Unveiling the Thermoelectric Performances of Zn1−xFexSe Nanoparticles Prepared by the Hydrothermal Method
Abstract
:1. Introduction
2. Results and Discussion
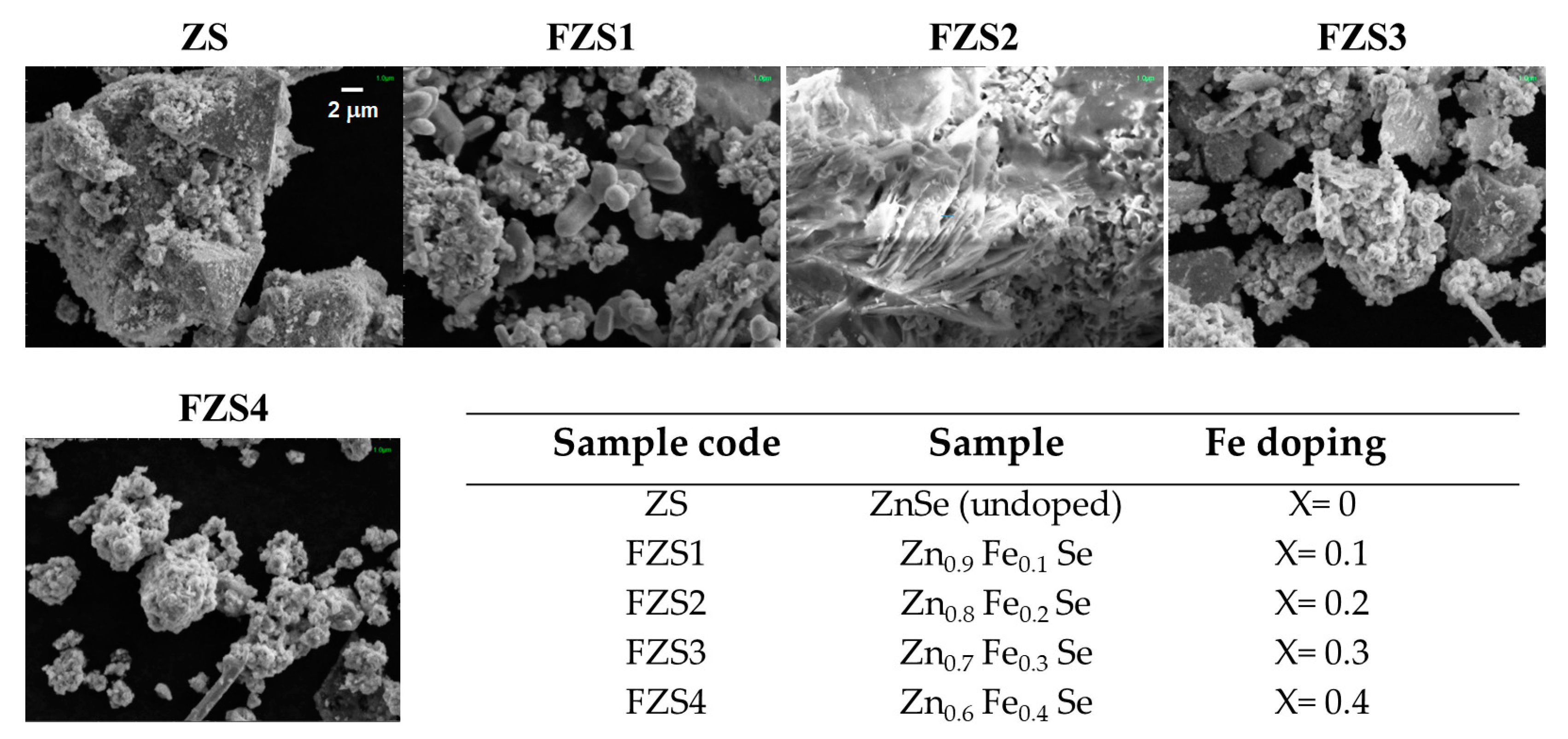
2.1. Scanning Electron Microscopy Investigation
2.2. X-ray Diffraction Study
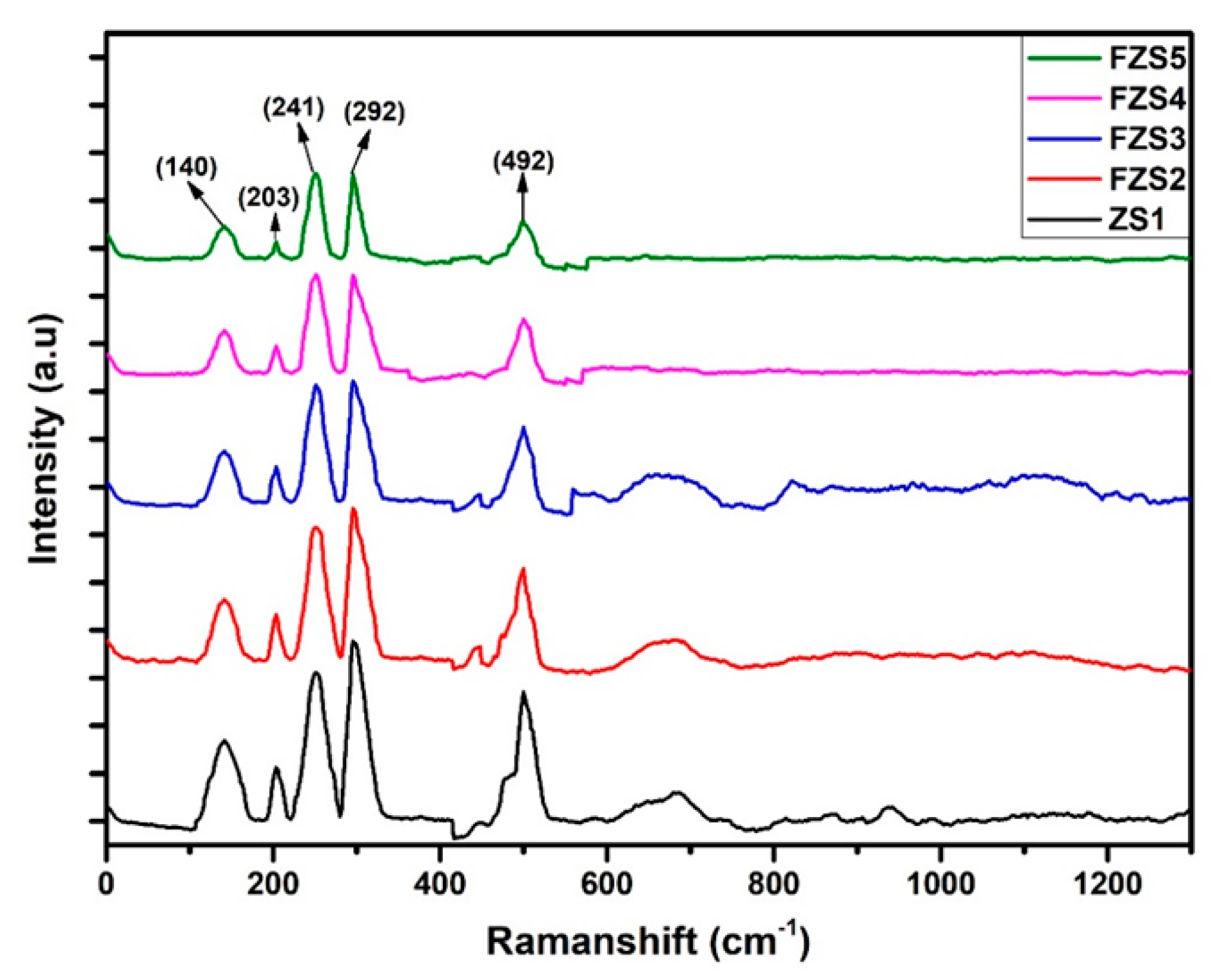
2.3. Raman Characterization
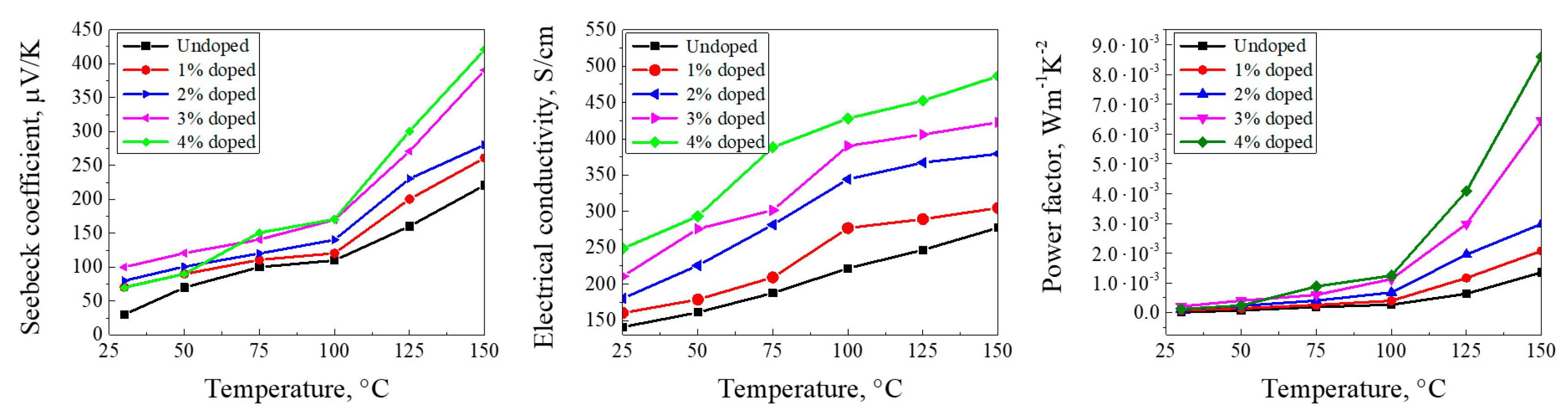
2.4. Electrical and Thermoelectric Measurements
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Q.; Li, H.; Ma, Y.; Zhai, T. ZnSe nanostructures: Synthesis, properties and applications. Prog. Mater. Sci. 2016, 83, 472–535. [Google Scholar] [CrossRef]
- Feng, B.; Cao, J.; Yang, J.; Yang, S.; Han, D. Characterization and photocatalytic activity of ZnSe nanoparticles synthesized by a facile solvothermal method, and the effects of different solvents on these properties. Mater. Res. Bull. 2014, 60, 794–801. [Google Scholar] [CrossRef]
- Elsaeedy, H.I.; Hassan, A.A.; Yakout, H.A.; Qasem, A. The significant role of ZnSe layer thickness in optimizing the performance of ZnSe/CdTe solar cell for optoelectronic applications. Opt. Laser Technol. 2021, 141, 107139. [Google Scholar] [CrossRef]
- Benstaali, W.; Bentata, S.; Abbad, A.; Belaidi, A. Ab-initio study of magnetic, electronic and optical properties of ZnSe doped-transition metals. Mater. Sci. Semicond. Process. 2013, 6, 231–237. [Google Scholar] [CrossRef]
- Godlewski, M.; Guziewicz, E.; Kopalko, K.; Łusakowska, E.; Dynowska, E.; Godlewski, M.M.; Goldys, E.; Phillips, M.R. Origin of white color light emission in ALE-grown ZnSe. J. Lumin. 2003, 102, 455–459. [Google Scholar] [CrossRef]
- Lin, T.K.; Chang, S.J.; Chiou, Y.Z.; Wang, C.K.; Chang, S.P.; Lam, K.T.; Sun, Y.S. Homoepitaxial ZnSe MIS photodetectors with SiO2 and BST insulator layers. SolidState Electron. 2006, 50, 750. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Li, X. Ultrasensitive NO2 Detection Utilizing Mesoporous ZnSe/ZnO Heterojunction-Based Chemiresistive-Type Sensors. ACS Appl. Mater. Interfaces 2019, 11, 29029–29040. [Google Scholar] [CrossRef]
- Li, P.; He, T. Recent advances in zinc chalcogenide-based nanocatalysts for photocatalytic reduction of CO2. J. Mater. Chem. A 2021, 9, 23364–23381. [Google Scholar] [CrossRef]
- Gul, S.; Cooper, J.K.; Glans, P.-A.; Guo, J.; Yachandra, V.K.; Yano, J.; Zhang, J.Z. Effect of Al3+ co-doping on the dopant local structure, optical properties, and exciton dynamics in Cu+-doped ZnSe nanocrystals. ACS Nano 2013, 7, 8680–8692. [Google Scholar] [CrossRef]
- Norris, D.J.; Efros, A.L.; Erwin, S.C. Doped nanocrystals. Science 2008, 319, 1776–1779. [Google Scholar] [CrossRef]
- Chen, M.; Cui, X.; Xiao, X.; Xu, Y.; Cui, J.; Guo, J.; Liu, C.; Guo, H. Mid-infrared emission of transition metal Co2+-doped ZnSe nanocrystals at room temperature via hydrothermal preparation. ACS Appl. Nano Mater. 2019, 2, 2844–2853. [Google Scholar] [CrossRef]
- Myoung, N.; Martyshkin, D.V.; Fedorov, V.V.; Sergey, B. Energy scaling of 4.3 μm room temperature Fe: ZnSe laser. Opt. Lett. 2011, 36, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- DiSalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Zou, J.; Chen, Z.-G. Advanced thermoelectric design: From materials and structures to devices. Chem. Rev. 2020, 120, 7399–7515. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Lo, S.-H.; Zhang, Y.; Sun, H.; Tan, G.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature 2014, 508, 373–377. [Google Scholar] [CrossRef]
- Chang, C.; Wu, M.H.; He, D.S.; Pei, Y.L.; Wu, C.F.; Wu, X.F.; Yu, H.L.; Zhu, F.Y.; Wang, K.D.; Chen, Y.; et al. 3D charge and 2D phonon transports leading to high out-of-plane ZT in n-type SnSe crystals. Science 2018, 360, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, M.Y.R.; Lee, H.; Wang, D.; Ren, Z.; Fleurial, J.P.; Gogna, P. Nanocomposites to enhance ZT in thermoelectrics. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Prete, D.; Erdman, P.A.; Demontis, V.; Zannier, V.; Ercolani, D.; Sorba, L.; Beltram, F.; Rossella, F.; Taddei, F.; Roddaro, S. Thermoelectric conversion at 30 K in InAs/InP nanowire quantum dots. Nano Lett. 2019, 19, 3033–3039. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Lee, J.; Lee, W.; Li, D. Thermoelectrics of nanowires. Chem. Rev. 2019, 119, 9260–9302. [Google Scholar] [CrossRef]
- Peri, L.; Prete, D.; Demontis, V.; Zannier, V.; Rossi, F.; Sorba, L.; Beltram, F.; Rossella, F. Giant reduction of thermal conductivity and enhancement of thermoelectric performance in twinning superlattice InAsSb nanowires. Nano Energy 2022, 103 Pt A, 107700. [Google Scholar] [CrossRef]
- Rocci, M.; Demontis, V.; Prete, D.; Ercolani, D.; Sorba, L.; Beltram, F.; Pennelli, G.; Roddaro, S.; Rossella, F. Suspended InAs Nanowire-Based Devices for Thermal Conductivity Measurement Using the 3 ω Method. J. Mater. Eng. Perform. 2018, 27, 6299–6305. [Google Scholar] [CrossRef]
- Prete, D.; Dimaggio, E.; Demontis, V.; Zannier, V.; Rodriguez-Douton, M.J.; Guazzelli, L.; Beltram, F.; Sorba, L.; Pennelli, G.; Rossella, F. Electrostatic Control of the Thermoelectric Figure of Merit in Ion-Gated Nanotransistors. Adv. Funct. Mater. 2021, 31, 2104175. [Google Scholar] [CrossRef]
- Flamee, S.; Dierick, R.; Cirillo, M.; Van Genechten, D.; Aubertab, T.; Hens, Z. Synthesis of metal selenide colloidal nanocrystals by the hot injection of selenium powder. Dalton Trans. 2013, 42, 12654–12661. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Sun, C.C.; Xue, C.; Jiang, Y.T.; Zhang, J.; Zhao, L.J. Structure and optical properties of iron doped ZnSe microspheres. Opt. Mater. 2021, 114, 110989. [Google Scholar] [CrossRef]
- Liu, X.Y.; Mo, M.S.; Zeng, J.H.; Qian, Y.T. Large-scale synthesis of ultra-long wire-like single-crystal selenium arrays. J. Cryst. Growth 2003, 259, 144. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Li, L. Study on Raman spectra of zinc selenide nanopowders synthesized by hydrothermal method. J. Alloys Compd. 2010, 506, 327–330. [Google Scholar] [CrossRef]
- Qiao, F.; Qi, Q.; Wang, Z.; Xu, K.; Ai, S. Double enzymatic cascade reactions within FeSe–Pt@ SiO2 nanospheres: Synthesis and application toward colorimetric biosensing of H2O2 and glucose. Analyst 2015, 140, 6684–6691. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibsong, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Wiley: Hoboken, NJ, USA, 1974; Chapter 9. [Google Scholar]
- Javid, M.A.; Rafi, M.; Ali, I.; Hussain, F.; Imran, M.; Ali, N. Synthesis and study of structural properties of Sn doped ZnO nanoparticles. Mater. Sci. Pol. 2016, 34, 741–746. [Google Scholar] [CrossRef] [Green Version]
- Yon, V.; Rochat, N.; Charles, M.; Nolot, E.; Gergaud, P. X-ray Diffraction Microstrain Analysis for Extraction of Threading Dislocation Density of GaN Films Grown on Silicon, Sapphire, and SiC Substrates. Phys. Status Solidi B 2020, 257, 1900579. [Google Scholar] [CrossRef]
- Kasar, R.R.; Deshpande, N.G.; Gudage, Y.G.; Vyas, J.C.; Sharma, R. Studies and correlation among the structural, optical and electrical parameters of spray-deposited tin oxide (SnO2) thin films with different substrate temperatures. Phys. B Condens. Matter 2008, 403, 3724–3729. [Google Scholar] [CrossRef]
- Divya, R.; Manikandan, N.; Girisun, T.S.; Vinitha, G. Investigations on the structural, morphological, linear and third order nonlinear optical properties of manganese doped zinc selenide nanoparticles for optical limiting application. Opt. Mater. 2020, 100, 109641. [Google Scholar] [CrossRef]
- Yadav, K.; Dwivedi, Y.; Jaggi, N. Structural and optical properties of Ni doped ZnSe nanoparticles. J. Lumin. 2015, 158, 181–187. [Google Scholar] [CrossRef]
- Bellucci, S.; Chiaretti, M.; Onorato, P.; Rossella, F.; Grandi, M.; Galinetto, P.; Sacco, I.; Micciulla, F. Micro-Raman study of the role of sterilization on carbon nanotubes for biomedical applications. Nanomedicine 2010, 5, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Caridad, J.; Closkey, D.; Rossella, F.; Bellani, V.; Donegan, J.; Krstić, V. Effective wavelength scaling of and damping in plasmonic helical antennae. ACS Photonics 2015, 2, 675–679. [Google Scholar] [CrossRef]
- Caridad, J.; Rossella, F.; Bellani, V.; Grandi, M.; Diez, E. Automated detection and characterization of graphene and few-layer graphite via Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 286–293. [Google Scholar] [CrossRef]
- Taylor, W. The Raman spectra of cubic zinc selenide and telluride. Phys. Lett. A 1967, 24, 556–558. [Google Scholar] [CrossRef]
- Hoffmann, A.; Heitz, R.; Broser, I. Fe3+ as near-infrared luminescence center in ZnS. Phys. Rev. B 1990, 41, 5806–5816. [Google Scholar] [CrossRef]
- Liang, B.; Hou, L.P.; Zou, S.Y.; Zhang, L.; Guo, Y.C.; Liu, Y.T.; Farooq, M.U.; Shi, L.J.; Liu, R.B.; Zou, B.S. The aggregation of Fe3+ and their d–d radiative transitions in ZnSe: Fe3+ nanobelts by CVD growth. RSC Adv. 2018, 8, 3133–3139. [Google Scholar] [CrossRef]
- Liu, Y.; Calcabrini, M.; Yu, Y.; Lee, S.; Chang, C.; David, J.; Ghosh, T.; Spadaro, M.C.; Xie, C.; Cojocaru-Mirédin, O.; et al. Defect Engineering in Solution-Processed Polycrystalline SnSe Leads to High Thermoelectric Performance. ACS Nano 2022, 25, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.H.; Wei, M.; Luo, J.T.; Li, F.; Liang, G.X.; Liang, Y.; Hao, J.; Ma, H.L.; Zhang, X.H.; Fan, P. An enhanced power factor via multilayer growth of Ag-doped skutterudite CoSb3 thin films. Inorg. Chem. Front. 2018, 5, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Neophytou, N.; Zianni, X.; Kosina, H.; Frabboni, S.; Lorenzi, B.; Narducci, D. Simultaneous increase in electrical conductivity and Seebeck coefficient in highly boron-doped nanocrystaline Si. Nanotechnology 2013, 24, 205402. [Google Scholar] [CrossRef]
- Ali, H.T.; Jacob, J.; Isram, M.; Mahmood, K.; Yusuf, M.; Mehboob, K.; Ikram, S.; Amin, N.; Javaid, K. Effect of Aluminum (Al) Concentration on the Thermoelectric Performance of Zinc Aluminum Selenium (ZnAlSe) Alloy. J. Supercond. Nov. Magn. 2021, 34, 2199–2206. [Google Scholar] [CrossRef]
- Jacob, J.; Mahmood, K.; Usman, M.Y.; Rehman, U.; Ali, A.; Ashfaq, A.; Amin, N.; Ikram, S.; Hussain, S. Modulation of thermoelectric properties of bulk ZnAlO by annealing in oxygen environment. Phys. B Condens. Matter 2019, 572, 247–250. [Google Scholar] [CrossRef]
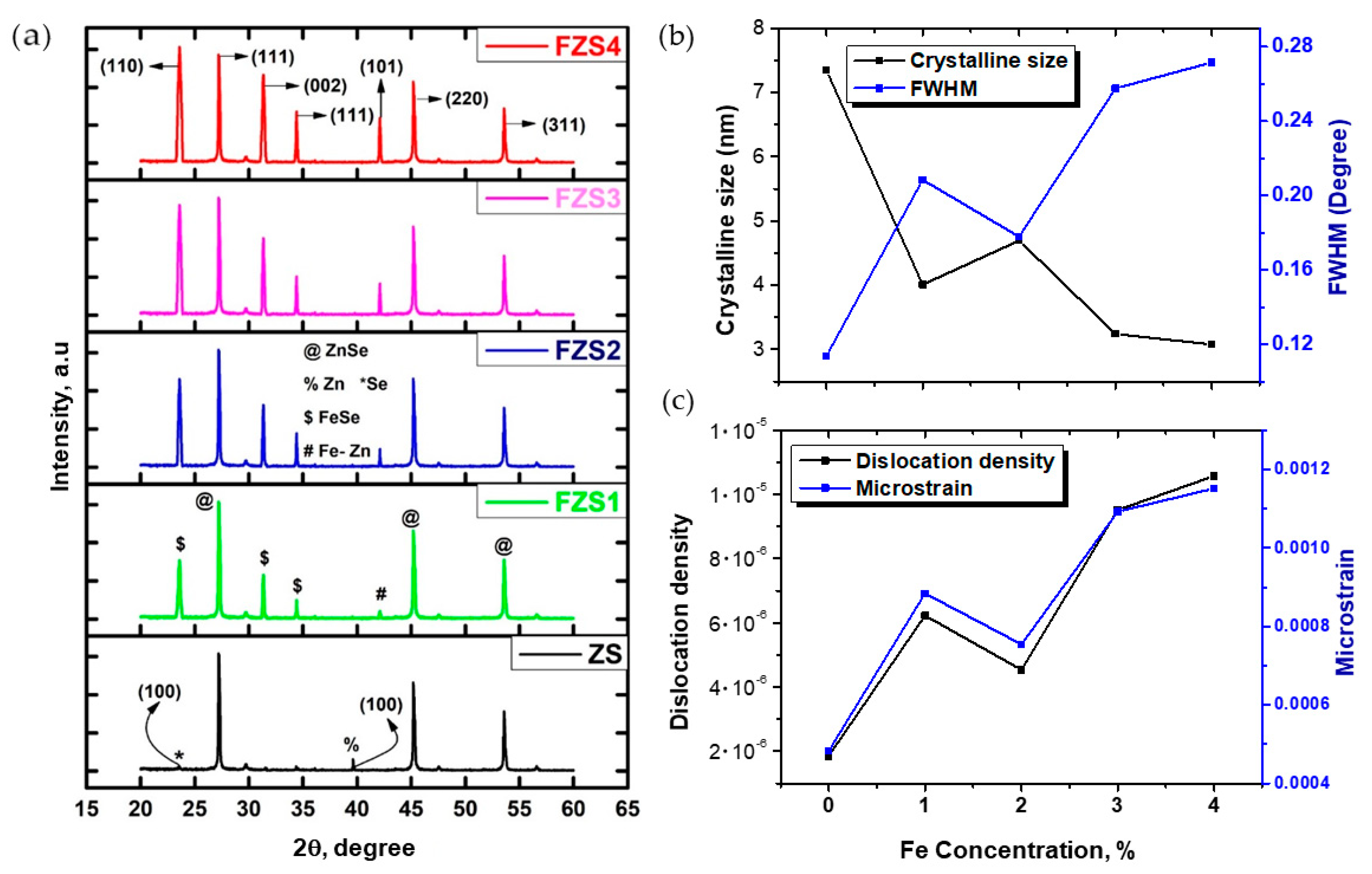
| Sample | FWHM (Degree) | Crystalline Size (D) (nm) | Interplanar Spacing (dhkl) (Å) | Dislocation Density (δ) 10− (Line m−2) | Microstrain (ε) 10−4 (Line −2 m−4) |
|---|---|---|---|---|---|
| ZS1 | 0.11 | 7.34 | 3.27 | 1.85 | 4.82 |
| FZS1 | 0.20 | 4.01 | 3.27 | 6.23 | 8.84 |
| FZS2 | 0.17 | 4.70 | 3.27 | 4.54 | 7.54 |
| FZS3 | 0.26 | 3.24 | 3.27 | 9.51 | 10.92 |
| FZS4 | 0.27 | 3.08 | 3.27 | 10.57 | 11.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isram, M.; Demontis, V.; Magrin Maffei, R.; Abbas Khan, N.; di Bona, A.; Benedetti, S.; Amin, N.; Mahmood, K.; Rossella, F. Unveiling the Thermoelectric Performances of Zn1−xFexSe Nanoparticles Prepared by the Hydrothermal Method. Inorganics 2023, 11, 286. https://doi.org/10.3390/inorganics11070286
Isram M, Demontis V, Magrin Maffei R, Abbas Khan N, di Bona A, Benedetti S, Amin N, Mahmood K, Rossella F. Unveiling the Thermoelectric Performances of Zn1−xFexSe Nanoparticles Prepared by the Hydrothermal Method. Inorganics. 2023; 11(7):286. https://doi.org/10.3390/inorganics11070286
Chicago/Turabian StyleIsram, Muhammad, Valeria Demontis, Riccardo Magrin Maffei, Najaf Abbas Khan, Alessandro di Bona, Stefania Benedetti, Nasir Amin, Khalid Mahmood, and Francesco Rossella. 2023. "Unveiling the Thermoelectric Performances of Zn1−xFexSe Nanoparticles Prepared by the Hydrothermal Method" Inorganics 11, no. 7: 286. https://doi.org/10.3390/inorganics11070286






