Flexible Co(II)-and Ni(II)-Based Cationic 2D Metal–Organic Frameworks Based on a Charge-Neutral (O,O)-Donor Bridge
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
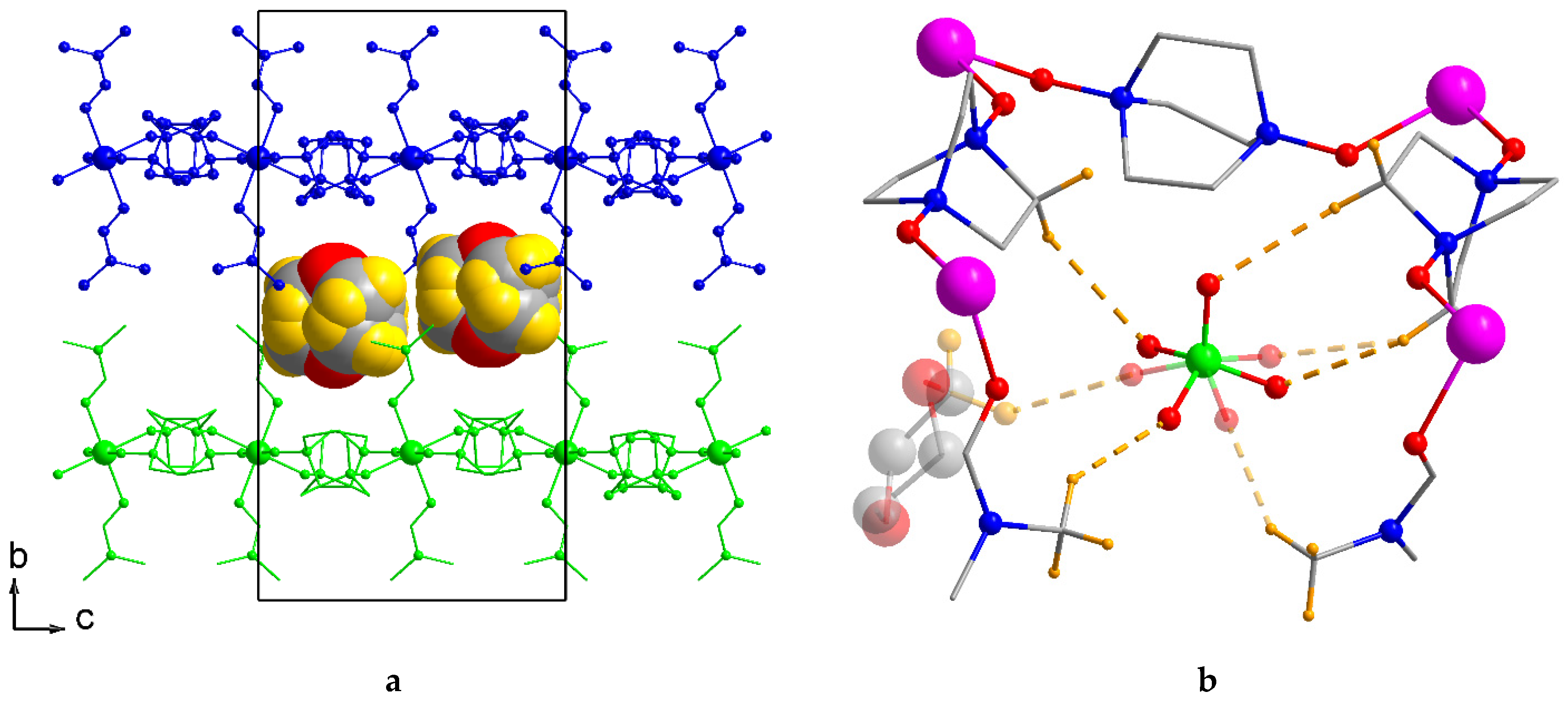
2.2. Crystal Structure Description
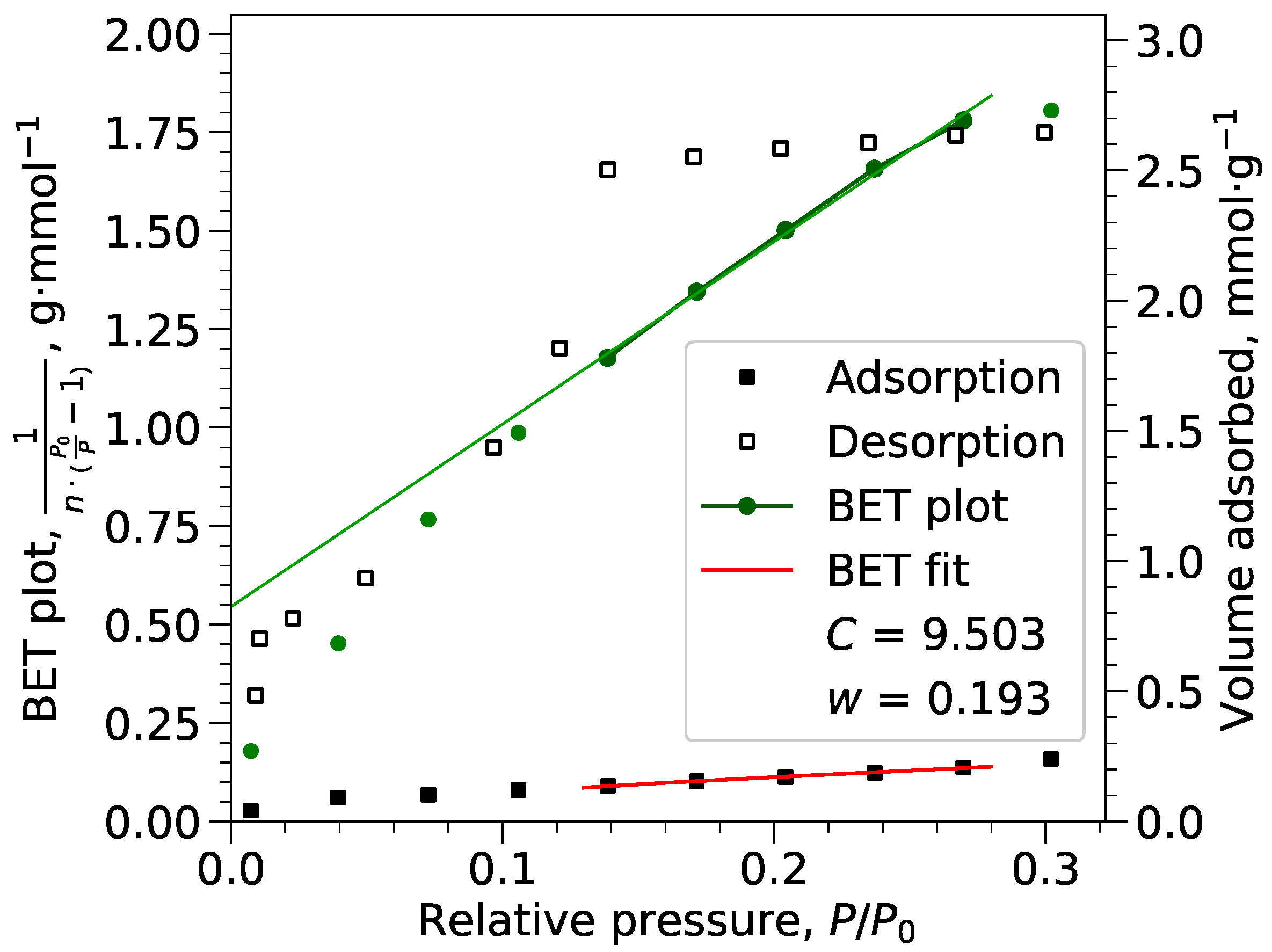
2.3. Adsorption Measurements
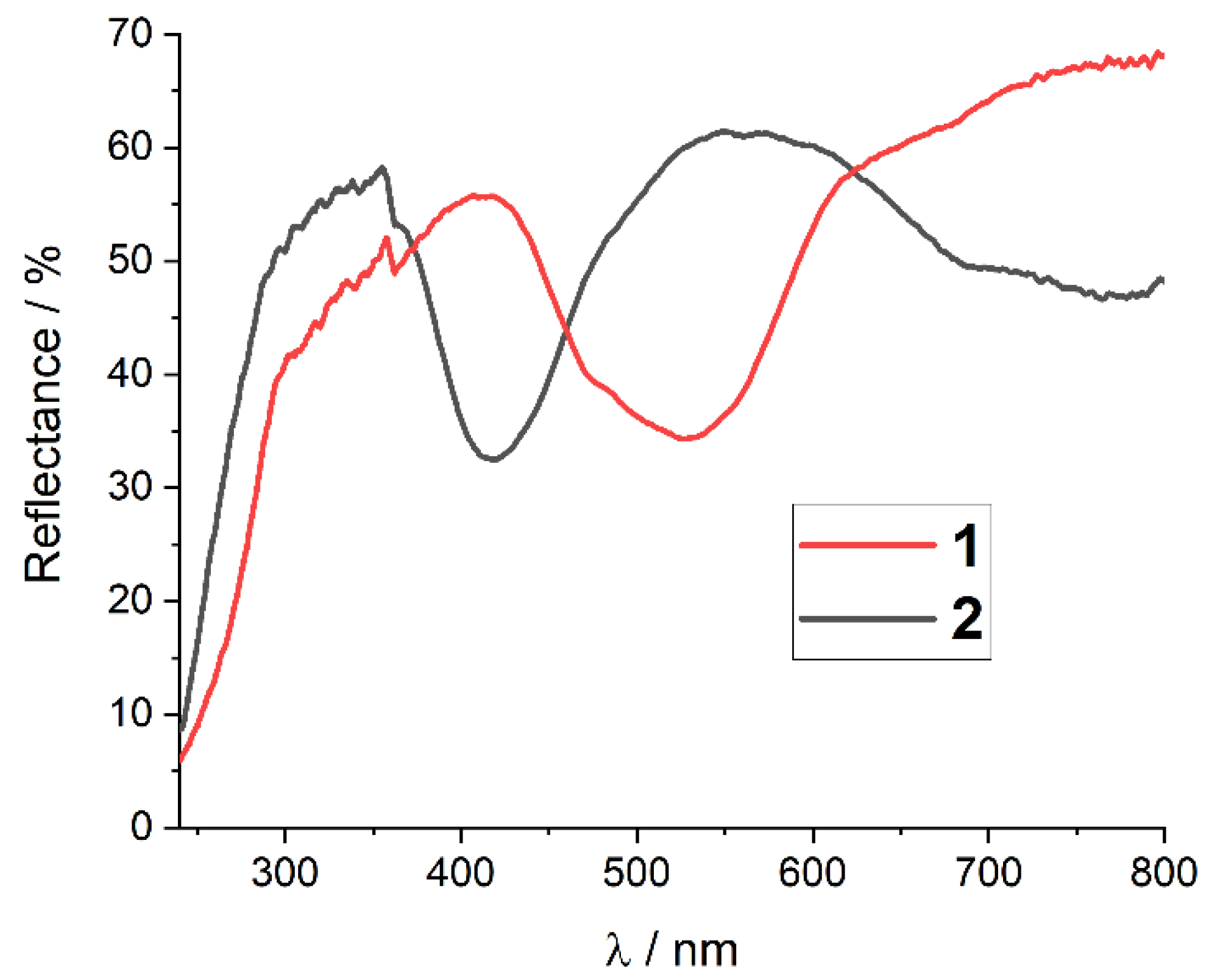
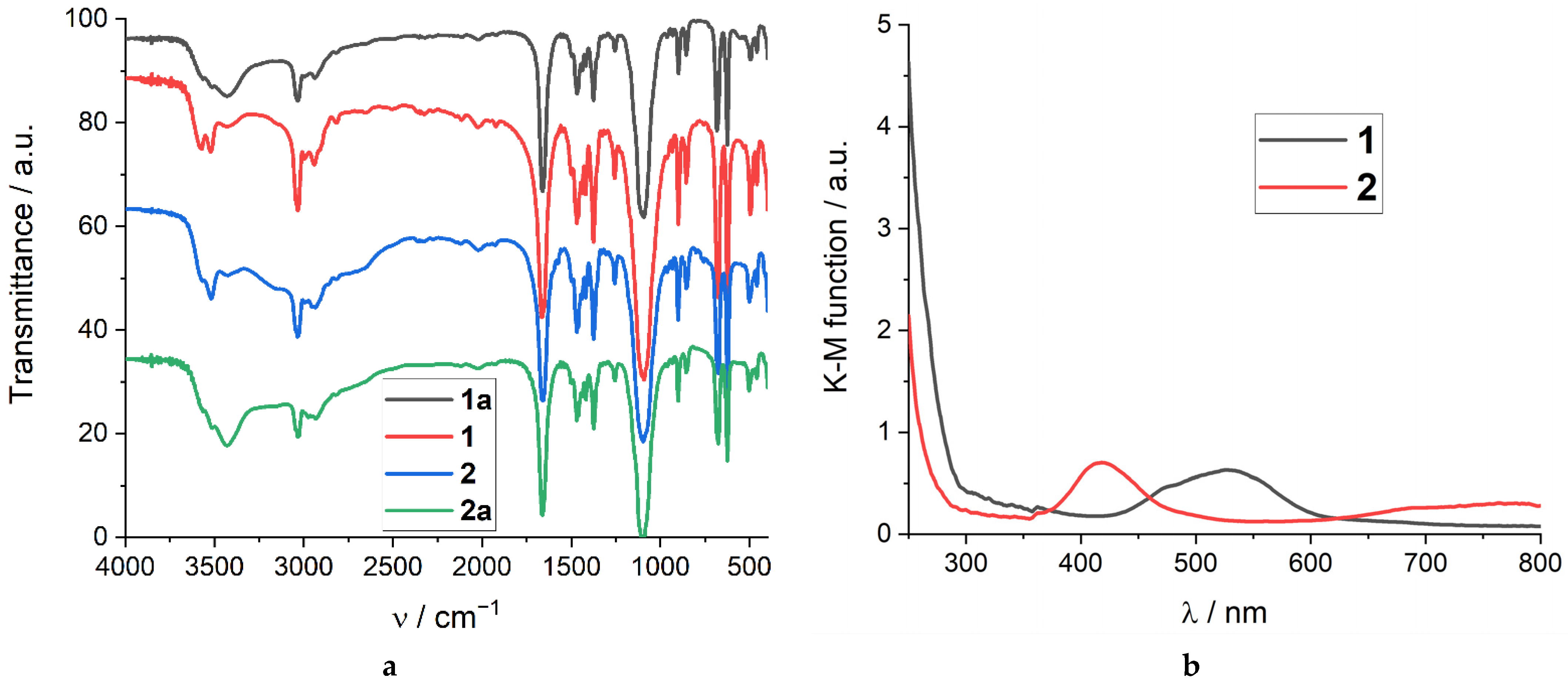
2.4. Spectroscopic Characterization
2.5. Magnetization Measurements
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthetic Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Additional Characterization Data

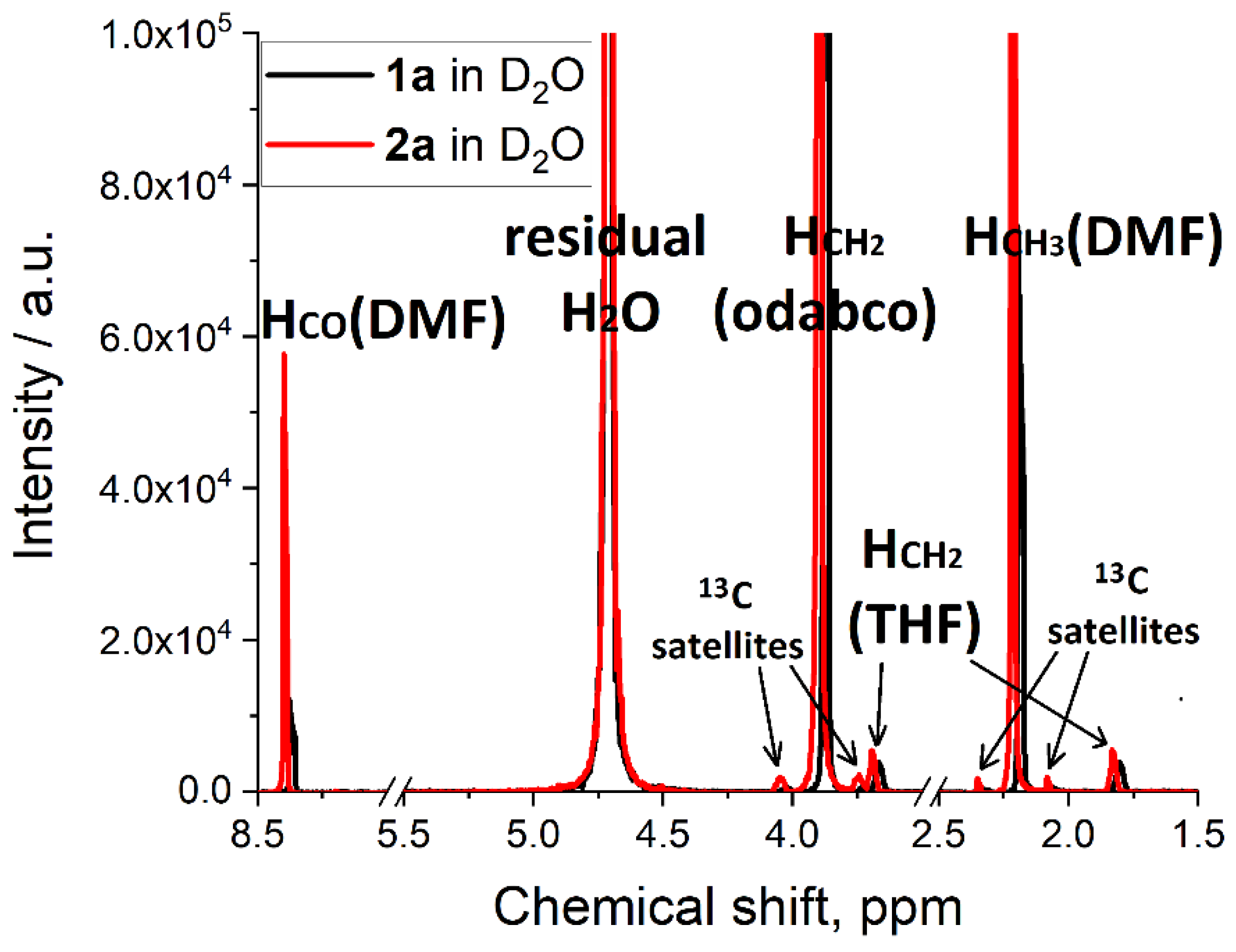
| Peak Position, ppm | Integral, a.u. | Assignment | Amount in One Molecule | Molecular Units, a.u. * |
|---|---|---|---|---|
| 8.37 (1a) 8.39 (2a) | 1.000 | HCO (DMF) | 1 | 1.44 DMF (1a) 1.43 DMF (2a) |
| 2.19 (1a) 2.22 (2a) | 6.519 (1a) 6.635 (2a) | HCH3 (DMF) | 6 | |
| 3.89 (1a) 3.89 (2a) | 18.133 (1a) 18.075 (2a) | HCH2 (odabco) | 12 | 2.00 odabco (1a and 2a) |
| 3.67 (1a) 3.69 (2a) | 0.488 (1a) 0.179 (2a) | HCH2O (THF) | 4 | 0.17 THF (1a) 0.06 THF (2a) |
| 1.80 (1a) 1.82 (2a) | 0.516 (1a) 0.176 (2a) | HCH2CH2 (THF) | 4 |
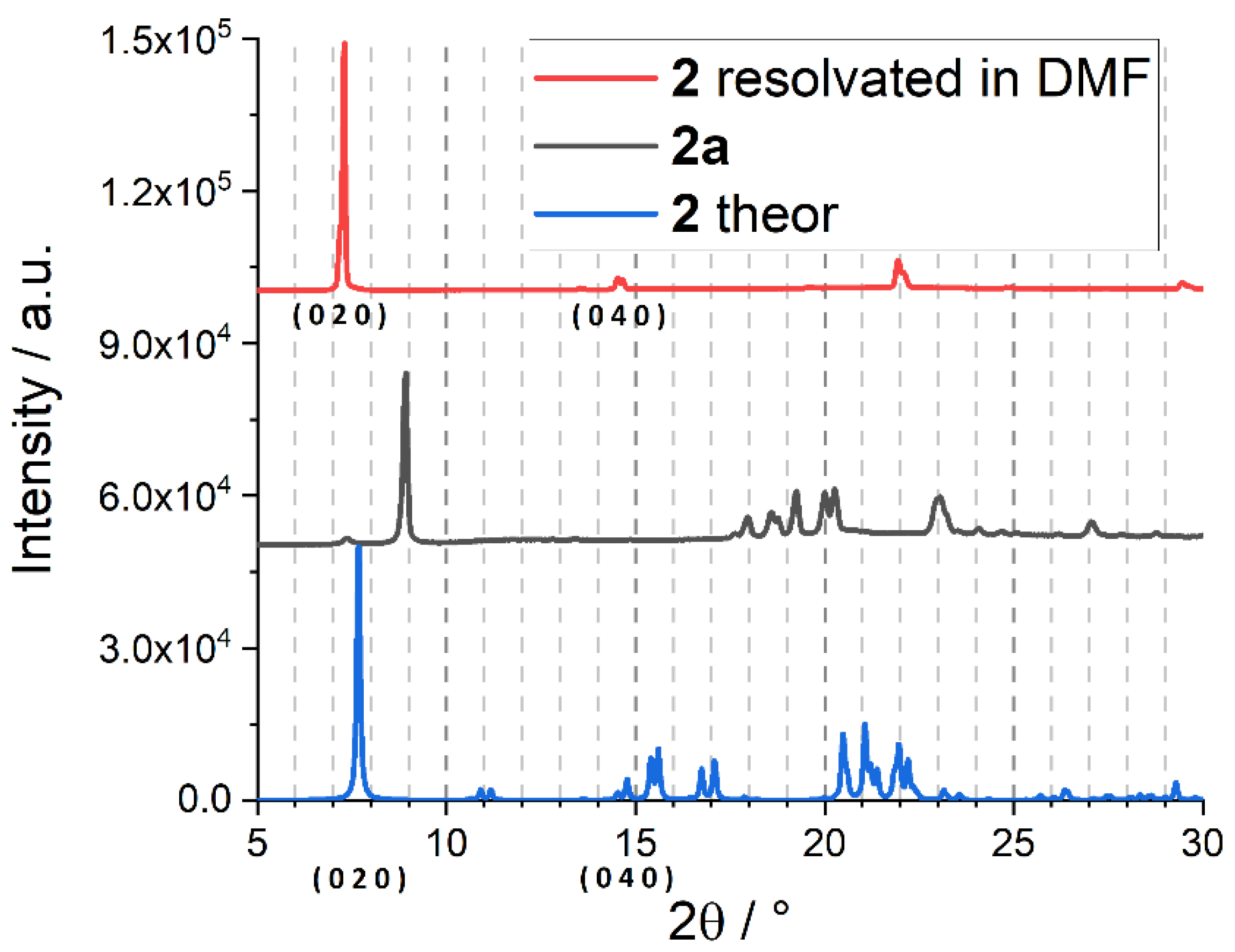
Appendix B. Adsorption Supplementary Data
Appendix B.1. BET Calculations

Appendix B.2. Langmuir Calculations
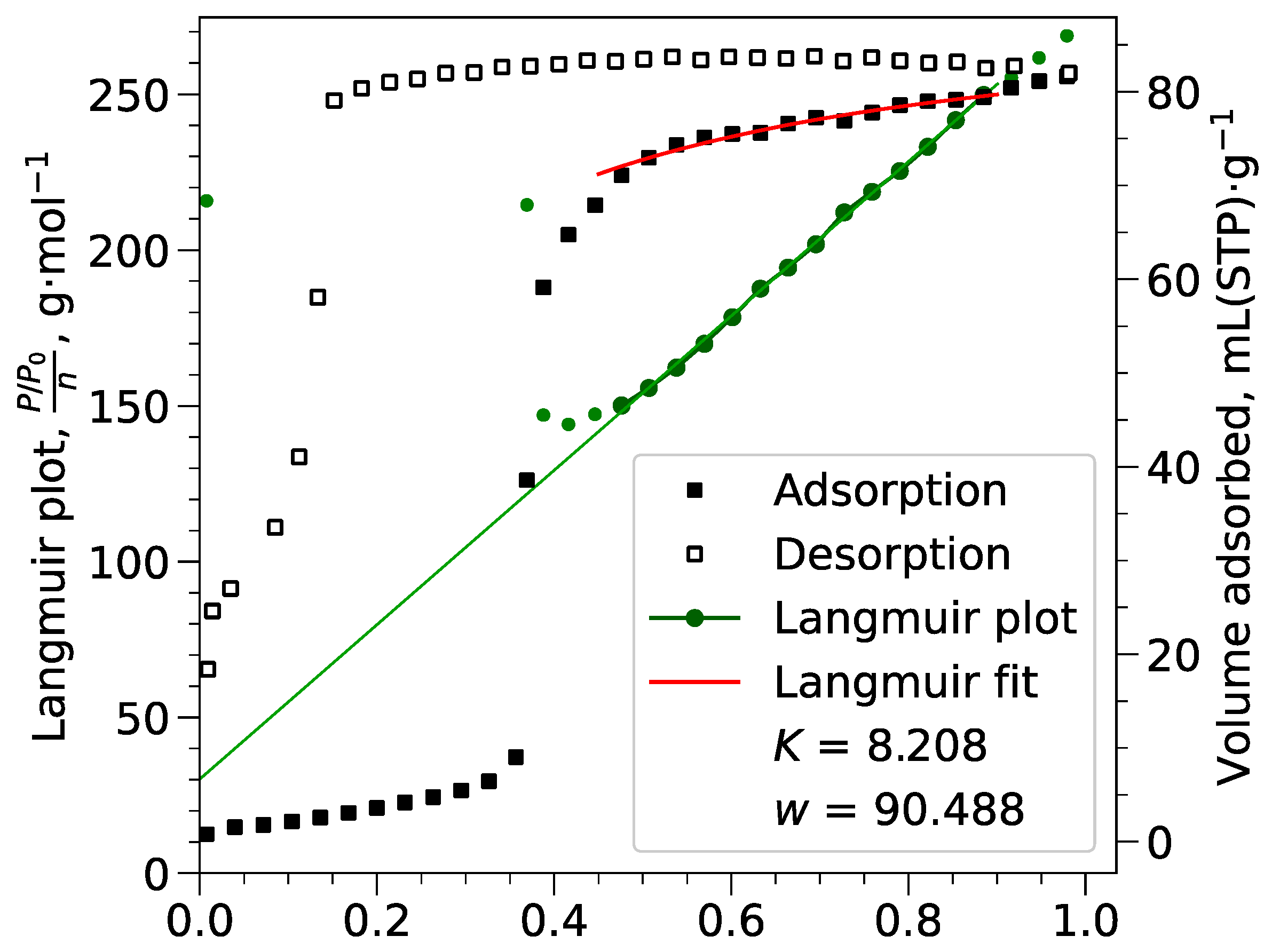
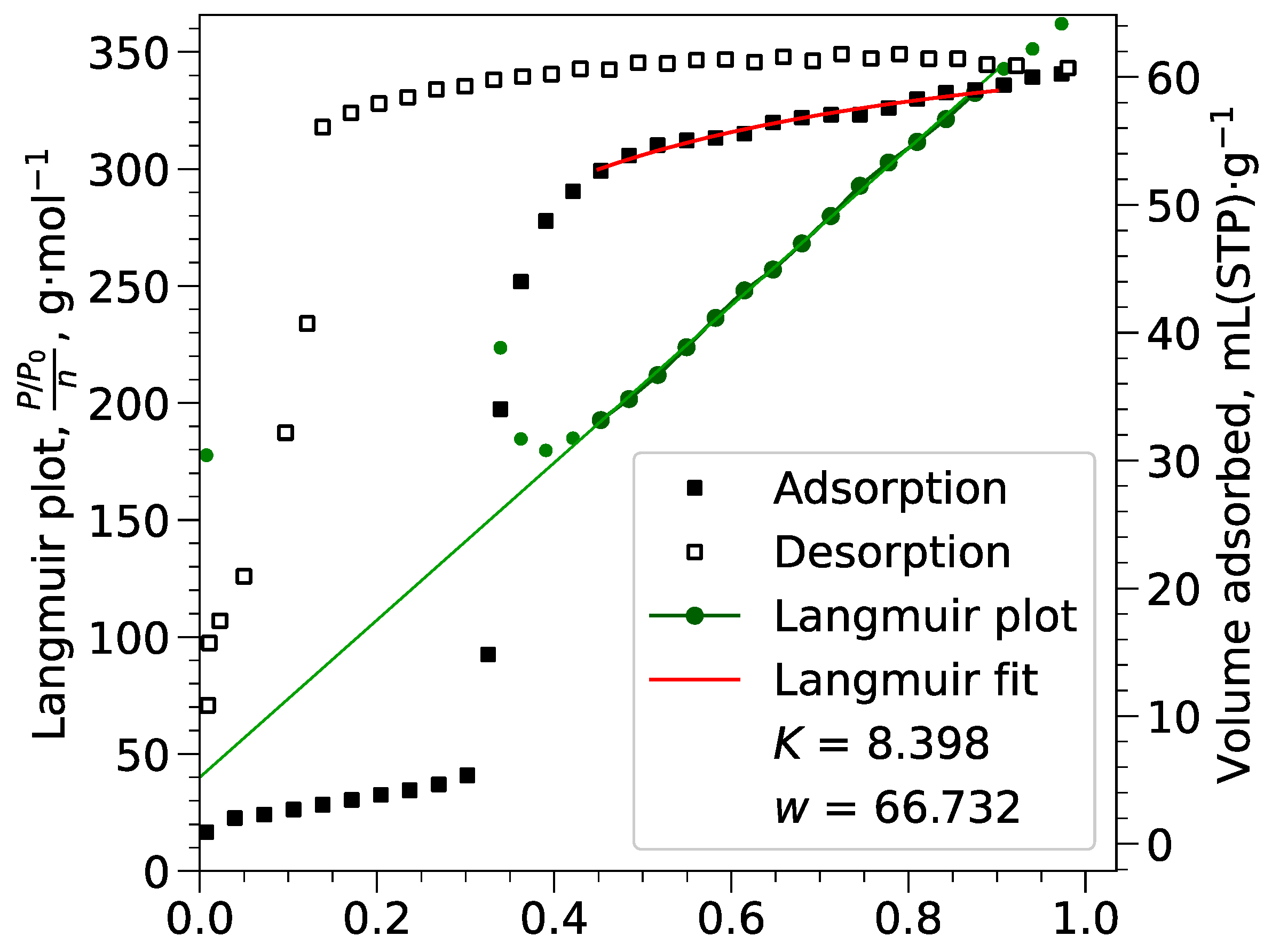
Appendix B.3. Pore Volumes
Appendix C. The Crystallographic Data
| 1 | 2 | |
|---|---|---|
| Chemical formula | C22H46Cl2CoN6O16 | C22H46Cl2N6NiO16 |
| Mr/g·mol–1 | 780.48 | 780.26 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c |
| Temperature/K | 237 | 130 |
| a/Å | 12.1805(6) | 12.1891(3) |
| b/Å | 23.3427(14) | 23.0318(6) |
| c/Å | 12.1557(5) | 11.9842(3) |
| α/° | 90 | 90 |
| b/° | 90.975 (4) | 91.577 (2) |
| γ/° | 90 | 90 |
| V/Å3 | 3455.7(3) | 3363.13(15) |
| Z | 4 | 4 |
| F(000) | 1636 | 1640 |
| D(calc.)/g·cm–3 | 1.500 | 1.541 |
| μ/mm–1 | 0.73 | 0.81 |
| Crystal size/mm | 0.40 × 0.40 × 0.06 | 0.27 × 0.25 × 0.07 |
| θ range for data collection/° | 1.87 ≤ θ ≤ 25.35 | 3.34 ≤ θ ≤ 25.34 |
| No. of reflections: measured/independent/observed [I > 2σ(I)] | 7638/3175/2606 | 7249/3081/2696 |
| Rint | 0.0255 | 0.0170 |
| Index ranges | –14 ≤ h ≤ 14 –28 ≤ k ≤ 23 –14 ≤ l ≤ 12 | –14 ≤ h ≤ 14 –27 ≤ k ≤ 19 –13 ≤ l ≤ 14 |
| Final R indices [I > 2σ(I)] | R1 = 0.0474 wR2 = 0.1125 | R1 = 0.0295 wR2 = 0.0757 |
| Final R indices (all data) | R1 = 0.0627 wR2 = 0.1211 | R1 = 0.0362 wR2 = 0.0788 |
| Goodness-of-fit on F2 | 1.034 | 1.058 |
| Largest diff. peak, hole/e·Å–3 | 0.48, –0.42 | 0.34, –0.44 |
References
- Ghasempour, H.; Wang, K.-Y.; Powell, J.A.; ZareKarizi, F.; Lv, X.-L.; Morsali, A.; Zhou, H.-C. Metal–organic frameworks based on multicarboxylate linkers. Coord. Chem. Rev. 2021, 426, 213542. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Y.; Su, Z.; Zhang, X.; Zhang, W.; Srinivas, K. Organic carboxylate-based MOFs and derivatives for electrocatalytic water oxidation. Coord. Chem. Rev. 2021, 428, 213619. [Google Scholar] [CrossRef]
- Desai, A.V.; Sharma, S.; Let, S.; Ghosh, S.K. N-donor linker based metal-organic frameworks (MOFs): Advancement and prospects as functional materials. Coord. Chem. Rev. 2019, 395, 146–192. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-Organic Frameworks: Synthetic Methods and Potential Applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, S.; Dam, G.K.; Ghosh, S.K. Neutral Nitrogen Donor Ligand-based MOFs for Sensing Applications. Chem. Asian J. 2021, 16, 2569–2587. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Shahnawaz Khan, M.; Shahid, M.; Ahmad, M. A bifunctionalised Pb-based MOF for iodine capture and dye removal. Dalton Trans. 2023, 52, 4501–4516. [Google Scholar] [CrossRef]
- Lysova, A.A.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. Efficient separation of methane, ethane and propane on mesoporous metal-organic frameworks. Chem. Eng. J. 2023, 453, 139642. [Google Scholar] [CrossRef]
- Li, X.L.; Fang, D.; Lieu, W.Y.; Chen, C.; Shahnawaz Khan, M.; Li, D.-S.; Tian, B.; Shi, Y.; Yang, H.Y. Metal–Organic-Framework-Derived 3D Hierarchical Matrixes for High-Performance Flexible Li–S Batteries. ACS Appl. Mater. Interfaces 2023, 15, 20064–20074. [Google Scholar] [CrossRef]
- Shi, Y.; Zou, Y.; Shahnawaz Khan, M.; Zhang, M.; Yan, J.; Zheng, X.; Wang, W.; Xie, Z. Metal–organic framework-derived photoelectrochemical sensors: Structural design and biosensing technology. J. Mater. Chem. C 2023, 11, 3692–3709. [Google Scholar] [CrossRef]
- Matyukhina, A.K.; Zorina-Tikhonova, E.N.; Goloveshkin, A.S.; Babeshkin, K.A.; Efimov, N.N.; Kiskin, M.A.; Eremenko, I.L. Field-Induced Slow Magnetic Relaxation in CoII Cyclopropane-1,1-dicarboxylates. Molecules 2022, 27, 6537. [Google Scholar] [CrossRef]
- Tepper, R.; Schulze, B.; Bellstedt, P.; Heidler, J.; Görls, H.; Jäger, M.; Schubert, U.S. Halogen-bond-based cooperative ion-pair recognition by a crown-ether-embedded 5-iodo-1,2,3-triazole. Chem. Commun. 2017, 53, 2260–2263. [Google Scholar] [CrossRef] [PubMed]
- Czugler, M.; Pintér, I. Sodium halide complexes of ribose derivatives and their unusual crystal structures. Carbohydr. Res. 2011, 346, 1610–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marabello, D.; Antoniotti, P.; Benzi, P.; Beccari, F.; Canepa, C.; Cariati, E.; Cioci, A.; Lo Presti, L. Crystal structure or chemical composition of salt–sugar-based metal–organic frameworks: What are the nonlinear optical properties due to? Acta Crystallogr. 2021, B77, 506–514. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, H.; Liao, Q.; Tang, T.; Liu, J.; Liu, Z.; Xie, L.; Yan, J. Preparation of two metal organic frameworks (K-β-CD-MOFs and Cs-β-CD-MOFs) and the adsorption research of myricetin. Polyhedron 2021, 196, 114983. [Google Scholar] [CrossRef]
- Koshevoy, E.I.; Samsonenko, D.G.; Berezin, A.S.; Fedin, V.P. Metal-Organic Coordination Polymers Formed from γ-Cyclodextrin and Divalent Metal Ions. Eur. J. Inorg. Chem. 2019, 2019, 4321–4327. [Google Scholar] [CrossRef]
- Govindaraj, M.; Huang, W.-C.; Lee, C.-Y.; Lakshmanan, V.; Liu, Y.-H.; So, P.B.; Lin, C.-H.; Chen, J.-D. Structural Diversity of Mercury(II) Halide Complexes Containing Bis-pyridyl-bis-amide with Bulky and Angular Backbones: Ligand Effect and Metal Sensing. Int. J. Mol. Sci. 2022, 23, 7861. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Liang, F.; Hu, Z.; Wu, Y. Co-crystal AX·(H3C3N3O3) (A = Na, Rb, Cs; X = Br, I): A series of strongly anisotropic alkali halide cyanurates with a planar structural motif and large birefringence. Dalton Trans. 2021, 50, 11555–11561. [Google Scholar] [CrossRef]
- Li, Z.-F.; Liang, L.-L.; Wu, F.; Zhou, F.-G.; Ni, X.-L.; Feng, X.; Xiao, X.; Zhang, Y.-Q.; Xue, S.-F.; Zhu, Q.-J.; et al. An approach to networks based on coordination of alkyl-substituted cucurbit[5]urils and potassium ions. CrystEngComm 2013, 15, 1994–2001. [Google Scholar] [CrossRef]
- Shen, N.; Yang, Z.; Liu, S.; Dai, X.; Xiao, C.; Taylor-Pashow, K.; Li, D.; Yang, C.; Li, J.; Zhang, Y.; et al. 99TcO4− removal from legacy defense nuclear waste by an alkaline-stable 2D cationic metal organic framework. Nat. Commun. 2020, 11, 5571. [Google Scholar] [CrossRef]
- Ma, J.; Wang, C.-C.; Zhao, Z.-X.; Wang, P.; Li, J.-J.; Wang, F.-X. Adsorptive capture of perrhenate (ReO4−) from simulated wastewater by cationic 2D-MOF BUC-17. Polyhedron 2021, 202, 115218. [Google Scholar] [CrossRef]
- Yu, C.-X.; Chen, J.; Zhang, Y.; Song, W.-B.; Li, X.-Q.; Chen, F.-J.; Zhang, Y.-J.; Liu, D.; Liu, L.-L. Highly efficient and selective removal of anionic dyes from aqueous solution by using a protonated metal-organic framework. J. Alloys Compd. 2021, 853, 157383. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, L.; Yang, Y.; Zhang, K.; Cai, Y.; Xu, Y.; Gai, Y.; Xiong, K. Viologen-Based Cationic Metal–Organic Framework for Antibiotics Detection and MnO4− Removal in Water. Cryst. Growth Des. 2022, 22, 3991–3997. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Qin, J.-S.; Pang, J.; Zhang, P.; Zhang, Y.; Huang, Y.; Drake, H.F.; Liu, W.R.; Zhou, H.-C. Stepwise Assembly of Turn-on Fluorescence Sensors in Multicomponent Metal–Organic Frameworks for in Vitro Cyanide Detection. Angew. Chem. Int. Ed. 2020, 59, 9319–9323. [Google Scholar] [CrossRef]
- Shi, P.-F.; Hu, H.-C.; Zhang, Z.-Y.; Xiong, G.; Zhao, B. Heterometal–organic frameworks as highly sensitive and highly selective luminescent probes to detect I− ions in aqueous solutions. Chem. Commun. 2015, 51, 3985–3988. [Google Scholar] [CrossRef]
- Li, G.; Liu, W.-S.; Yang, S.-L.; Zhang, L.; Bu, R.; Gao, E.-Q. Anion-Afforded Functions of Ionic Metal–Organic Frameworks: Ionochromism, Anion Conduction, and Catalysis. Inorg. Chem. 2022, 61, 902–910. [Google Scholar] [CrossRef]
- He, H.; Hashemi, l.; Hu, M.-L.; Morsali, A. The role of the counter-ion in metal-organic frameworks’ chemistry and applications. Coord. Chem. Rev. 2018, 376, 319–347. [Google Scholar] [CrossRef]
- Noori, Y.; Akhbari, K. Post-synthetic ion-exchange process in nanoporous metal–organic frameworks; an effective way for modulating their structures and properties. RSC Adv. 2017, 7, 1782–1808. [Google Scholar] [CrossRef] [Green Version]
- Dybtsev, D.N.; Kovalenko, K.A.; Mironov, Y.V.; Fedin, V.P.; Férey, G.; Yakovleva, N.A.; Berdonosova, E.A.; Klyamkin, S.N.; Kogan, E.V. Reversible sorption of hydrogen on the novel hybrid material based on mesoporous chromium(III) terephthalate with included rhenium clusters. Russ. Chem. Bull. 2009, 58, 1623–1626. [Google Scholar] [CrossRef]
- Kovalenko, K.A.; Ruban, N.V.; Adonin, S.A.; Korneev, D.V.; Erenburg, S.B.; Trubina, S.V.; Kvashnina, K.; Sokolov, M.N.; Fedin, V.P. Bi(III) immobilization inside MIL-101: Enhanced photocatalytic performance. New J. Chem. 2017, 41, 2255–2260. [Google Scholar] [CrossRef] [Green Version]
- Noro, S.-I.; Mizutani, J.; Hijikata, Y.; Matsuda, R.; Sato, H.; Kitagawa, S.; Sugimoto, K.; Inubushi, Y.; Kubo, K.; Nakamura, T. Porous coordination polymers with ubiquitous and biocompatible metals and a neutral bridging ligand. Nat. Commun. 2015, 6, 5851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-R.; Lin, X.-L.; Chen, B.; Zheng, H.-D.; Chen, Z.-R.; Li, H.-H.; Zheng, S.-T. Thermal-Responsive Polyoxometalate–Metalloviologen Hybrid: Reversible Intermolecular Three-Component Reaction and Temperature-Regulated Resistive Switching Behaviors. Angew. Chem. Int. Ed. 2021, 60, 16911–16916. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Zakrzewski, J.J.; Reczyński, M.; Sieklucka, B. Multi-colour uranyl emission efficiently tuned by hexacyanidometallates within hybrid coordination frameworks. Chem. Commun. 2019, 55, 3057–3060. [Google Scholar] [CrossRef]
- Zakrzewski, J.J.; Sieklucka, B.; Chorazy, S. Europium(III) Photoluminescence Governed by d8–d10 Heterometallophilic Interactions in Trimetallic Cyanido-Bridged Coordination Frameworks. Inorg. Chem. 2020, 59, 1393–1404. [Google Scholar] [CrossRef]
- Wang, D.-H.; Zhao, L.-M.; Lin, X.-Y.; Wang, Y.-K.; Zhang, W.-T.; Song, K.-Y.; Li, H.-H.; Chen, Z.-R. Iodoargentate/iodobismuthate-based materials hybridized with lanthanide-containing metalloviologens: Thermochromic behaviors and photocurrent responses. Inorg. Chem. Front. 2018, 5, 1162–1173. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.-R.; Li, Y.-L.; Li, H.-H.; Chen, Z.-R.; Jiang, R. Encapsulating Halometallates into 3-D Lanthanide-Viologen Frameworks: Controllable Emissions, Reversible Thermochromism, Photocurrent Responses, and Electrical Bistability Behaviors. Inorg. Chem. 2019, 58, 13862–13880. [Google Scholar] [CrossRef]
- Long, D.-L.; Lake, A.J.; Champness, N.R.; Wilson, C.; Schröder, M. Unprecedented Seven- and Eight-Connected Lanthanide Coordination Networks. Angew. Chem. Int. Ed. 2001, 40, 2443–2447. [Google Scholar] [CrossRef]
- Ma, F.; Sun, R.; Sun, A.-H.; Xiong, J.; Sun, H.-L.; Gao, S. Regulating the structural dimensionality and dynamic properties of a porous dysprosium coordination polymer through solvent molecules. Inorg. Chem. Front. 2020, 7, 930–938. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Y.-R.; Song, K.-Y.; Lin, X.-L.; Li, H.-H.; Chen, Z.-R. Molecular Nonvolatile Memory Based on [α-GeW12O40]4−/Metalloviologen Hybrids Can Work at High Temperature Monitored by Chromism. Chem. Mater. 2021, 33, 2178–2186. [Google Scholar] [CrossRef]
- Qian, J.-F.; Tian, W.-J.; Yang, S.; Sun, Z.-H.; Chen, L.; Wei, M.-J.; Wu, Z.; He, M.-Y.; Zhang, Z.-H.; Mei, L. Auxiliary Ligand-Dependent Adaptive Regulation of Uranyl Coordination in Mixed-Ligand Uranyl Compounds of Flexible Biphenyltetracarboxylic Acid. Inorg. Chem. 2020, 59, 17659–17670. [Google Scholar] [CrossRef] [PubMed]
- Toma, O.; Mercier, N.; Allain, M.; Meinardi, F.; Forni, A.; Botta, C. Mechanochromic Luminescence of N,N′-Dioxide-4,4′-bipyridine Bismuth Coordination Polymers. Cryst. Growth Des. 2020, 20, 7658–7666. [Google Scholar] [CrossRef]
- Ma, F.; Xiong, J.; Meng, Y.-S.; Yang, J.; Sun, H.-L.; Gao, S. Rational construction of a porous lanthanide coordination polymer featuring reversible guest-dependent magnetic relaxation behavior. Inorg. Chem. Front. 2018, 5, 2875–2884. [Google Scholar] [CrossRef]
- Hon, P.K.; Mak, T.C.W. Isolation and crystal structures of 1:3 molecular complexes of triethylenediamineN,N′-dioxide with hydrogen peroxide and water. J. Crystallogr. Spectrosc. Res. 1987, 17, 419–429. [Google Scholar] [CrossRef]
- Abasheeva, K.D.; Demakov, P.A.; Fedin, V.P. Diverse Hydrogen-Bonded Structural Motifs in 1,4-Diazabicyclo[2.2.2]octane N,N′-Dioxide Salts with Oxoanions. Molbank 2022, 2022, M1508. [Google Scholar] [CrossRef]
- Sun, F.-X.; Zhu, G.-S.; Fang, Q.-R.; Qiu, S.-L. A novel 3D metal-organic framework with the pcu topology constructed from 1,4-diaza-bicyclo[2.2.2]octane-N,N′-dioxide. Inorg. Chem. Comm. 2007, 10, 649. [Google Scholar] [CrossRef]
- Demakov, P.A.; Romanov, A.S.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Synthesis and structure of manganese(II) coordination polymers with 1,4-diazabicyclo[2.2.2]octane N, N′-dioxide: Solvent and template effects. Russ. Chem. Bull. 2020, 69, 1511–1519. [Google Scholar] [CrossRef]
- Abasheeva, K.D.; Demakov, P.A.; Dybtsev, D.N.; Fedin, V.P. Crystal Structure of Coordination Cobalt(II) and Zinc(II) polymers with 1,4-Diazabicyclo[2.2.2]Octane N,N′-Dioxide. J. Struct. Chem. 2022, 63, 1349–1357. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ovchinnikova, A.A.; Fedin, V.P. SYNTHESIS, structure, and optical properties of the lanthanum(III) cationic coordination polymer with 1,4-Diazabicyclo[2.2.2]Octane N,N′-Dioxide. J. Struct. Chem. 2023, 64, 199–207. [Google Scholar] [CrossRef]
- Demakov, P.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Zinc(II) metal-organic frameworks with 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide: Control of the parameters of the cationic porous framework and optical properties. Russ. Chem. Bull. 2022, 71, 83–90. [Google Scholar] [CrossRef]
- Demakov, P.A.; Yudina, Y.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Crystal structure of zinc coordination polymers based on 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide: Effect of hydrophobicity of carboxylate ligands. J. Struct. Chem. 2021, 62, 403–411. [Google Scholar] [CrossRef]
- Chen, L.Z.; Sun, J. Reversible ferroelectric phase transition of 1,4-diazabicyclo[2,2,2]octane N,N′-dioxide di(perchlorate). Inorg. Chem. Comm. 2017, 76, 67–70. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Zhang, Y.; Noro, S.-I.; Kubo, K.; Yoshitake, M.; Liu, Z.-Q.; Cai, H.-L.; Fu, D.-W.; Yoshikawa, H.; Awaga, K.; et al. Molecule-displacive ferroelectricity in organic supramolecular solids. Sci. Rep. 2013, 3, 2249. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Ji, Q.; Wang, X.; Pan, Q.; Cao, X.; Xu, G. Two novel metal–organic coordination polymers based on ligand 1,4-diazabicyclo[2.2.2]octane N,N′-dioxide with phase transition, and ferroelectric and dielectric properties. CrystEngComm 2017, 19, 5907–5914. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Wu, R.; Cai, Y.; Zhou, Z.-H. Unusual N-oxide formation in the peroxidation of cobalt(II) ethylenediaminetetraacetates. Dalton Trans. 2017, 46, 1290–1296. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Feng, X.; Meihaus, K.R.; Meng, X.; Zhang, Y.; Li, L.; Liu, J.-L.; Pedersen, K.S.; Keller, L.; Shi, W.; et al. Coercive Fields Above 6 T in Two Cobalt(II)–Radical Chain Compounds. Angew. Chem. Int. Ed. 2020, 59, 10610–10618. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.; Kuznetsova, O.; Fursova, E.; Letyagin, G.; Romanenko, G.; Bogomyakov, A.; Zueva, E. Simultaneous Introduction of Two Nitroxides in the Reaction: A New Approach to the Synthesis of Heterospin Complexes. Inorg. Chem. 2017, 56, 14567–14576. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Pu, M.; Sang, X.; Li, S.; Liu, X.; Wu, Y.-D.; Feng, X. Asymmetric Catalytic (2+1) Cycloaddition of Thioketones to Synthesize Tetrasubstituted Thiiranes. Angew. Chem. Int. Ed. 2022, 61, e202201151. [Google Scholar] [CrossRef]
- Belov, A.S.; Vologzhanina, A.V.; Fedorov, Y.V.; Kuznetsov, E.V.; Voloshin, Y.Z. Unexpected transformation of mono- to bis-macrobicyclic dimethylglyoximate framework in a chloroform solution: Photochemical, MALDI-TOF MS and X-ray diffraction studies. Inorg. Chem. Commun. 2013, 35, 242–246. [Google Scholar] [CrossRef]
- Ran, J.-W.; Tong, Y.-P. Crystal Structures, Magnetic Properties, and Theoretical Investigations of Three Linear Cluster Complexes with Pyridine-2-Amidoxime. J. Struct. Chem. 2018, 59, 1433–1439. [Google Scholar] [CrossRef]
- Sniekers, J.; Geysens, P.; Vander Hoogerstraete, T.; Van Meervelt, L.; Fransaer, J.; Binnemans, K. Cobalt(ii) liquid metal salts for high current density electrodeposition of cobalt. Dalton Trans. 2018, 47, 4975–4986. [Google Scholar] [CrossRef]
- Yin, X.-J.; Zhu, L.-G. Structural variation from trinuclears to 1D chains: Syntheses, structures and properties. Appl. Organomet. Chem. 2019, 33, e4796. [Google Scholar] [CrossRef]
- Desiraju, G.R. C–H⋯O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem. Commun. 2005, 2005, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Zavras, A.; Fry, J.A.; Beavers, C.M.; Talbo, G.H.; Richards, A.F. 2-Pyridylmethylphosphonic acid: A flexible, multi-dentate ligand for metal phosphonates. CrystEngComm 2011, 13, 3551–3561. [Google Scholar] [CrossRef]
- Goodgame, D.M.L.; Menzer, S.; Smith, A.M.; Williams, D.J. Formation of a novel three-dimensional network of 48-membered rings in the compound [Ni(m-XBP)3](ClO4)2. J. Chem. Soc. Chem. Commun. 1994, 1994, 1825–1826. [Google Scholar] [CrossRef]
- Pal Singh Pannu, A.; Kapoor, P.; Hundal, G.; Kapoor, R.; Martinez-Ripoll, M.; Singh Hundal, M. A self assembled 3-D network propagated by coordination polymerization and H-bonding: Synthesis and X-ray crystal structure of [{Co(L)2(H2O)2}(ClO4)2(CH3COCH3)2(H2O)2]n, where L = N,N-diisopropylisonicotinamide. J. Coord. Chem. 2011, 64, 1566–1577. [Google Scholar] [CrossRef]
- Shi, J.-M.; Zhang, E.-X.; Wu, C.-J.; Yi, L.; Ma, J.-P. Synthesis, crystal structure and magnetism study on a three-dimensional Co(II) coordination polymer with 2,5-dimethylpyrazine-1,4-dioxide as bridging ligand. J. Coord. Chem. 2007, 60, 1667–1672. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Du, M.; Lu, W.-J.; Mak, T.C.W. Anion-controlled formation of diverse porous cobalt(II) coordination polymers with 1,2-bis(4-pyridyl)ethane-N,N′-dioxide. Inorg. Chem. Commun. 2005, 8, 623–625. [Google Scholar] [CrossRef]
- Zhou, G.X.; Wang, X.Y.; Yan, G.; Liao, G.Y.; Xia, H. Syntheses, crystal structures, and properties of various one- dimensional coordination polymers based on macrocyclic metallic tectons and dicarboxylic acid ligand. Russ. J. Coord. Chem. 2016, 42, 461–469. [Google Scholar] [CrossRef]
- Su, Z.; Bai, Z.-S.; Xu, J.; Okamura, T.; Liu, G.-X.; Chu, Q.; Wang, X.-F.; Sun, W.-Y.; Ueyama, N. Synthesis, structure and property of cobalt(II) complexes with 3,5-di(1H-imidazol-1-yl)benzoic acid. CrystEngComm 2009, 11, 873–880. [Google Scholar] [CrossRef]
- Wang, Q.; Ou, G.-C. Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane-κ4N,N′,N″,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate—Methanol (1/2), C27H49ClN4NiO12. Z. Krist. New Cryst St. 2022, 237, 745–747. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, Z.; Sun, W. Synthesis and Crystal Structure of an Unprecedented Supramolecular Complex [Co(µ2-ClO4)2(H2O)2]·2MA. Chin. J. Chem. 2009, 27, 501–504. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Kumar Maity, D.; Dey, A.; Ghosh, S.; Halder, A.; Pratim Ray, P.; Ghoshal, D. Set of Multifunctional Azo Functionalized Semiconducting Cd(II)-MOFs Showing Photoswitching Property and Selective CO2 Adsorption. Inorg. Chem. 2018, 57, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible Metal–Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Férey, G.; Serre, C. Large Breathing Effects in Three-Dimensional Porous Hybrid Matter: Facts, Analyses, Rules and Consequences. Chem. Soc. Rev. 2009, 38, 1380. [Google Scholar] [CrossRef] [PubMed]
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T.-H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655. [Google Scholar] [CrossRef]
- Demakov, P.A.; Volynkin, S.S.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. A Selenophene-Incorporated Metal–Organic Framework for Enhanced CO2 Uptake and Adsorption Selectivity. Molecules 2020, 25, 4396. [Google Scholar] [CrossRef]
- Li, Z.; Ma, X.; Xiong, S.; Ye, Y.; Yao, Z.; Lin, Q.; Zhang, Z.; Xiang, S. Facile synthesis of oxidized activated carbons for high-selectivity and low-enthalpy CO2 capture from flue gas. New J. Chem. 2018, 42, 4495–4500. [Google Scholar] [CrossRef]
- Lukyanova, M.B.; Tkachev, V.V.; Lukyanov, B.S.; Pugachev, A.D.; Ozhogin, I.V.; Komissarova, O.A.; Aldoshin, S.M.; Minkin, V.I. Structure Investigation of New Condensation Products of 1,2,3,3-Tetramethylindolenium with Metoxysubstituted Diformylphenols. J. Struct. Chem. 2018, 59, 565–570. [Google Scholar] [CrossRef]
- Jian, Y.-F.; Fu, B.; Fu, S.; Liu, X. Synthesis, Crystal Structure, and Photoluminescence of a Lithium Perchlorate Complex with 18-Crown-6. J. Struct. Chem. 2019, 60, 1119–1125. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Tabatabaeian, K.; Hosseinzadeh, F. Contribution of Diffuse Reflectance Spectroscopy to Monitoring the Synthesis Improvement of Encapsulated Complexes. Russ. J. Coord. Chem. 2005, 31, 585–587. [Google Scholar] [CrossRef]
- Kashif, M.K.; Milhuisen, R.A.; Nippe, M.; Hellerstedt, J.; Zee, D.Z.; Duffy, N.W.; Halstead, B.; De Angelis, F.; Fantacci, S.; Fuhrer, M.S.; et al. Cobalt Polypyridyl Complexes as Transparent Solution-Processable Solid-State Charge Transport Materials. Adv. Energy Mat. 2016, 6, 1600874. [Google Scholar] [CrossRef] [Green Version]
- Jbali, W.; Selmi, W.; Jouffret, L.; Marzouki, R.; Zid, M.F. Synthesis, crystal structure, spectroscopic study and Hirshfeld surface analysis of [Ni(C6H8N2)3]Cl2·2(H2O). J. Mol. Struct. 2021, 1225, 129123. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Matin, M.A.; Akhter Simol, H.; Hosen, A. Environmentally Friendly Room Temperature Synthesis of 1-Tetralone over Layered Double Hydroxide-Hosted Sulphonato-Salen-Nickel(II) Complex. Green Sustain. Chem. 2023, 13, 9–22. [Google Scholar] [CrossRef]
- Demakov, P.A.; Bogomyakov, A.S.; Urlukov, A.S.; Andreeva, A.Y.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Transition Metal Coordination Polymers with Trans-1,4-Cyclohexanedicarboxylate: Acidity-Controlled Synthesis, Structures and Properties. Materials 2020, 13, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Lavrov, A.N.; Dybtsev, D.N.; Fedin, V.P. Coordination Polymers of Ni(II) with Thiophene Ligands: Synthesis, Structures, and Magnetic Properties. Russ. J. Coord. Chem. 2021, 47, 664–669. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]


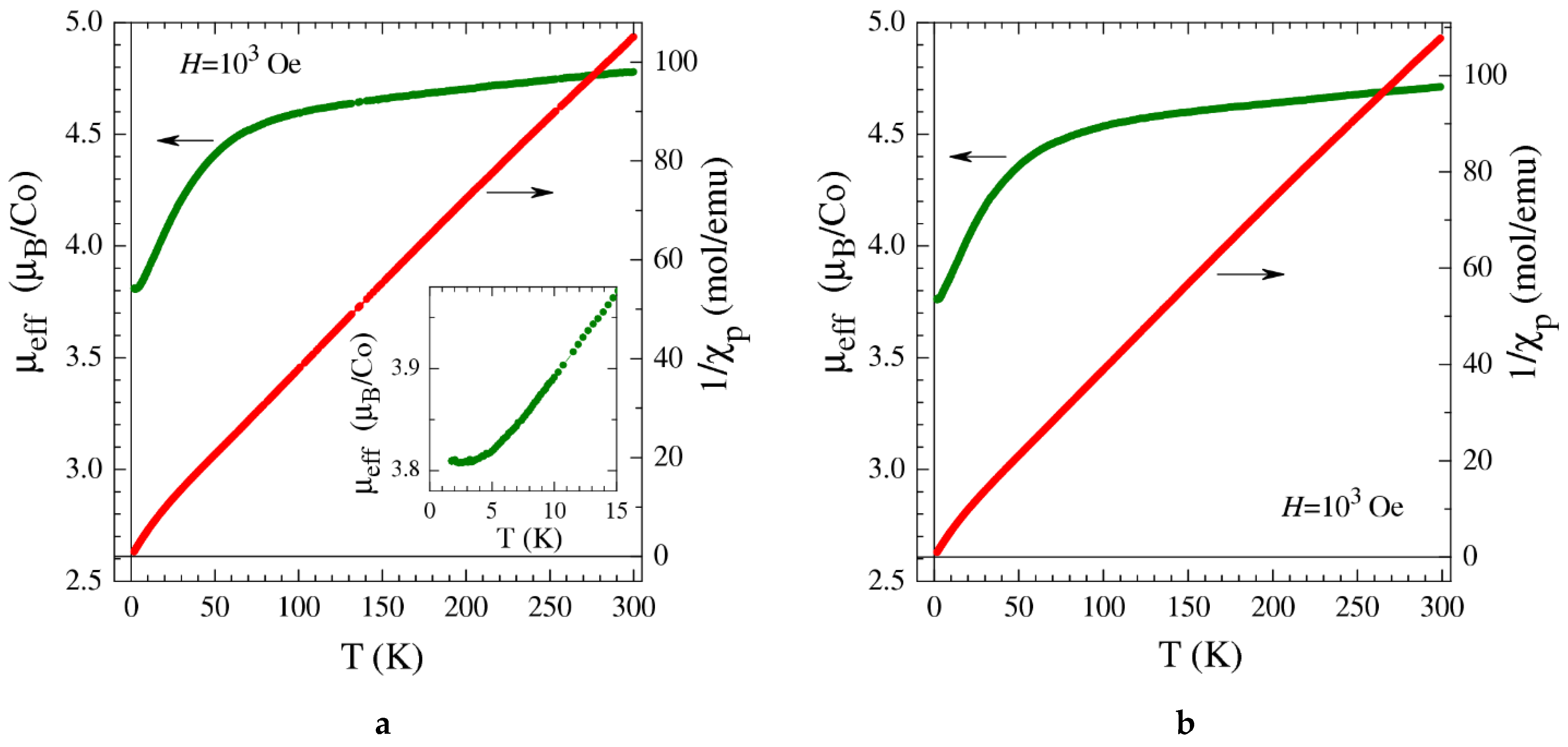
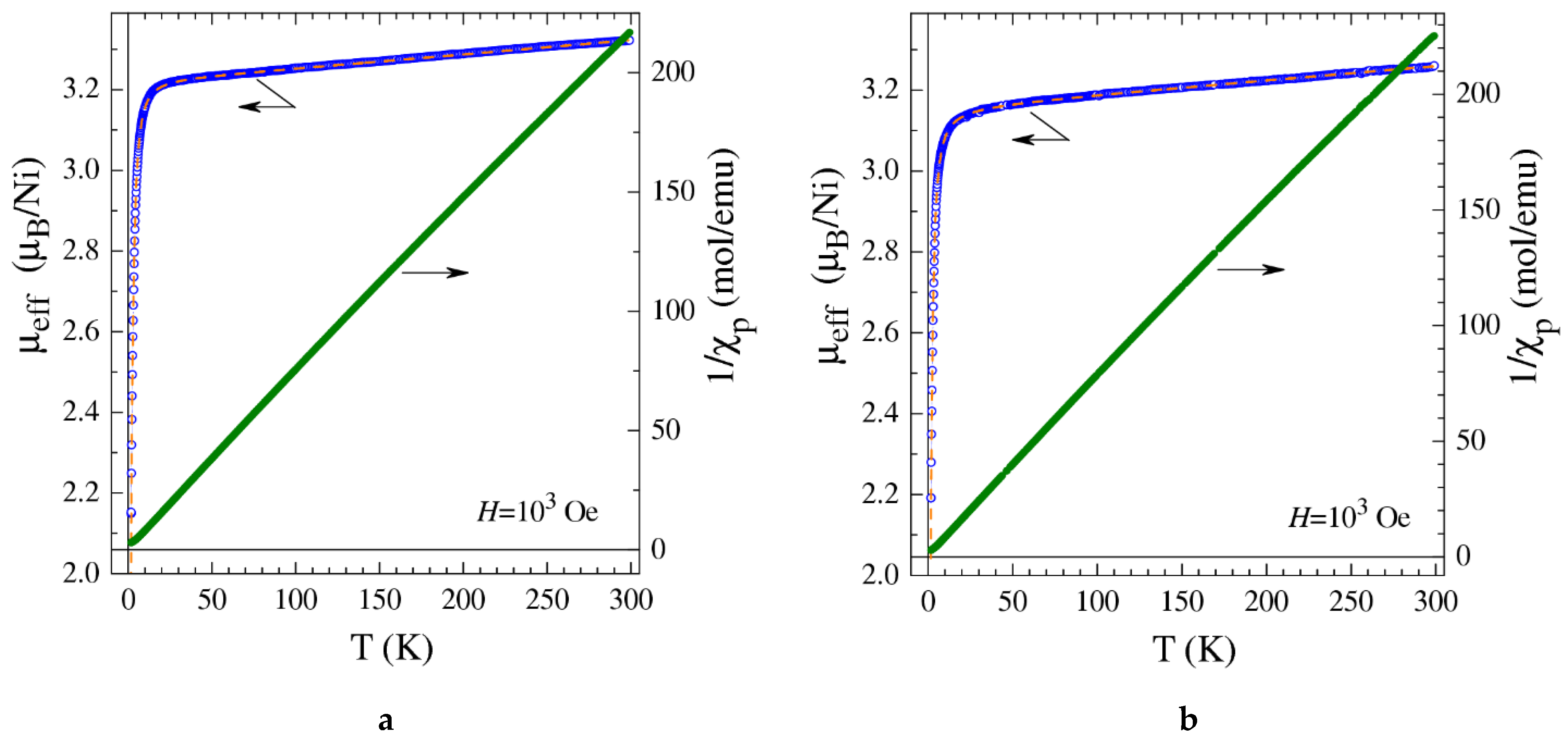
| Sample | Specific Surface Area/m2·g−1 | Vpore/cm3·g−1 | Vads(CO2) a/cm3(STP)·g−1 |
|---|---|---|---|
| 1a | 36.0 b/511 c | 0.007 b/0.103 a | 81.8 |
| 2a | 24.4 b/377 c | 0.006 b/0.076 a | 60.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demakov, P.A.; Kovalenko, K.A.; Lavrov, A.N.; Fedin, V.P. Flexible Co(II)-and Ni(II)-Based Cationic 2D Metal–Organic Frameworks Based on a Charge-Neutral (O,O)-Donor Bridge. Inorganics 2023, 11, 259. https://doi.org/10.3390/inorganics11060259
Demakov PA, Kovalenko KA, Lavrov AN, Fedin VP. Flexible Co(II)-and Ni(II)-Based Cationic 2D Metal–Organic Frameworks Based on a Charge-Neutral (O,O)-Donor Bridge. Inorganics. 2023; 11(6):259. https://doi.org/10.3390/inorganics11060259
Chicago/Turabian StyleDemakov, Pavel A., Konstantin A. Kovalenko, Alexander N. Lavrov, and Vladimir P. Fedin. 2023. "Flexible Co(II)-and Ni(II)-Based Cationic 2D Metal–Organic Frameworks Based on a Charge-Neutral (O,O)-Donor Bridge" Inorganics 11, no. 6: 259. https://doi.org/10.3390/inorganics11060259





