Review on the Chemistry of [M(NH3)n](XO4)m (M = Transition Metal, X = Mn, Tc or Re, n = 1–6, m = 1–3) Ammine Complexes
Abstract
:1. Introduction
2. Discussion
2.1. General Consideration on the Synthesis of Ammonia Complexed Transition Metal Tetraoxometallates (XO4−, X = Mn, Tc, Re)
- The low concentration of permanganate ion in the solution is advantageous and can easily reach if the permanganate complex prepared is sparingly soluble. In this case, the decreasing solubility with the salting out effect by using the excess of the most soluble salt (chloride, nitrate) of the complex cation and decreasing the temperature is advantageous. A starting salt in a metathesis reaction with permanganates (transition metal ammonia complex salt, e.g., chloride, nitrate, etc.) is to be selected among the most soluble salts and used as a concentrated solution as possible;
- The decrease in temperature diminishes the oxidation ability of permanganate ions and generally decreases the solubility, thus acting as a main driving force in the removal of the oxidizing anion from the solution. Therefore, the typical synthesis can be conducted at and below room temperature, with immediate cooling of the solution until freezing;
- The excess ammonia increases the pH and increases the concentration of the ammonia-complexed cations in the solution; thus, using the ammonia in excess is also a key step in these syntheses. It is the most important factor in decreasing the oxidation ability of permanganate ions.
2.2. Diammine Complexes
2.2.1. Preparation and Properties of Diamminesilver(I) Permanganate [Ag(NH3)2]MnO4
2.2.2. Diamminesilver(I) Perrhenate
2.2.3. [Diamminezinc(II)], [Diamminecadmium(II)], [Diamminecopper(II)], and [Diamminenickel(II)] Perrhenates, [M(NH3)2](ReO4)2 (M = Zn, Cd, Ni)
2.3. Triammine Complexes
Preparation and Properties of [Triamminesilver(I) Permanganate, [Ag(NH3)3]MnO4
2.4. Tetraammine Complexes
2.4.1. Tetraamminecopper(II) Permanganate
–4NH3, 3013–423 K –2O2, 423–773 K
2.4.2. Tetraamminezinc(II) and [Tetraamminecadmium(II)] Permanganates
2.4.3. [Tetraamminecopper(II)], [Tetraamminezinc(II)], and [Tetraamminecadmium(II)] Perrhenates
2.4.4. [Tetraamminenickel(II)] and [Tetraamminecobalt(II)] Perrhenates, [M(NH3)4](ReO4)2 (M = Ni, Co)
2.4.5. [Co(NH3)4CO3]MnO4
2.4.6. [Tetraamminemetal(II)] Permanganates, Pertechnetates, and Perrhenates of Platinum Group Metals, [M(NH3)4](XO4)2 (M = Pt, Pd, X= Mn, Tc, Re) and [Ru(NO)(OH)(NH3)4](ReO4)2
2.5. Pentaammine Complexes
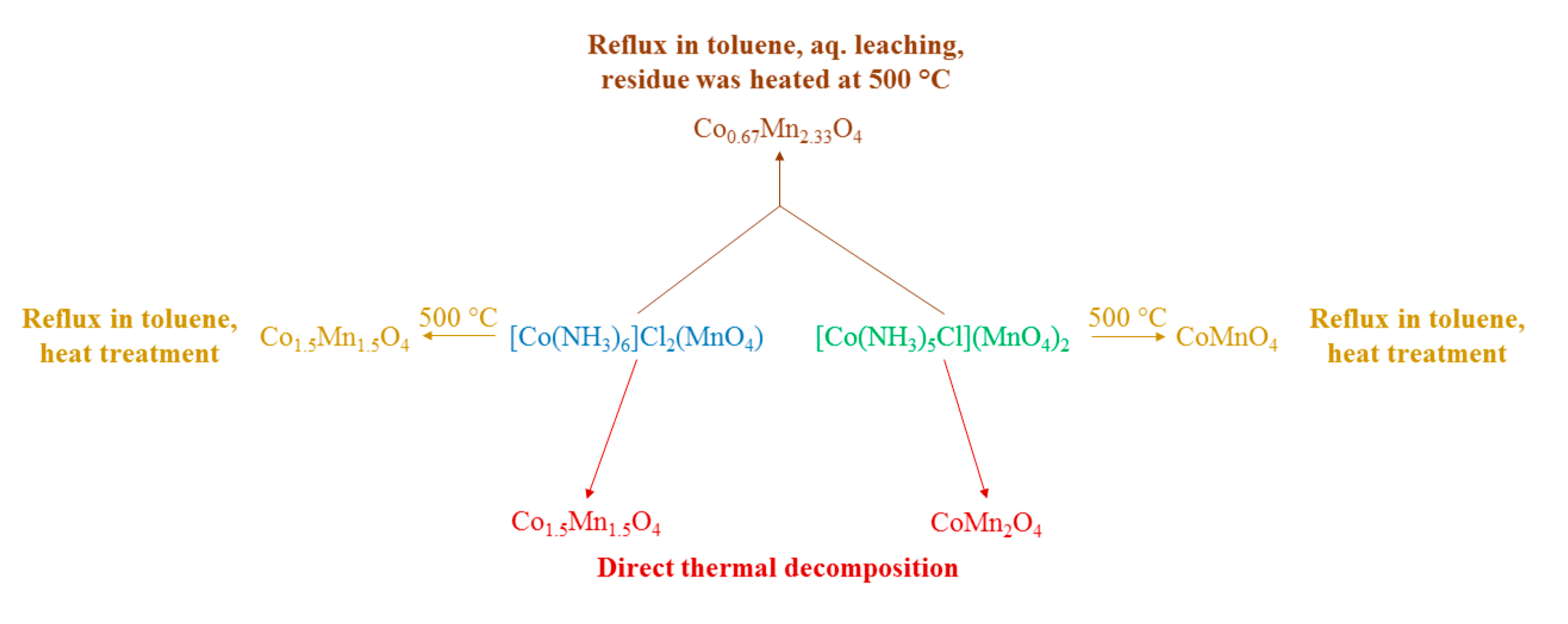
2.5.1. [(Chlorido)pentamminecobalt(III)] Permanganate
2.5.2. [Pentamminechlorometal(III)] Perrhenates, [M(NH3)5Cl](ReO4)2 (M = Co, Cr, Ru, Rh, Ir)
2.5.3. [Pentammine(aquo)cobalt(III)] Perrhenate Dihydrate
2.5.4. [Perrhenatopentamminecobalt(III)] Perrhenate, [Co(NH3)5OReO3](ReO4)2
2.5.5. [Co(NH3)5ReO4]X2 (X = Cl, ClO4, NO3)
2.5.6. [Pentaamminechloroplatinum(IV)] Perrhenate
2.6. Hexammine Complexes
2.6.1. [Ni(NH3)6](MnO4)2
2.6.2. [Hexaamminenickel(II)] Perrhenate, [Ni(NH3)6](ReO4)2
2.6.3. [Hexaamminemetal(III)] Permanganate, Pertechnetate, and Perrhenate, [M(NH3)6](XO4)3, (M = Cr, X = Mn, Re), M = Co, X = Tc, Re)
2.6.4. [Hexaammincobalt(III)] Permanganate, [Co(NH3)6](MnO4)3
2.6.5. [Hexaamminecobalt(III)] Dichloride Permanganate and [Hexaammincobalt(III)] dibromide Permanganate, [Co(NH3)6]X2(MnO4), X = Cl, Br
2.6.6. Potassium [Hexaamminecobalt(III)] Dichloride Dipermanganate
3. Conclusions
Funding
Conflicts of Interest
References
- Lazarenko, G.A.; Neokladnova, L.N.; Shablovskaya, S.F. Effect of metals on the thermal decomposition of cobalt(III) hexaammine complexes with a metal-containing anion. Koord. Khimiya 1981, 7, 1485–1488. [Google Scholar]
- Lagunova, V.I.; Filatov, E.Y.; Plyusnin, P.E.; Korenev, S.V. In Situ and Ex Situ Studies of Tetrammineplatinum(II) Chromate Thermolysis. Russ. J. Inorg. Chem. 2020, 65, 1566–1570. [Google Scholar] [CrossRef]
- Serebrennikova, P.S.; Komarov, V.Y.; Sukhikh, A.S.; Khranenko, S.P.; Zadesenets, A.V.; Gromilov, S.A.; Yusenko, K.V. [Nien3](MoO4)0.5(WO4)0.5 co-crystals as single-source precursors for ternary refractory Ni–Mo–W alloys. Nanomaterials 2021, 11, 3272. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.G.; Mogk, J.; Conrad, M.; Kraus, F. Octaammine EuII and YbII Azides and Their Thermal Decompositions to the Nitrides. Eur. J. Inorg. Chem. 2016, 26, 4162–4169. [Google Scholar] [CrossRef]
- Domonov, D.P.; Pechenyuk, S.I.; Semushina, Y.P.; Yusenko, K.V. Solid-state transformations in inner coordination sphere of [Co(NH3)6][Fe(C2O4)3]·3H2O as a route to access catalytically active Co-Fe materials. Materials 2019, 12, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusenko, K.V.; Pechenyuk, S.I.; Vikulova, E.S.; Semushina, Y.P.; Baidina, I.A.; Filatov, E.Y. Isostructurality and Thermal Properties in the Series of Double Complex Salts [M1(NH3)6][M2(C2O4)3]·3H2O (M1 = Co, Ir, M2 = Fe, Cr). J. Struct. Chem. 2019, 60, 1062–1071. [Google Scholar] [CrossRef]
- Farhadi, S.; Pourzare, K.; Bazgir, S. Co3O4 nanoplates: Synthesis, characterization and study of optical and magnetic properties. J. Alloys Compd. 2014, 587, 632–637. [Google Scholar] [CrossRef]
- Garkul’, I.A.; Zadesenets, A.V.; Plyusnin, P.E.; Filatov, E.Y.; Asanova, T.I.; Kozlov, D.V.; Korenev, S.V. Zinc(II) and Manganese(II) Oxalatopalladates as Precursors of Bimetallic Nanomaterials. Russ. J. Inorg. Chem. 2020, 65, 1571–1576. [Google Scholar] [CrossRef]
- Ilyushin, M.A.; Shugalei, I.V.; Tverjanovich, A.S.; Smirnov, A.V. Influence of the Mechanism of the Initial Stages of the Ligand Decomposition on the Initiating Ability of Cobalt(III) Ammine Tetrazolate Complexes. Russ. J. Inorg. Chem. 2020, 90, 640–647. [Google Scholar] [CrossRef]
- Béres, K.A.; Szilágyi, F.; Homonnay, Z.; Dürvanger, Z.; Bereczki, L.; Trif, L.; Petruševski, V.M.; Farkas, A.; Bayat, N.; Kótai, L. Structural, Spectroscopic, and Thermal Decomposition Features of [Carbonatotetraamminecobalt(III)] Iodide—Insight into the Simultaneous Solid-Phase Quasi-Intramolecular Redox Reactions. Inorganics 2023, 11, 68. [Google Scholar] [CrossRef]
- Béres, K.A.; Homonnay, Z.; Kvitek, L.; Dürvanger, Z.; Kubikova, M.; Harmat, V.; Szilágyi, F.; Czégény, Z.; Németh, P.; Bereczki, L.; et al. Thermally Induced Solid-Phase Quasi-Intramolecular Redox Reactions of [Hexakis(urea-O)iron(III)] Permanganate: An Easy Reaction Route to Prepare Potential (Fe,Mn)Ox Catalysts for CO2 Hydrogenation. Inorg. Chem. 2022, 61, 14403–14418. [Google Scholar] [CrossRef] [PubMed]
- Franguelli, F.P.; Béres, K.A.; Kótai, L. Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds. Inorganics 2021, 9, 79. [Google Scholar] [CrossRef]
- Holló, B.B.; Petruševski, V.M.; Kovács, G.B.; Franguelli, F.P.; Farkas, A.; Menyhárd, A.; Lendvay, G.; Sajó, I.E.; Nagy-Bereczki, L.; Pawar, R.P.; et al. Thermal and spectroscopic studies on a double-salt-type pyridine–silver perchlorate complex having κ1-O coordinated perchlorate ions. J. Therm. Anal. Calorim. 2019, 138, 1193–1205. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, G.B.; May, N.V.; Bombicz, P.A.; Klébert, S.; Németh, P.; Menyhárd, A.; Novodárszki, G.; Petrusevski, V.; Franguelli, F.P.; Magyari, J.; et al. An unknown component of a selective and mild oxidant: Structure and oxidative ability of a double salt-type complex having κ1O-coordinated permanganate anions and three- and four-fold coordinated silver cations. RSC Adv. 2019, 9, 28387–28398. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, T.; Magyari, J.; Sajo, I.E.; Pasinszki, T.; Homonnay, Z.; Szilagyi, I.M.; Farkas, A.; May, Z.; Effenberger, H.; Szakall, S.; et al. Evidence of quasi-intramolecular redox reactions during thermal decomposition of ammonium hydroxodisulfitoferriate(III), (NH4)2[Fe(OH)(SO3)2]·H2O. J. Therm. Anal. Calorim. 2018, 132, 493–502. [Google Scholar] [CrossRef] [Green Version]
- Sajó, I.E.; Kovács, G.B.; Pasinszki, T.; Bombicz, A.P.; May, Z.; Szilágyi, I.M.; Jánosity, A.; Banerji, K.K.; Kant, R.; Kótai, L. The chemical identity of ‘[Ag(py)2]MnO4‘ organic solvent soluble oxidizing agent and new synthetic routes for the preparation of [Ag(py)n]XO4 (X = Mn, Cl, Re, n = 2–4) complexes. J. Coord. Chem. 2018, 71, 2884–2904. [Google Scholar] [CrossRef] [Green Version]
- Korenev, S.V.; Venediktov, Y.V.; Shubin, Y.V.; Gromilov, S.A.; Yusenko, K.V. Synthesis and Structure of Binary Complexes of Platinum Group Metals—Precursors of Metallic Materials. J. Struct. Chem. 2003, 44, 45–59. [Google Scholar] [CrossRef]
- Chakravorti, M.C. The chemistry of coordinated perrhenateperrhenate (ReO4−). Coord. Chem. Revs. 1990, 106, 205–225. [Google Scholar] [CrossRef]
- Mansouri, M.; Atashi, H.; Tabrizi, F.F.; Mirzaei, A.A.; Mansouri, G. Kinetics studies of nano-structured cobalt-manganese oxide catalysts in Fischer–Tropsch synthesis. J. Ind. Eng. Chem. 2013, 19, 1177–1183. [Google Scholar] [CrossRef]
- Mansouri, G.; Mansouri, M. Synthesis and characterization of Co–Mn nanocatalyst prepared by thermal decomposition for Fischer–Tropsch reaction. Iran. J. Chem. Eng. 2018, 37, 1–9. [Google Scholar]
- Béres, K.A.; Homonnay, Z.; Barta Holló, B.; Gracheva, M.; Petruševski, V.M.; Farkas, A.; Dürvanger, Z.; Kótai, L. Synthesis, structure, and Mössbauer spectroscopic studies on the heat-induced solid-phase redox reactions of hexakis(urea-O)iron(III) peroxodisulfate. J. Mater. Res. 2022, 38, 1102–1118. [Google Scholar] [CrossRef]
- Sajo, I.E.; Bakos, L.P.; Szilagyi, I.M.; Lendvay, G.; Magyari, J.; Mohai, M.; Szegedi, A.; Farkas, A.; Janosity, A.; Klebert, S.; et al. Unexpected Sequential NH3/H2O Solid/Gas Phase Ligand Exchange and Quasi-Intramolecular Self-Protonation Yield [NH4Cu(OH)MoO4], a Photocatalyst Misidentified before as (NH4)2Cu(MoO4)2. Inorg. Chem. 2018, 57, 13679–13692. [Google Scholar] [CrossRef] [PubMed]
- Kótai, L.; Fodor, J.; Jakab, E.; Sajó, I.E.; Szabó, P.; Lónyi, F.; Valyon, J.; Gács, I.; Argay, G.Y.; Banerji, K.K. A thermally induced low-temperature intramolecular redox reaction of bis(pyridine)silver(I) permanganate and its hemipyridine solvate. Transit. Met. Chem. 2006, 31, 30–34. [Google Scholar] [CrossRef]
- Kótai, L.; Sajó, I.; Fodor, J.; Szabó, P.; Jakab, E.; Argay, G.Y.; Holly, S.; Gács, I.; Banerji, K.K. Reasons for and consequences of the mysterious behaviour of newly prepared hemipyridine solvate of bis(pyridine)silver(I) permanganate, Agpy2MnO4 0.5py. Transit. Met. Chem. 2005, 30, 939–943. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Filatov, E.Y.; Baidina, I.A.; Yusenko, K.V.; Zadesenetz, A.V.; Korenev, S.V. Synthesis of [M(NH3)5Cl](ReO4)2 (M = Cr, Co, Ru, Rh, Ir) and investigation of thermolysis products. crystal structure of [Rh(NH3)5Cl](ReO4)2. J. Struct. Chem. 2006, 47, 1103–1110. [Google Scholar] [CrossRef]
- Filatov, E.Y.; Shubin, Y.V.; Korenev, S.V. Study of nanoalloys formation mechanism from single-source precursors [M(NH3)5Cl](ReO4)2, M = Rh, Ir. Z. Kristallogr. Suppl. 2007, 26, 283–288. [Google Scholar] [CrossRef]
- Filatov, E.; Shubin, Y.; Sharafutdinov, M. In situ synchrotron X-ray diffraction study of formation mechanism of Rh0.33Re0.67 nanoalloy powder upon thermal decomposition of complex precursor. Z. Kristallogr. Suppl. 2008, 27, 185–192. [Google Scholar] [CrossRef]
- Solt, H.E.; Németh, P.; Mohai, M.; Sajó, I.E.; Klébert, S.; Franguelli, F.P.; Fogaca, L.A.; Pawar, R.P.; Kótai, L. Temperature-Limited Synthesis of Copper Manganites along the Borderline of the Amorphous/Crystalline State and Their Catalytic Activity in CO Oxidation. ACS OMEGA 2021, 6, 1523–1533. [Google Scholar] [CrossRef]
- Kótai, L.; Petruševski, V.M.; Bereczki, L.; Béres, K.A. Catalytic Properties of the Spinel-Like CuxMn3−xO4 Copper Manganese Oxides—An Overview. Catalysts 2023, 13, 129. [Google Scholar] [CrossRef]
- Nockemann, P.; Meyer, G. [Ag(NH3)2]ClO4: Kristallstrukturen, Phasenumwandlung, Schwingungsspektren. Z. Anorg. Allg. Chem. 2002, 628, 1636–1640. [Google Scholar] [CrossRef]
- Górska, N.; Mikuli, E.; Kótai, L. Spectroscopic, Structural and Thermal Characterization of Crystalline [Cr(OC(NH2)2)6]X3 (X = CIO4, BF4 and CI) Complexes. Eur. Chem. Bull. 2014, 3, 474–481. [Google Scholar]
- Bereczki, L.; Fogaca, L.A.; Durvanger, Z.; Harmat, V.; Kamaras, K.; Nemeth, G.; Hollo, B.B.; Petrusevski, V.M.; Bodis, E.; Farkas, A.; et al. Dynamic disorder in the high-temperature polymorph of bis[diamminesilver(I)] sulfate-reasons and consequences of simultaneous ammonia release from two different polymorphs. J. Coord. Chem. 2021, 74, 2144–2162. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Barta-Holló, B.; Petruševski, V.M.; Sajó, I.E.; Klébert, S.; Farkas, A.; Bódis, E.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal decomposition and spectral characterization of di[carbonatotetraamminecobalt(III)] sulfate trihydrate and the nature of its thermal decomposition products. J. Therm. Anal. Calorim. 2021, 145, 2907–2923. [Google Scholar] [CrossRef]
- Fogaca, L.; Éva, K.; Németh, G.; Kamarás, K.; Béres, K.A.; Németh, P.; Petruševski, V.; Bereczki, L.; Barta-Holló, B.; Sajó, I.E.; et al. Solid-Phase Quasi-Intramolecular Redox Reaction of [Ag(NH3)2]MnO4: An Easy Way to Prepare Pure AgMnO2. Inorg. Chem. 2021, 60, 3749–3760. [Google Scholar] [CrossRef] [PubMed]
- Hillebrecht, H.; Thiele, G.; Koppenhöfer, A.; Vahrenkamp, H. Kristallstruktur und Schwingungsspektren von Zn(NH3)4(ClO4)2/Crystal Structure and Vibrational Spectra of Zn(NH3)4(ClO4)2. Z. Naturforsch. 1994, 49B, 1163–1168. [Google Scholar] [CrossRef]
- Béres, K.A.; Sajó, I.E.; Lendvay, G.; Trif, L.; Petruševski, V.M.; Barta-Holló, B.; Korecz, L.; Franguelli, F.P.; László, K.; Szilágyi, I.M.; et al. Solid-Phase “Self-Hydrolysis” of [Zn(NH3)4MoO4@2H2O] Involving Enclathrated Water—An Easy Route to a Layered Basic Ammonium Zinc Molybdate Coordination Polymer. Molecules 2021, 26, 4022. [Google Scholar] [CrossRef]
- Sajó, I.E.; Kótai, L.; Keresztury, G.; Gács, I.; Pokol, G.Y.; Kristóf, J.; Soptrayanov, B.; Petrusevski, V.M.; Timpu, D.; Sharma, P.K. Studies on the chemistry of tetraamminezinc(II) dipermanganate ([Zn(NH3)4](MnO4)2). Low-temperature synthesis of the manganese zinc oxide (ZnMn2O4) catalyst precursor. Helv. Chim. Acta 2008, 91, 1646–1658. [Google Scholar] [CrossRef]
- Firozabadi, H.; Sardarian, A.R. An Efficient Conversion of Oximes to Their Corresponding Carbonyl Compounds with Bispyridinesilver Permanganate Under Mild Conditions. Synth. Commun. 1983, 13, 863–866. [Google Scholar] [CrossRef]
- Gulevskaya, A.V.; Pozharskii, A.F.; Lomachenkova, L.V. Chichibabin amination of 1,3-dimethyllumazine. Chem. Heterocycl. Compd. 1990, 26, 1316–1317. [Google Scholar] [CrossRef]
- Besedin, D.V.; Gulevskaya, A.V.; Pozharskii, A.F. Reaction of 6,8-dimethylpyrimido [4,5-c]pyridazine-5,7(6H,8H)-dione with α,ω-diamines as the first example of tandem nucleophilic substitution in neutral azines. Mendeleev. Commun. 2000, 10, 150–151. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Vessal, B.; Naderi, M. Bispyridinesilver permanganate[Ag(C5H5N)2]MnO4: An efficient oxidizing reagent for organic substrates. Tetrahedron Lett. 1982, 23, 1847–1850. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Sardarian, A.R. Facile Oxidation of Polycyclic Arenes and Acetylenic Hydrocarbons with Bis(pyridine)silver Permanganate and Bis(2,2′-bipyridyl)copper(II) Permanganate Under Mild and Neutral Conditions. Synthesis 1986, 11, 946–948. [Google Scholar] [CrossRef]
- Gulevskaya, A.V.; Maes, B.U.W.; Meyers, C. A Novel Synthetic Route to 2-Amino and 2-Alkylamino-1,3,5-Triazines Based on Nucleophilic Aromatic Substitution of Hydrogen: The First Reactions of 1,3,5-Triazine with Nucleophiles without Ring Decomposition. SYNLETT 2007, 1, 71–74. [Google Scholar] [CrossRef]
- Chandra, R.; Sarkar, A. A Facile Oxidation of 3,4-Diphenylthiophene-2,5-Dimethanol and Furan-2,5-Dimethanol to 3,4-Diphenylthiophene-2,5-Dicarboxylic Acid using Bis(pyridine)silver permanganate. Proc. Ind. Nat. Sci. Acad. 1994, 60A, 465. [Google Scholar]
- Anjaneyuu, A.S.R.; Umasundari, P.; Sastry, C.V.M. Synthetic Experiments in Lignans. Part 10. Use of Bis (pyridine) silver Permanganate as a New Reagent for Synthesis of 1-Phenylnaphthalene Lactones. Ind. J. Chem. 1986, B25, 955. [Google Scholar]
- Poonam, A.; Rao, A.; Sharma, G.; Kotai, L.; Sajo, I.E.; Sharma, P.K. Kinetics and mechanism of the oxidation of aliphatic secondary alcohols by tetrakis(pyridine)silver dichromate. Int. J. Chem. 2015, 4, 13–21. [Google Scholar]
- Panwar, S.; Soni, U.; Goswami, G.; Kotai, L.; Sajo, I.E.; Sharma, P.K. Structure-Reactivity Correlation in the Oxidation of Substituted Benzyl Alcohols by Tetrakis (pyridine) Silver Dichromate. Int. J. Chem. 2014, 3, 288–299. [Google Scholar]
- Prasad, R.L.; Kushwaha, A.; Kumar, R.; Szilágyi, I.M.; Kótai, L. Solid-state thermal degradation behaviour of 1-D coordination polymers of Ni(II) and Cu(II) bridged by conjugated ligand. J. Therm. Anal. Calorim. 2013, 114, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Meena, A.K.; Daiya, A.; Sharma, A.; Banerji, J.; Sajo, S.; Kótai, L.; Sharma, V. Oxidation of some organic sulficles by tetrakis (pyridine) silver dichromate: A kinetic & mechanistic approach. Int. J. Chem. 2012, 1, 55–65. [Google Scholar]
- Banerji, J.; Kótai, L.; Sharma, P.K.; Banerji, K.K. Kinetics and mechanism of the oxidation of substituted benzaldehydes with bis(pyridine) silver permanganate. Eur. Chem. Bull. 2012, 1, 135–140. [Google Scholar]
- Purohit, T.; Banerji, J.; Kotai, L.; Sajo, I.; Banerji, K.K.; Sharma, P.K. Kinetics and mechanism of the oxidation of substituted benzaldehydes with bis(pyridine)silver permanganate. Indian J. Chem. Sect. A 2012, 89, 1045–1052. [Google Scholar]
- Banerji, J.; Kótai, L.; Banerji, K.K. Kinetics and mechanism of oxidation of formic and oxalic acids by bis(pyridine) silver permanganate. J. Indian Chem. Soc. 2009, 48, 797–800. [Google Scholar]
- Banerji, J.; Banerji, K.K.; Kotai, L.; Sharma, D.; Sharma, P.K. Kinetics and mechanism of the oxidation of organic sulfides with bis(pyridine)silver permanganate. J. Indian Chem. Soc. 2011, 88, 1879–1886. [Google Scholar]
- Meena, A.K.; Daiya, A.; Sharma, A.; Banerji, J.; Kotai, L.; Sharma, V. Oxidation of some vicinal and non-vicinal diols by tetrakis(pyridine)silver dichromate: A kinetic and mechanistic study. J. Indian Chem. Soc. 2011, 88, 1887–1893. [Google Scholar]
- Kumar, A.; Mishra, P.; Kotai, L.; Banerji, K.K. Kinetics and mechanism of the oxidative regeneration of carbonyl compounds from oximes by tetraamminecopper (II) permanganate. Indian J. Chem. Sect. A 2003, 42, 72–74. [Google Scholar]
- Shukla, R.; Kotai, L.; Sharma, P.K.; Banerji, K.K. Kinetics and mechanism of the oxidative regeneration of carbonyl compounds from phenylhydrazones by tetramminecopper(2+)bis-(permanganate). J. Chem. Res. Synop. 2003, 4, 184–185. [Google Scholar] [CrossRef]
- Hetmanczyk, L.; Hetmanczyk, J. Phase transition, thermal dissociation and dynamics of NH3 ligands in [Cd(NH3)4](ReO4)2. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 164, 24–32. [Google Scholar] [CrossRef]
- Hetmanczyk, L.; Hetmanczyk, J. Comparison of vibrational dynamics, thermal behaviour, and phase transition in [Ni(NH3)4](ReO4)2 and [Ni(NH3)6](ReO4)2. J. Therm. Anal. Calorim. 2015, 119, 1415–1428. [Google Scholar] [CrossRef] [Green Version]
- Kótai, L.; Németh, P.; Kocsis, T.; Sajó, I.E.; Pasinszki, T.; Szilágyi, M.A.; Kant, R.; Pawar, R.P.; Sharma, P.K. A new route to synthesize controlled-size MMn2O4-type transition metal (M = Cd, Zn, Cu) nanomanganites. Nano Stud. 2016, 13, 7–13. [Google Scholar]
- Kótai, L.; Sajó, I.E.; Jakab, E.; Keresztury, G.; Németh, C.; Gács, I.; Menyhárd, A.; Kristóf, J.; Hajba, L.; Petrusevski, V.M.; et al. Studies on the Chemistry of [Cd(NH3)4](MnO4)2. A Low Temperature Synthesis Route of the CdMn2O4+x Type NOx and CH3SH Sensor Precursors. Z. Anorg. Allg. Chem. 2012, 638, 177–186. [Google Scholar] [CrossRef]
- Bereczki, L.; Petruševski, V.M.; Franguelli, F.P.; Béres, K.A.; Farkas, A.; Holló, B.B.; Czégény, Z.; Szilágyi, I.M.; Kótai, L. [Hexaamminecobalt(III)] Dichloride Permanganate—Structural Features and Heat-Induced Transformations into (CoII,MnII)(CoIII,MnIII)2O4 Spinels. Inorganics 2022, 10, 252. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Kováts, É.; Czégény, Z.; Bereczki, L.; Petruševski, V.M.; Holló, B.B.; Béres, K.A.; Farkas, A.; Szilágyi, I.M.; Kótai, L. Multi-Centered Solid-Phase Quasi-Intramolecular Redox Reactions of [(Chlorido)Pentaamminecobalt(III)] Permanganate—An Easy Route to Prepare Phase Pure CoMn2O4 Spinel. Inorganics 2022, 10, 18. [Google Scholar] [CrossRef]
- Kótai, L.; Banerji, K.K. An improved method for the preparation of high-purity permanganate salts. Synth. React. Inorg. Met. Org. Chem. 2001, 31, 491–495. [Google Scholar] [CrossRef]
- Kótai, L.; Sajó, I.E.; Gács, I.; Sharma, P.K.; Banerji, K.K. Convenient routes for the preparation of barium permanganate and other permanganate salts. Z. Anorg. Allg. Chem. 2007, 633, 1257–1260. [Google Scholar] [CrossRef]
- Kótai, L.; Gács, I.; Sajó, I.E.; Sharma, P.K.; Banerji, K.K. Beliefs and facts in permanganate chemistry—An overview on the synthesis and the reactivity of simple and complex permanganates. Trends Inorg. Chem. 2009, 11, 25–104. [Google Scholar] [CrossRef]
- Kótai, L.; Keszler, Á.; Pató, J.; Holly, S.; Banerji, K.K. The reaction of barium manganate with acids and their precursors. Indian J. Chem. Sect. A. 1999, 38, 966–968. [Google Scholar]
- Bruni, G.; Levi, G. Gli ammoniacati del sali d’argento. Note I. Gazz. Chim. Ital. 1916, 46, 17–42. [Google Scholar]
- Wilke-Dörfurt, E.; Gunzert, T. Über Neue Salze der Perrheniumsäure. Z. Anorg. Allg. Chem. 1933, 215, 369–387. [Google Scholar] [CrossRef]
- Klobb, T. Combinaisons de l’ammoniaque avec les permanganates metalliques. Compt. Rend. Hebd. Seanc. Acad. Sci. 1886, 103, 384–385. [Google Scholar]
- Scagliari, G.; Marangoni, A. Isomorfismo fra perclorati e permanganate. Gazz. Chim. Ital. 1915, 45, 42–44. [Google Scholar]
- Kótai, L.; Gács, I.; Kazinczy, B.; Sajó, I.E.; Sreedhar, B. Quasi-intramolecular acid-base interactions in aqueous solutions of metal-complexes of basic ligands I. Generalized theoretical considerations on the deammoniation of [MLm]Xn type ammonia complexes. Transit. Met. Chem. 2003, 28, 292–295. [Google Scholar] [CrossRef]
- Kótai, L.; Horváth, T.; Szentmihályi, K.; Keszler, Á. Evidence for quasi-intramolecular acid-base reactions in solutions of transition metal ammine complexes. Transit. Met. Chem. 2000, 25, 293–294. [Google Scholar] [CrossRef]
- Kotai, L.; Argay, G.; Holly, S.; Keszler, A.; Pukanszky, B.; Banerji, K.K. Study on the Existence of Hydrogen Bonds in ammonium permanganate. Z. Anorg. Allg. Chem. 2001, 627, 114–118. [Google Scholar] [CrossRef]
- Christensen, O.T. Untersuchungen über Manganverbindungen. I. Über Ammoniumpermanganat. Z. Anorg. Allgem. Chem. 1900, 24, 203–2019. [Google Scholar] [CrossRef] [Green Version]
- Kótai, L.; Banerji, K.K.; Sajó, I.; Kristóf, J.; Sreedhar, B.; Holly, S.; Keresztury, G.; Rockenbauer, A. An unprecedented-type intramolecular redox reaction of solid tetraamminecopper (2+) bis (permanganate) ((Cu(NH3)4)(MnO4)2)—A low-temperature synthesis of copper dimanganese tetraoxide-type (CuMn2O4) nanocrystalline catalyst precursors. Helv. Chim. Acta 2002, 85, 2316–2327. [Google Scholar] [CrossRef]
- Sharma, M.; Songara, U.; Swami, P.; Purohit, P.; Vyas, S.; Sharma, P.K. Structure-reactivity correlation in oxidation of substituted benzyl alcohols by tetramminecopper(II) bis(permanganate. Int. J. Chem. 2016, 5, 74–82. [Google Scholar]
- Fogaça, L.A.; Bereczki, L.; Petruševski, V.M.; Barta-Holló, B.; Franguelli, F.P.; Mohai, M.; Béres, K.A.; Sajó, I.E.; Szilágyi, I.M.; Kotai, L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics 2021, 9, 38. [Google Scholar] [CrossRef]
- Mahroua, O.; Alili, B.; Ammari, A.; Bellal, B.; Bradai, D.; Trari, M. On the physical and semiconducting properties of the crednerite AgMnO2 prepared by sol-gel auto-ignition. Ceram. Int. 2019, 45, 10511–10517. [Google Scholar] [CrossRef]
- Koriche, N.; Bouguelia, A.; Mohammedi, M.; Trari, M. Synthesis and physical properties of new oxide AgMnO2. J. Mater. Sci. 2007, 42, 4778–4784. [Google Scholar] [CrossRef]
- Béres, K.A.; Petruševski, V.M.; Barta-Holló, B.; Németh, P.; Fogaça, L.A.; Franguelli, F.P.; Farkas, A.; Menyhárd, A.; Szilágyi, I.M.; Kótai, L. AgNO3·NH4NO3—An enigmatic double-salt type “decomposition intermediate” of diamminesilver(I) permanganate. Z. Anorg. Allg. Chem. 2021, 647, 1166–1174. [Google Scholar] [CrossRef]
- Chakravorti, M.C.; Sarkar, M.B. Rhenium. Part XVII: A Few Complexes of Copper Containing Coordinated Perrhenate, [ReO4]. J. Indian Chem. Soc. 1983, 60, 617–714. [Google Scholar]
- Chakravorti, M.C.; Sarkar, M.B.; Bharadwaj, P.K. Rhenium part XV. Tetragonal complexes of nickel(II) containing coordinated perrhenate [ReO4]−. Transit. Met. Chem. 1981, 6, 211–214. [Google Scholar] [CrossRef]
- Chakravorti, M.C.; Sarkar, M.B. Rhenium. Part XVI. Ammine and pyridine compounds of zinc and cadmium containing coordinated perrhenate. Transit. Met. Chem. 1982, 7, 19–22. [Google Scholar] [CrossRef]
- Ephraim, F. Ueber die Natur der Nebenvalenzen XIX. Ammoniakate des Silbers. Ber. Dtsch. Chem. Ges. 1918, 51, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Bruni, G.; Levi, G. Gli ammoniacati del sali d’argento. Note III. Gazz. Chim. Ital. 1917, 47, 259–272. [Google Scholar]
- Müller, A.; Böschen, I.; Baran, E.J.; Aymonino, P.J. Über Tetramminmetallchalkogenometallate. Monatsh. Chem. 1973, 104, 836–847. [Google Scholar] [CrossRef]
- Müller, A.; Böschen, I.; Sievert, W. Notizen: Zur Kristallstruktur von [Zn(NH3)4](OsO3N)2 und [Cd(NH3)4](OsO3N)2. Z. Naturfosch. 1970, 25, 311–312. [Google Scholar] [CrossRef]
- Pitzer, K.S. The Crystal Structure of Tetramminocadmium Perrhenate, Cd(NH3)4(ReO4). Z. Krist. 1935, 92, 131–135. [Google Scholar] [CrossRef]
- Khranenko, S.P.; Shusharina, E.A.; Gromilov, S.A.; Smolentsev, A.I. Crystal structure of [Cu(NH3)4](ReO4)2. J. Struct. Chem. 2009, 50, 1201–1203. [Google Scholar] [CrossRef]
- Zadesenets, A.V.; Khranenko, S.P.; Shubin, Y.V.; Baidina, I.A.; Korenev, S.V. Complex Salts [Pd(NH3)4](ReO4)2 and [Pd(NH3)4](MnO4)2: Synthesis, Structure, and Thermal Properties. Russ. J. Coord. Chem. 2006, 32, 374–379. [Google Scholar] [CrossRef]
- Rochon, F.D.; Kong, P.C.; Melanson, R. [Tetraammineplatinum(ll) Bis[pertechnetate(VIl)]. Acta Cryst. 1990, 46C, 8–10. [Google Scholar]
- Korolkov, I.V.; Zadesenets, A.V.; Gromilov, S.A.; Yusenko, K.V.; Baidina, I.A.; Korenev, S.V. Metal solid solutions obtained by thermolysis of Pt and Re salts. Crystal structure of [Pt(NH3)4](ReO4)2. J. Struct. Chem. 2006, 47, 489–498. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Kuznetsov, V.Y.; Popova, R.A.; Zalkind, O.A. Synthesis and study of tetraammineplatinum(II) perrhenate. Zhurnal Neorg. Khimii 1979, 24, 3306–3308. [Google Scholar]
- Seferiadis, N.; Dubler, E.; Oswald, H.R. Structure of Tetraamminecopper(II) Dipermanganate. Acta Cryst. 1986, 42C, 942–945. [Google Scholar] [CrossRef]
- Müller, A.; Christophliemk, P.; Tossidiss, I. Struktur thermisches verhalten und eigenschaften von tetramminkobalt(II)-perrhenat [Co(NH3)4](ReO4)2; elektronen-und schwingungsspektre. J. Mol. Struct. 1973, 15, 289–299. [Google Scholar] [CrossRef]
- Klobb, T. Combinaisons de l’ammoniaque avec les permanganates métalliques. Bull. Soc. Chim. Fr. 1890, 3, 508. [Google Scholar]
- Gorbunov, V.V.; Shmagin, L.F. Burning of copper (II) tetramine salts. Combust. Explos. Shock Waves 1972, 8, 429–431. [Google Scholar] [CrossRef]
- Poineau, F.; Mausolf, E.; Kerlin, W.; Czerwinski, K. Hexaammine-cobalt(III) pertechnetate: Preparation, structure and solubility. J. Radioanal. Nucl. Chem. 2016, 311, 775–778. [Google Scholar] [CrossRef]
- Kotai, L.; Kazinczy, B.; Keszler, Á.; Holly, S.; Gács, I.; Banerji, K.K. Three Reagents in One: Ammonium Permanganate in the Oxidation of Benzyl Alcohol. Z. Nat. 2001, 56B, 823–825. [Google Scholar] [CrossRef]
- Briscoe, H.V.A.; Robinson, P.L.; Rudge, A.J. Perrhenates of copper, nickel and cobalt and the ammines of these compounds. J. Chem. Soc. 1931, 1, 2211–2213. [Google Scholar] [CrossRef]
- Zagorodnyaya, A.N.; Abisheva, Z.S.; Aitekeyeva, S.N.; Bukurov, T.N.; Sapukov, I.A. Synthesis and properties of tetraamminecopper diperrhenate. Komplex Ispolz. Miner. Sirya 2003, 1, 16–23. [Google Scholar]
- Zagorodnyaya, A.N.; Abisheva, Z.S. Rhenium recovery from ammonia solutions. Hydrometallurgy 2002, 65, 69–76. [Google Scholar] [CrossRef]
- Tellez, C. Espectro infrarrojo de [Zn(NH3)4](ReO4)2 con substitucion isotopica 14N/15N. Semina 1983, 4, 410–411. [Google Scholar]
- Zagordnyaya, A.N.; Abisheva, Z.S.; Sadikanova, S.E.; Kvyatkovskaya, M.N.; Kokoveshnikova, T.A.; Sapukov, I.A. Hermal decomposition of tetraamminecadmium diperrhenate. Izv. Nat. Akad. Nauk Respub. Kazakh. Ser. Khim. 2005, 1, 54–60. [Google Scholar]
- Tellez, C. Metal-ligand vibrations of [Cd(NH3)4](ReO4)2 with 110/116Cd and H/D isotope substitution. Semina 1980, 6, 73–74. [Google Scholar]
- Zagordnyaya, A.N.; Ponomareva, E.I.; Abisheva, Z.S. Extraction technology for rhenium recovery from chloride-sulfate zinc-cadmium solutions. In Solvent Extraction in the Process Industries: ISEC93; Logsdail, D.H., Slater, M.J., Eds.; Elsevier Applied Science: Amsterdam, The Netherlands, 1993; pp. 167–174. [Google Scholar]
- Zagorodnyaya, A.N.; Abisheva, Z.S.; Kvyatkovskaya, M.N.; Kokoveshnikova, T.A.; Kasymova, A.S.; Sadykanova, S.E. Thermal decomposition of tetraamminecopper diperrhenate. Dokl. Nat. Akad. Nauk Respub. Kazakh. 2004, 3, 89–96. [Google Scholar]
- Bibikova, V.I.; Vasilevskaya, I.I.; Vasilyeva, A.G.; Niselson, L.A. Thermal decomposition of ammonium perrhenate to obtain rhenium heptoxide. J. Appl. Chem. (Zh. Prikl. Khim.) 1973, 43, 1115–1116. [Google Scholar]
- Ratner, Y.E.; Tsvetkov, Y.V.; Berezkina, L.G. Thermal dissociation and reduction of ammonium perrhenate. Zhurnal Neorg. Khimii 1968, 13, 1516–1519. [Google Scholar]
- Zagorodnyaya, A.N.; Abisheva, Z.S.; Kvyatkovskaya, M.N.; Kokoveshnikova, T.A.; Kasymova, A.S.; Sadykanova, S.E.; Bochevskaya, E.G. Thermal decomposition of tetraamminzinc diperrhenate. Komplex Ispolz. Miner. Sirya 2005, 1, 22–29. [Google Scholar]
- Müller, A.; Böschen, I.; Baran, E.J. Uber Hexamminmetallchalkogenometallate. Monatsh. Chem. 1973, 104, 821–835. [Google Scholar] [CrossRef]
- Minacheva, L.K.; Kokunova, V.N.; Sergienko, V.S.; Kokunov, Y.V. Crystal structure of hydroxonitrosotetraamineruthenium(IV) perrhenate [Ru(NO)(OH)(NH)](ReO4)2. Zhurnal Neorg. Khimii 2001, 46, 1293–1296. [Google Scholar]
- Baidina, I.A.; Filatov, E.Y.; Makotchenko, E.V.; Smolentsev, A.I. Synthesis and structure investigation of Co(III) complex salts with the perrhenate anion. J. Struct. Chem. 2012, 53, 112–118. [Google Scholar] [CrossRef]
- Yusenko, K.V.; Baidina, I.A.; Gromilov, S.A.; Korenev, S.V. Nvestigation of pentaamminechloroplatinum(IV) perrhenate dihydrate. J. Struct. Chem. 2007, 48, 578–582. [Google Scholar] [CrossRef]
- Krestov, G.A.; Yatsimirsikii, K.B. Thermodynamical characteristics of (chloro)(pentaamine)cobalt type complex compounds. Zhurnal Neorg. Khimii 1961, 6, 2294–2303. [Google Scholar]
- Sacconi, B.L.; Sabatini, A.; Cans, P. Infrared Spectra from of Some Metal-Ammine Complexes. Inorg. Chem. 1964, 3, 1772–1774. [Google Scholar] [CrossRef]
- Grinberg, A.A.; Varshavskii, Y.S. The frequency of coordinated ammonia deformation mode and its relationship with the chemical properties of transition metal ammonia complexes. Primen. Molekul. Spektr. Khim. 1966, 1, 104–107. [Google Scholar]
- Lenz, E.; Murmann, K.R. The Preparation and Kinetics of Hydrolysis of Pentaammineperrhenatocobalt(III) Ion. Inorg. Chem. 1968, 7, 1880–1885. [Google Scholar] [CrossRef]
- Liss, I.B.; Murmann, R.K. Complex Ion Kinetics. Reaction Rates on Ion-Exchange Resins Compared to Those in Water. Inorg. Chem. 1975, 14, 2314–2317. [Google Scholar] [CrossRef]
- Lenz, E.; Murmann, R.K. Pentaammineperrhenatocobalt(III) salts. Inorg. Synth. 1970, 12, 214–218. [Google Scholar]
- Baran, E.J.; Aymonino, P.J. Über Hexamminkobalt(lll)-permanganat. Z. Anorg. Allgem. Chem. 1968, 362, 215–219. [Google Scholar] [CrossRef]
- Willke-Dörfurt, E.; Balz, G.; Weinhard, A. Beitrag zur Kenntnis der Fluorsulfonsäure. Z. Anorg. Allgem. Chem. 1929, 185, 417–424. [Google Scholar] [CrossRef]
- Klobb, T. De quelques permanganates nouveaux. Bull. Soc. Chim. 1887, 47, 240–244. [Google Scholar]
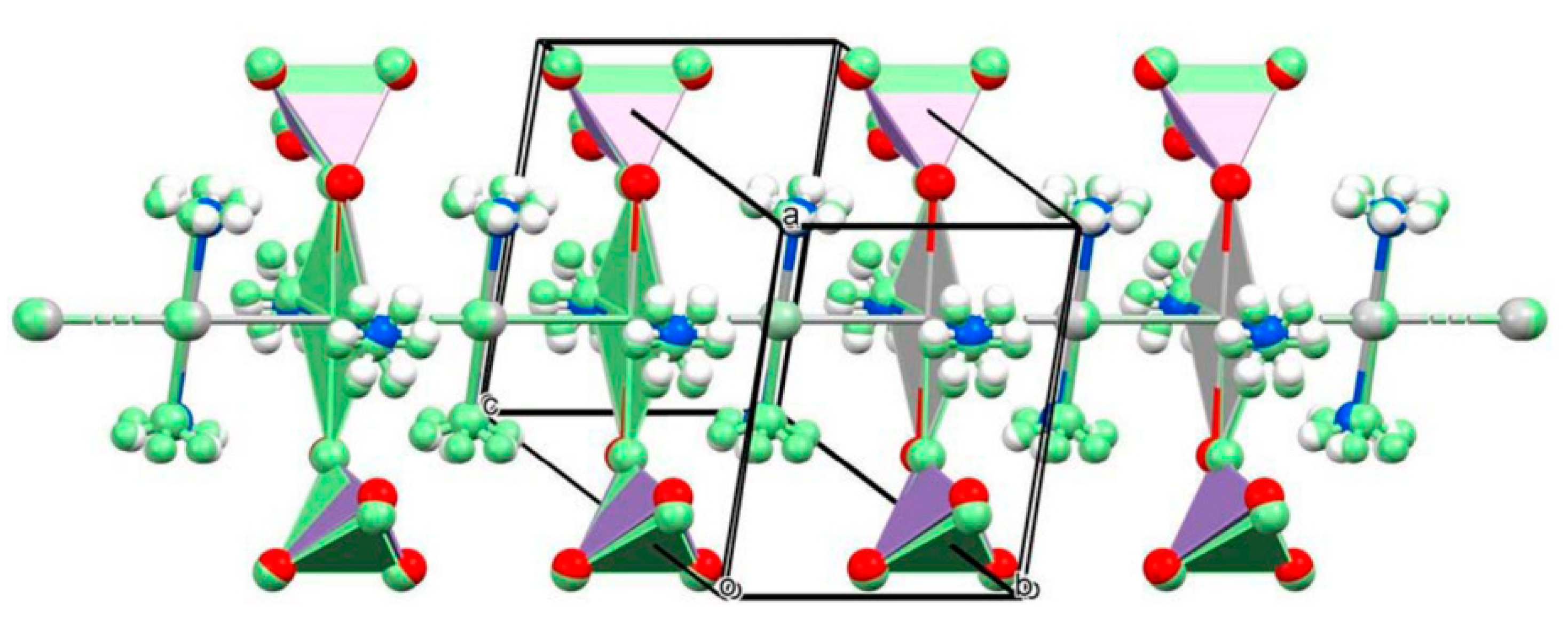


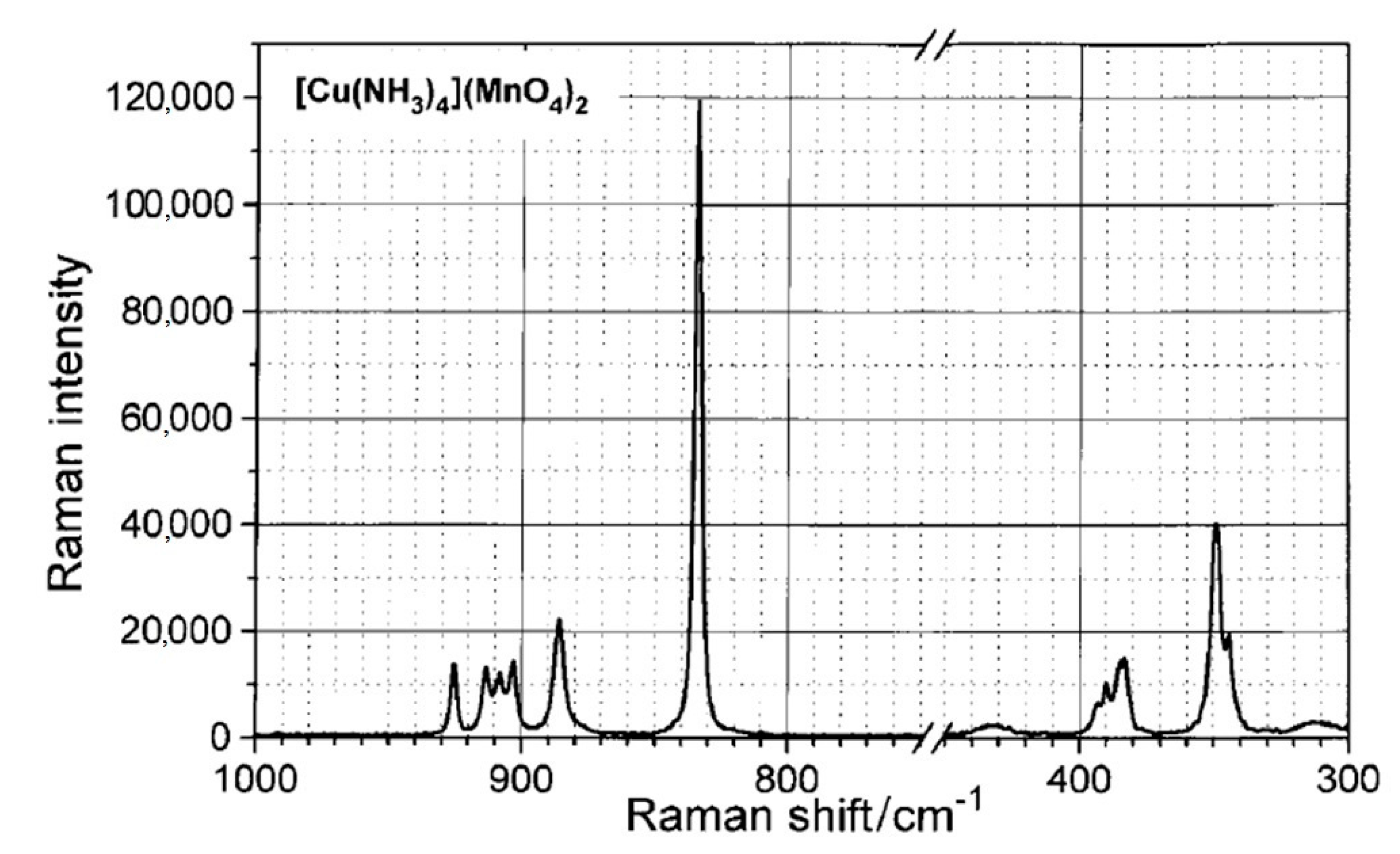
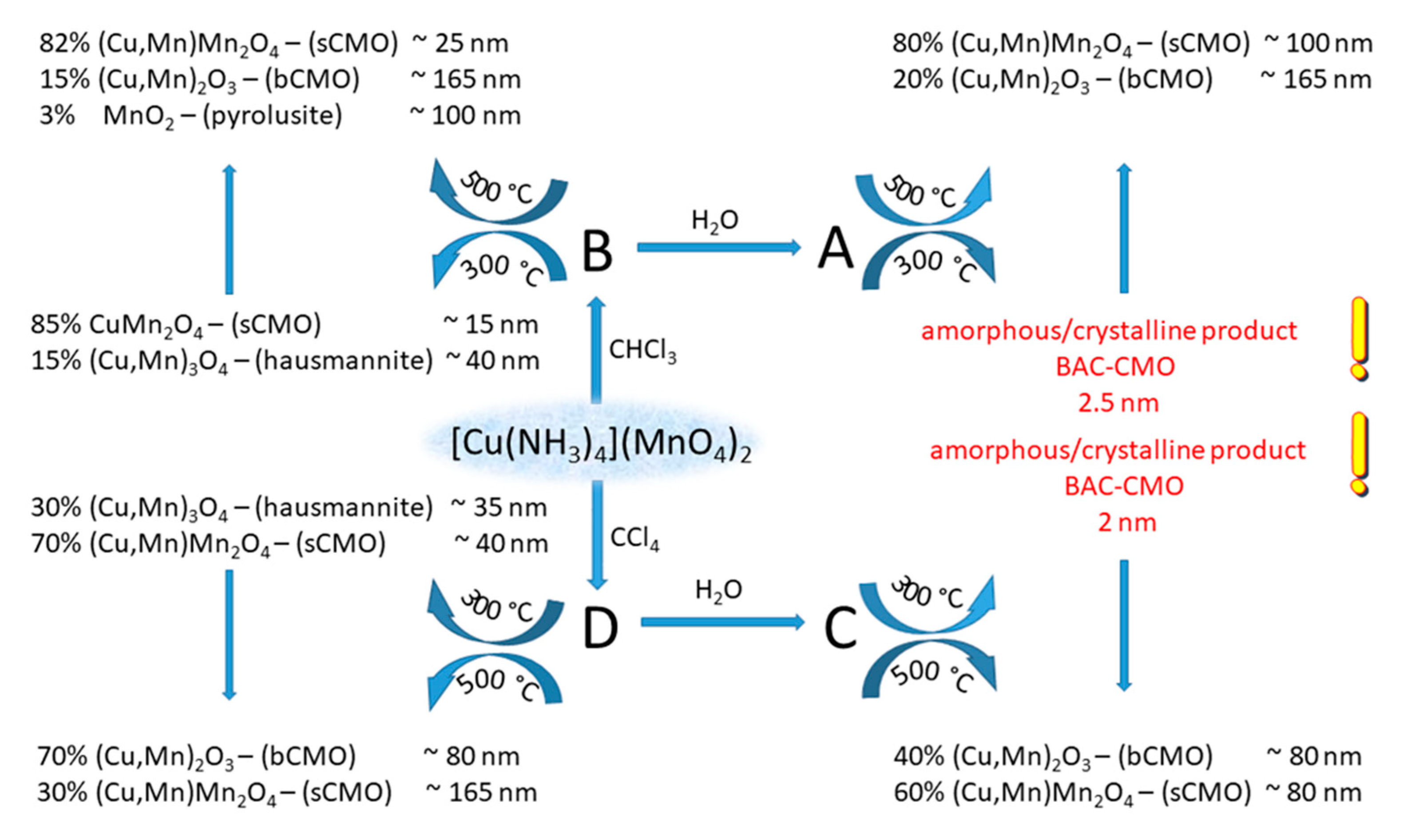
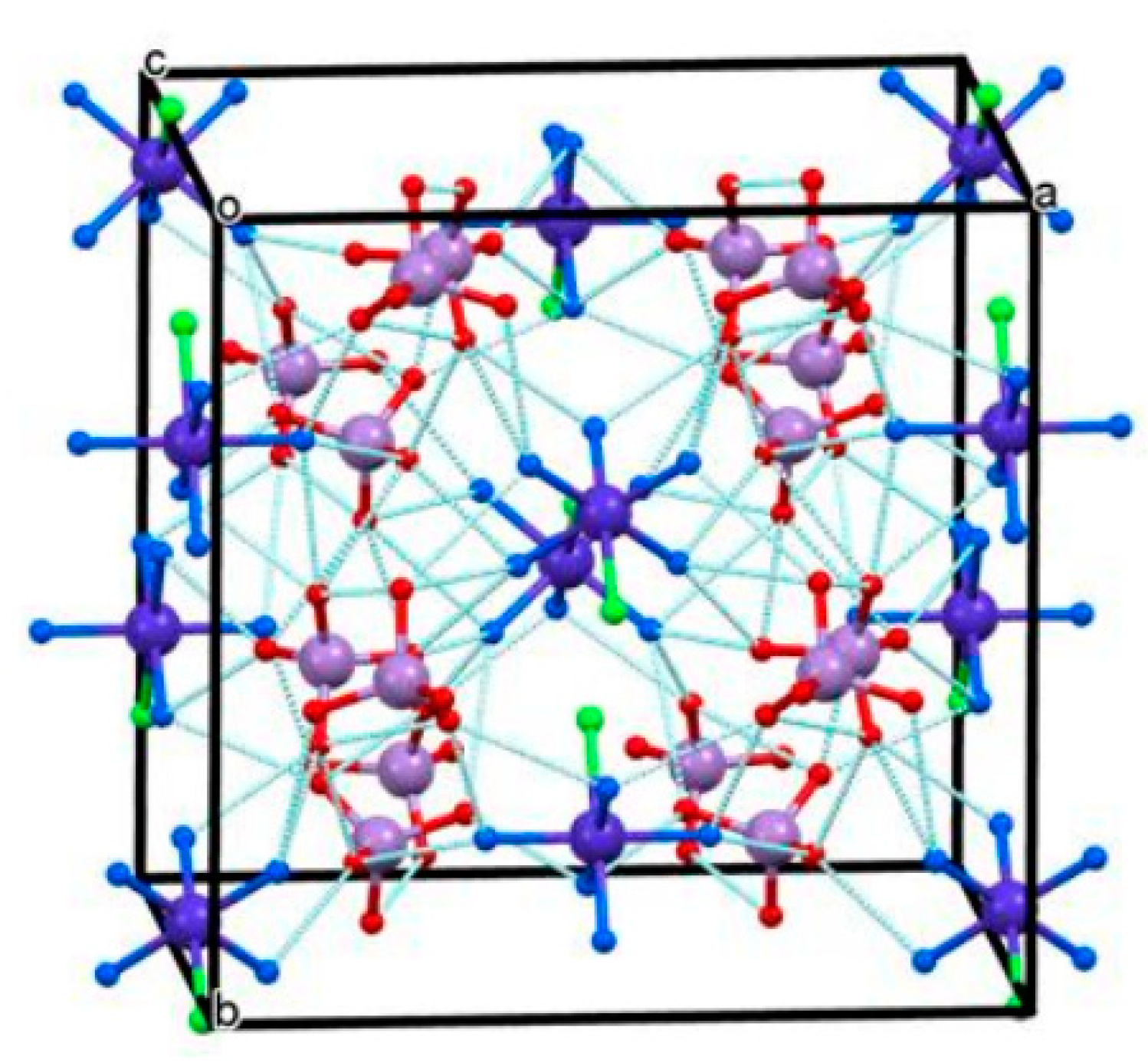


| Compound | T, K | a, b, c, Å | α,β,γ, ° | Space Group | Z | V, Å3 | Dcalcd, g/mL | Ref. |
|---|---|---|---|---|---|---|---|---|
| M = Cu, X = Mn | 298 | 5.413 9.093 10.749 | 96.18 | P21/m | 2 | 526.0 | 2.33 | [94] |
| M = Cu, X = Re | 150 | 6.5167; 6.7790; 7.4627 | 67.336; 80.004 70.687 | P-1 | 1 | 3.661 | [92] | |
| M = Zn, X = Mn | 298 | 10.335 | F4-3m | 4 | [89] | |||
| M = Zn, X = Re | 10.53 | F4-3m | 4 | 3.60 | [86] | |||
| 10.66 | F4-3m | 4 | [87] | |||||
| M = Cd, X = Mn | 298 | 10.432 | F4-3m | 4 | [60] | |||
| 10.44 | 2.41 | [86] | ||||||
| M = Cd, X = Re | 10.53 | F4-3m | 4 | [88] | ||||
| 10.54 | [87] | |||||||
| 10.67 | 3.71 | [86] | ||||||
| M = Ni, X = Re | 9.2 5.2 6.7 | 1 | 3.22 | [82] | ||||
| M = Co, X = Re | 10.54 | F4-3m | 4 | 3.56 | [95] | |||
| M = Pd, X = Mn | 5.1746 7.5861 7.7217 | 69.313 78.872 76.883 | P1 | 1 | 274.1 | 2.50 | [90] | |
| M = Pd, X = Re | 5.1847 7.7397 7.9540 | 69.531 79.656 77.649 | P-1 | 1 | 290.19 | 4.37 | [90] | |
| M = Pt, X = Tc | 5.179 7.725 7.935 | 69.33 79.74 77.41 | P-1 | 1 | 3.396 | [91] | ||
| M = Pt, X = Re | 298 | 5.1847 7.7397 7.9540 | 69.531 79.656 77.649 | P1 | 1 | 290.19 | 4.370 | [92] |
| 12.70 8.91 5.09 | 104.1 | C2/m or Cm | 2 | 4.55 | [93] |
| Compound | T, K | a, b, c | α, β, γ | Space Group | Z | V, Å3 | Dcalcd., g/mL | Ref. |
|---|---|---|---|---|---|---|---|---|
| M = Co, Y = H2O, X = Re, n = 3, ×2H2O | 150 | 9.9797 12.6994 14.7415 | 102.870 | C2/c | 4 | 1821.35 | 3.456 | [113] |
| M = Co, Y = Cl, X = Re, n = 2, ×2H2O | 14.9446 14.6562 12.2434 | Cmc21 | 8 | 2681.68 | 3.368 | [113] | ||
| M = Co, Y = Cl, X = Re, n = 2; ×0.5H2O | 293 | 8.0086 12.9839 17.5122 | 91.858 | P21/sn | 4 | 1820.01 | 3.462 | [113] |
| M = Co, Y = Cl, X = Re, n = 2 | 293 | 14.974 14.688 12.2434 | 2708.5 | 3.33 | [25] | |||
| M = Rh, Y = Cl, X = Re, n = 2 | 293 | 15.0740 14.7290 12.3470 | Cmc21 | 1430.5 | 3.19 | [25] | ||
| M = Cr, Y = Cl, X = Re, n = 2 | 293 | 15.071 14.806 12.439 | 2775.7 | 3.30 | [25] | |||
| M = Ru, Y = Cl, X = Re, n = 2 | 293 | 15.053 14.793 12.445 | 2741.3 | 3.54 | [25] | |||
| M = Rh, Y = Cl, X = Re, n = 2 | 293 | 15.033 14.723 12.331 | 2729.2 | 3.55 | [25] | |||
| M = Ir, Y = Cl, X = Re, n = 2 | 293 | 15.059 14.718 12.359 | 2739.2 | 3.98 | [25] | |||
| M = Co, Y = Cl, X = Mn, n = 2 | 14.2753 14.2816 12.2342 | Cmc21 | 8 | 2494.24 | 2.216 | [62] | ||
| M = Pt, X = Cl, X = Re, n = 3, ×2H2O | 10.3476 12.8955 14.3536 | P21/n | 4 | 1847.94 | 3.962 | [114] |
| Compound | T, K | a, b, c | α, β, γ | Space Group | Z | V, Å3 | Dcalcd., g/mL | Ref. |
|---|---|---|---|---|---|---|---|---|
| M = Co, X = Re, n = 3, ×2H2O | 293 | 14.9446 14.6562 12.2434 | Cmc21 | 8 | 2681.68 | 3.368 | [113] | |
| M = Co, X = Mn, n = 3 | 11.39 | Td2--F4-3m | 4 | 1477.6 | 2.33 | [111] | ||
| 11.39 | 4 | 1477.65 | [121] | |||||
| M = Co, X = Tc, n = 3, ×2H2O | 8.0266 12.6275 17.6438 | 91.320 | P21/n | [98] | ||||
| M = Cr, X = Mn, n = 3 | 11.45 | Td2-F4-3m | 4 | 1501.1 | 2.26 | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrotra, R.N. Review on the Chemistry of [M(NH3)n](XO4)m (M = Transition Metal, X = Mn, Tc or Re, n = 1–6, m = 1–3) Ammine Complexes. Inorganics 2023, 11, 308. https://doi.org/10.3390/inorganics11070308
Mehrotra RN. Review on the Chemistry of [M(NH3)n](XO4)m (M = Transition Metal, X = Mn, Tc or Re, n = 1–6, m = 1–3) Ammine Complexes. Inorganics. 2023; 11(7):308. https://doi.org/10.3390/inorganics11070308
Chicago/Turabian StyleMehrotra, Raj Narain. 2023. "Review on the Chemistry of [M(NH3)n](XO4)m (M = Transition Metal, X = Mn, Tc or Re, n = 1–6, m = 1–3) Ammine Complexes" Inorganics 11, no. 7: 308. https://doi.org/10.3390/inorganics11070308





