrossRef]
- Adhikari, B.; Kraatz, H.-B. Redox-triggered changes in the self-assembly of a ferrocene–peptide conjugate. Chem. Commun. 2014, 50, 5551–5553. [Google Scholar] [CrossRef] [Green Version]
- Moriuchi, T.; Hirao, T. Dipeptide-induced chirality organization. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 23–40. [Google Scholar] [CrossRef]
- Kirin, S.I.; Wissenbach, D.; Metzler-Nolte, N. Unsymmetrical 1,n′-disubstituted ferrocenoyl peptides: Convenient one pot synthesis and solution structures by CD and NMR spectroscopy. New J. Chem. 2005, 29, 1168–1173. [Google Scholar] [CrossRef]
- Kovačević, M.; Kodrin, I.; Cetina, M.; Kmetič, I.; Murati, T.; Čakić Semenčić, M.; Roca, S.; Barišić, L. The conjugates of ferrocene-1,1′-diamine and amino acids. A novel synthetic approach and conformational analysis. Dalton Trans. 2015, 44, 16405–16420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čakić Semenčić, M.; Siebler, D.; Heinze, K.; Rapić, V. Bis- and Trisamides Derived From 1′-Aminoferrocene-1-carboxylic Acid and α-Amino Acids: Synthesis and Conformational Analysis. Organometallics 2009, 28, 2028–2037. [Google Scholar] [CrossRef]
- Herrick, R.S.; Jarret, R.M.; Curran, T.P.; Dragoli, D.R.; Flaherty, M.B.; Lindyberg, S.E.; Slate, R.A.; Thornton, L.C. Ordered conformations in bis(amino acid) derivatives of 1,1′-ferrocenedicarboxylic acid. Tetrahedron Lett. 1996, 37, 5289–5292. [Google Scholar] [CrossRef]
- Kovač, V.; Čakić Semenčić, M.; Kodrin, I.; Roca, S.; Rapić, V. Ferrocene-dipeptide conjugates derived from aminoferrocene and 1-acetyl-1′-aminoferrocene: Synthesis and conformational studies. Tetrahedron 2013, 69, 10497–10506. [Google Scholar] [CrossRef]
- Čakić Semenčić, M.; Kodrin, I.; Barišić, L.; Nuskol, M.; Meden, A. Synthesis and Conformational Study of Monosubstituted Aminoferrocene- Based Peptides Bearing Homo-and Heterochiral Pro-Ala Sequences. Eur. J. Inorg. Chem. 2017, 2017, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Nuskol, M.; Studen, B.; Meden, A.; Kodrin, I.; Čakić Semenčić, M. Tight Turn in Dipeptide Bridged Ferrocenes: Synthesis, X-Ray Structural, Theoretical and Spectroscopic Studies. Polyhedron 2019, 161, 137–144. [Google Scholar] [CrossRef]
- Nuskol, M.; Šutalo, P.; Đaković, M.; Kovačević, M.; Kodrin, I.; Čakić Semenčić, M. Testing the Potential of the Ferrocene Chromophore as a Circular Dichroism Probe for the Assignment of the Screw-Sense Preference of Tripeptides. Organometallics 2021, 40, 1351–1362. [Google Scholar] [CrossRef]
- Nuskol, M.; Šutalo, P.; Kodrin, I.; Čakić Semenčić, M. Sensing of the Induced Helical Chirality by the Chiroptical Response of the Ferrocene Chromophore. Eur. J. Inorg. Chem. 2022, 2022, e202100880. [Google Scholar] [CrossRef]
- Venkatraman, J.; Shankaramma, S.C.; Balaram, P. Design of folded peptides. Chem. Rev. 2001, 101, 3131–3152. [Google Scholar] [CrossRef]
- Clayden, J.; Castellanos, A.; Solà, J.; Morris, G.A. Quantifying End-to-End Conformational Communication of Chirality through an Achiral Peptide Chain. Angew. Chem. Int. Ed. 2009, 48, 5962–5965. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, C.; Crisma, M.; Formaggio, F.; Peggion, C. Control of peptide conformation by the Thorpe-Ingold effect (C alpha-tetrasubstitution). Biopolymers 2001, 60, 396–419. [Google Scholar] [CrossRef] [PubMed]
- Barišić, L.; Čakić, M.; Mahmoud, K.A.; Liu, Y.-N.; Kraatz, H.-B.; Pritzkow, H.; Kirin, S.I.; Metzler-Nolte, N.; Rapić, V. Helically Chiral Ferrocene Peptides Containing 1′-Aminoferrocene-1-Carboxylic Acid Subunits as Turn Inducers. Chem. Eur. J. 2006, 12, 4965–4980. [Google Scholar] [CrossRef]
- Hirata, T.; Ueda, A.; Oba, M.; Doi, M.; Demizu, Y.; Kurihara, M.; Nagano, M.; Suemune, H.; Tanaka, M. Amino equatorial effect of a six-membered ring amino acid on its peptide 310- and α-helices. Tetrahedron 2015, 71, 2409–2420. [Google Scholar] [CrossRef] [Green Version]
- Doi, M.; Asano, A.; Komura, E.; Ueda, Y. The structure of an endomorphin analogue incorporating 1-aminocyclohexane-1-carboxlylic acid for proline is similar to the β-turn of Leu-enkephalin. Biochemical. Biophysical. Res. Commun. 2002, 297, 138–142. [Google Scholar] [CrossRef]
- Fabiano, N.; Valle, G.; Crisma, M.; Toniolo, C.; Saviano, M.; Lombardi, A.; Isernia, C.; Pavone, V.; Di Blasio, B.; Pedone, C.; et al. Conformational versatility of the Nα-acylated tripeptide amide tail of oxytocin. Int. J. Pept. Protein Res. 1993, 42, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, V.S.; Cameron, T.S. Proline-containing β-turns. Int. J. Pept. Protein Res. 1988, 31, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Jayakumar, R. Role of N-t-Boc group in helix initiation in a novel tetrapeptide. J. Pept. Res. 2002, 59, 249–256. [Google Scholar] [CrossRef]
- Wuthrich, K. NMR of Proteins and Nucleic Acids; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Kovačević, M.; Čakić Semenčić, M.; Kodrin, I.; Roca, S.; Perica, J.; Mrvčić, J.; Stanzer, D.; Molčanov, K.; Milašinvić, V.; Brkljačić, L.; et al. Biological Evaluation and Conformational Preferences of Ferrocene Dipeptides with Hydrophobic Amino Acids. Inorganics 2023, 11, 29. [Google Scholar] [CrossRef]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O Hydrogen Bonds on the Basis of the Charge Density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Schrödinger LLC. MacroModel; Schrödinger LLC: New York, NY, USA, 2019. [Google Scholar]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Keith, T.A. AIMAll; Version 19.02.13; TK Gristmill Software: Overland Park, KS, USA, 2017. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuskol, M.; Šutalo, P.; Kovačević, M.; Kodrin, I.; Čakić Semenčić, M. Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore. Inorganics 2023, 11, 225. https://doi.org/10.3390/inorganics11060225
Nuskol M, Šutalo P, Kovačević M, Kodrin I, Čakić Semenčić M. Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore. Inorganics. 2023; 11(6):225. https://doi.org/10.3390/inorganics11060225
Chicago/Turabian StyleNuskol, Marko, Petar Šutalo, Monika Kovačević, Ivan Kodrin, and Mojca Čakić Semenčić. 2023. "Central-to-Helical-to-Axial Chirality Transfer in Chiroptical Sensing with Ferrocene Chromophore" Inorganics 11, no. 6: 225. https://doi.org/10.3390/inorganics11060225