Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye from Aqueous Media
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
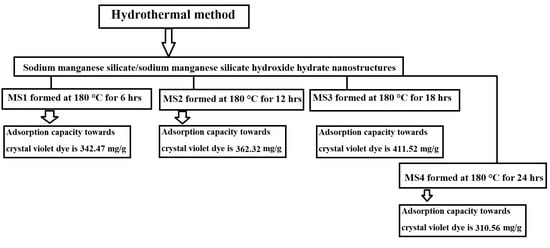
2.2. Synthesis of Sodium Manganese Silicate/Sodium Manganese Silicate Hydroxide Hydrate
2.3. Characterization
2.4. Disposal of Crystal Violet Dye from Aqueous Media
3. Results and Discussion
3.1. Discussion of Characterization of the Synthesized Samples
3.2. Disposal of Crystal Violet Dye from Aqueous Media
3.2.1. Effect of Solution pH
3.2.2. Effect of Contact Time
3.2.3. Effect of Temperature
3.2.4. Effect of Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ouachtak, H.; El Guerdaoui, A.; El Haouti, R.; Haounati, R.; Ighnih, H.; Toubi, Y.; Alakhras, F.; Rehman, R.; Hafid, N.; Addi, A.A.; et al. Combined Molecular Dynamics Simulations and Experimental Studies of the Removal of Cationic Dyes on the Eco-Friendly Adsorbent of Activated Carbon Decorated Montmorillonite Mt@AC. RSC Adv. 2023, 13, 5027–5044. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Luan, F. Air Pollution, Water Pollution and Robots: Is Technology the Panacea. J. Environ. Manag. 2023, 330, 117170. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-Based Materials and Their Adsorptive Removal Efficiency for Dyes: A Review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Min, X.; Tang, C.J.; Sillanpää, M.; Zhao, F. Recent Advances in Membrane Filtration for Heavy Metal Removal from Wastewater: A Mini Review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and Application of Polyethyleneimine (PEI)-based Composite/Nanocomposite Material for Heavy Metals Removal from Wastewater: A Critical Review. J. Hazard. Mater. Adv. 2022, 8, 100158. [Google Scholar] [CrossRef]
- Sankar Sana, S.; Haldhar, R.; Parameswaranpillai, J.; Chavali, M.; Kim, S.C. Silver Nanoparticles-Based Composite for Dye Removal: A Comprehensive Review. Clean. Mater. 2022, 6, 100161. [Google Scholar] [CrossRef]
- Wang, J.; Sha, X.; Chen, X.; Zhuo, H.; Xie, W.; Zhou, Z.; He, X.; Wu, L.; Li, B. Removal and Distribution of Antibiotics and Resistance Genes in Conventional and Advanced Drinking Water Treatment Processes. J. Water Process Eng. 2022, 50, 103217. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, N.; Hossini, H. Sonochemical Processes for Antibiotics Removal from Water and Wastewater: A Systematic Review. Chem. Eng. Res. Des. 2023, 189, 401–439. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, M.; Lee, X.; Feng, Q.; Cheng, N.; Zhang, X.; Wang, S.; Wang, B. Enhanced Removal of Sulfonamide Antibiotics from Water by Phosphogypsum Modified Biochar Composite. J. Environ. Sci. 2023, 130, 174–186. [Google Scholar] [CrossRef]
- Salahshoori, I.; Namayandeh, M.; Ghasemi, S.; Masoomeh, S.; Mirnezami, S.; Nobre, M.A.L.; Ali, H. Assessing Cationic Dye Adsorption Mechanisms on MIL-53 (Al) Nanostructured MOF Materials Using Quantum Chemical and Molecular Simulations: Toward Environmentally Sustainable Wastewater Treatment. J. Water Process Eng. 2023, 55, 104081. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, J.; Song, H.; Yang, F.; Shen, L.; Zhu, L. Enhanced Adsorption of Dyes by Functionalized UiO-66 Nanoparticles: Adsorption Properties and Mechanisms. J. Mol. Struct. 2023, 1292, 136111. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Wang, W.; Lin, R.; Liu, X. Carboxylated Cellulose-Based Aerogel with Cellular Pores Prepared by Stir Freezing for Cationic Dye Adsorption. Process Saf. Environ. Prot. 2023, 177, 807–817. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Q.; Huang, B.; Qi, J.; Wang, R.; Zhou, Q.; Chen, Q.; Zhu, L.; Jin, J.; Kong, Y. Removal Performance and Adsorption Kinetics of Dyes by a Co-Based Metal Organic Framework. Microporous Mesoporous Mater. 2023, 360, 112665. [Google Scholar] [CrossRef]
- Abbou, B.; Lebkiri, I.; Ouaddari, H.; El, A.; Ezzahra, F.; Kadiri, L.; Ouass, A.; Lebkiri, A.; Housseine, E. Improved Removal of Methyl Orange Dye by Adsorption Using Modified Clay: Combined Experimental Study Using Surface Response Methodology. Inorg. Chem. Commun. 2023, 155, 111127. [Google Scholar] [CrossRef]
- Thue, P.S.; Sophia, A.C.; Lima, E.C.; Wamba, A.G.N.; de Alencar, W.S.; dos Reis, G.S.; Rodembusch, F.S.; Dias, S.L.P. Synthesis and Characterization of a Novel Organic-Inorganic Hybrid Clay Adsorbent for the Removal of Acid Red 1 and Acid Green 25 from Aqueous Solutions. J. Clean. Prod. 2018, 171, 30–44. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Shen, Z.; Li, X.; Zhou, Q.; Sun, Y.; Wang, T.; Liu, Y.; Gao, Q. Gradient Adsorption of Methylene Blue and Crystal Violet onto Compound Microporous Silica from Aqueous Medium. ACS Omega 2020, 5, 28382–28392. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Freeze-Thaw as a Route to Build Manageable Polysaccharide Cryogel for Deep Cleaning of Crystal Violet. Chem. Eng. J. 2020, 396, 125354. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V.K. Adsorption of Hazardous Dye Crystal Violet from Wastewater by Waste Materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Nabil, G.M.; Khalifa, M.A.; El-Mallah, N.M.; Hassouba, H.M. Effective Removal of Crystal Violet and Methylene Blue Dyes from Water by Surface Functionalized Zirconium Silicate Nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103009. [Google Scholar] [CrossRef]
- Mahmood, O.A.A.Q.; Waisi, B.I. Crystal Violet Dye Removal from Aqueous Water Using Polyacrylonitrile Precursor Beads. Mater. Today Proc. 2021, 42, 2185–2192. [Google Scholar] [CrossRef]
- Sanakousar, M.F.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Jayanna, B.K.; Mounesh; Shridhar, A.H.; Prakash, K. Efficient Photocatalytic Degradation of Crystal Violet Dye and Electrochemical Performance of Modified MWCNTs/Cd-ZnO Nanoparticles with Quantum Chemical Calculations. J. Hazard. Mater. Adv. 2021, 2, 100004. [Google Scholar] [CrossRef]
- Wasim, M.; Sabir, A.; Shafiq, M.; Khan, R.U. Mussel Inspired Surface Functionalization of Polyamide Membranes for the Removal and Adsorption of Crystal Violet Dye. Dye. Pigment. 2022, 206, 110606. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and Removal of Crystal Violet Dye from Aqueous Solution by Modified Rice Husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef] [PubMed]
- Muhiuddin, G.; Bibi, I.; Nazeer, Z.; Majid, F.; Kamal, S.; Kausar, A.; Raza, Q.; Alwadai, N.; Ezzine, S.; Iqbal, M. Synthesis of Ni Doped Barium Hexaferrite by Microemulsion Route to Enhance the Visible Light-Driven Photocatalytic Degradation of Crystal Violet Dye. Ceram. Int. 2023, 49, 4342–4355. [Google Scholar] [CrossRef]
- Gad, Y.H.; Helal, R.H.; Radi, H.; El-nemr, K.F.; Khozemy, E.E. Preparation and Application of Irradiated Polyvinyl Alcohol/Starch/Pumice Composites for Adsorption of Basic Dye: Isotherm and Kinetics Study. Int. J. Biol. Macromol. 2023, 249, 126106. [Google Scholar] [CrossRef] [PubMed]
- Türk, F.N.; Arslano, H. Use and Applications of Metal-Organic Frameworks (MOF) in Dye Adsorption: Review. J. Environ. Chem. Eng. 2023, 11, 110568. [Google Scholar] [CrossRef]
- He, H.; Wen, J.; Zhao, Q.; Ke, G.; Yang, H. The Adsorption Activity and Mechanism of Common Tourmalines for Typical Anionic and Cationic Dyes. Chem. Phys. 2023, 571, 111938. [Google Scholar] [CrossRef]
- Thakur, V.; Sharma, P.; Awasthi, A.; Guleria, A.; Singh, K. Utility of Acrylic Acid Grafted Lignocellulosic Waste Sugarcane Bagasse for the Comparative Study of Cationic and Anionic Dyes Adsorption Applications. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100824. [Google Scholar] [CrossRef]
- Wei, J.; Jia, J.; Liao, T. Highly Selective Adsorption of Dyes by Functional Hypercrosslinked-Polymers Prepared in a Facile and Chemically Stable Manner. J. Environ. Chem. Eng. 2023, 11, 110555. [Google Scholar] [CrossRef]
- Shafiq, F.; Liu, C.; Zhou, H.; Chen, H.; Yu, S.; Qiao, W. Adsorption Mechanism and Synthesis of Adjustable Hollow Hydroxyapatite Spheres for Efficient Wastewater Cationic Dyes Adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2023, 672, 131713. [Google Scholar] [CrossRef]
- Sarabadan, M.; Bashiri, H.; Mousavi, S.M. Removal of Crystal Violet Dye by an Efficient and Low Cost Adsorbent: Modeling, Kinetic, Equilibrium and Thermodynamic Studies. Korean J. Chem. Eng. 2019, 36, 1575–1586. [Google Scholar] [CrossRef]
- Jayasantha Kumari, H.; Krishnamoorthy, P.; Arumugam, T.K.; Radhakrishnan, S.; Vasudevan, D. An Efficient Removal of Crystal Violet Dye from Waste Water by Adsorption onto TLAC/Chitosan Composite: A Novel Low Cost Adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Boujraf, S.; Taouda, H.; Zerrouq, F. Assessment of Adsorption Kinetics for Removal Potential of Crystal Violet Dye from Aqueous Solutions Using Moroccan Pyrophyllite. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 23, 20–28. [Google Scholar] [CrossRef]
- Muthukumaran, C.; Sivakumar, V.M.; Thirumarimurugan, M. Adsorption Isotherms and Kinetic Studies of Crystal Violet Dye Removal from Aqueous Solution Using Surfactant Modified Magnetic Nanoadsorbent. J. Taiwan Inst. Chem. Eng. 2016, 63, 354–362. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Adsorption of Crystal Violet onto Functionalised Multi-Walled Carbon Nanotubes: Equilibrium and Kinetic Studies. Ecotoxicol. Environ. Saf. 2016, 134, 390–397. [Google Scholar] [CrossRef]
- Loganathan, M.; Raj, A.S.; Murugesan, A.; Kumar, P.S. Effective Adsorption of Crystal Violet onto Aromatic Polyimides: Kinetics and Isotherm Studies. Chemosphere 2022, 304, 135332. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Peighambardoust, S.J.; Peighambardoust, S.H.; Pateiro, M.; Lorenzo, J.M. Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study. Molecules 2021, 26, 2241. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Synthesis of High-Performance Nickel Hydroxide Nanosheets/Gadolinium Doped-α-MnO2 Composite Nanorods as Cathode and Fe3O4/GO Nanospheres as Anode for an All-Solid-State Asymmetric Supercapacitor. J. Energy Chem. 2021, 64, 475–484. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.; Chen, Y.; Fang, Y.; Luo, H. Preparation of a Spindle δ-MnO2@Fe/Co-MOF-74 for Effective Adsorption of Arsenic from Water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127378. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Abdelrahman, E.A.; Hassanien, M.M.; Ibrahim, W.A. Application of Mesoporous Silica Nanoparticles Modified with Dibenzoylmethane as a Novel Composite for Efficient Removal of Cd(II), Hg(II), and Cu(II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2182–2196. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.M.; El-Azabawy, R.E. Efficient Removal of Methylene Blue Dye from Aqueous Media Using Fe/Si, Cr/Si, Ni/Si, and Zn/Si Amorphous Novel Adsorbents. J. Mater. Res. Technol. 2019, 8, 5301–5313. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Naglah, A.M.; Saad, F.A.; Abdelrahman, E.A. Modification of Silica Nanoparticles with 1-Hydroxy-2-Acetonaphthone as a Novel Composite for the Efficient Removal of Ni(II), Cu(II), Zn(II), and Hg(II) Ions from Aqueous Media. Arab. J. Chem. 2022, 15, 104010. [Google Scholar] [CrossRef]
- Cheriaa, J.; Khaireddine, M.; Rouabhia, M.; Bakhrouf, A. Removal of Triphenylmethane Dyes by Bacterial Consortium. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]

















| Structure formula |  |
| Chemical formula | C25H30ClN3 |
| Molar mass | 407.99 g/mol |
| Solubility | Soluble in water |
| Maximum Wavelength | 590 nm |
| Samples | % Mn | % Si | % Na | % O |
|---|---|---|---|---|
| MS1 | 28.97 | 32.04 | 9.20 | 29.79 |
| MS2 | 35.03 | 30.44 | 8.08 | 26.45 |
| MS3 | 31.07 | 28.26 | 11.53 | 29.14 |
| MS4 | 27.17 | 28.06 | 11.95 | 32.82 |
| Surface Properties | Sample | |||
|---|---|---|---|---|
| MS1 | MS2 | MS3 | MS4 | |
| BET surface area (m2/g) | 41.58 | 46.15 | 58.25 | 39.69 |
| Average pore size (nm) | 2.81 | 3.27 | 3.24 | 3.09 |
| Total pore volume (cc/g) | 0.0584 | 0.0755 | 0.0945 | 0.0714 |
| Adsorbent | QExp (mg/g) | Qe (mg/g) | Rate Constants | R2 | RSS | ||||
|---|---|---|---|---|---|---|---|---|---|
| First Order | Second Order | kF (1/min) | kS (g/mg.min) | First Order | Second Order | First Order | Second Order | ||
| MS1 | 337.82 | 280.56 | 348.43 | 0.0087 | 5.05 × 10−5 | 0.9763 | 0.9981 | 0.0019 | 8.95 × 10−5 |
| MS2 | 355.28 | 281.27 | 350.88 | 0.0088 | 6.10 × 10−5 | 0.9799 | 0.9992 | 0.0017 | 3.72 × 10−5 |
| MS3 | 407.04 | 304.53 | 416.67 | 0.0106 | 6.29 × 10−5 | 0.9849 | 0.9992 | 0.0018 | 2.71 × 10−5 |
| MS4 | 307.22 | 267.77 | 302.11 | 0.0069 | 4.49 × 10−5 | 0.9782 | 0.9981 | 0.0011 | 1.18 × 10−4 |
| Adsorbent | QExp (mg/g) | Qe (mg/g) | Rate Constants | R2 | χ2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| First Order | Second Order | kF (1/min) | kS (g/mg.min) | First Order | Second Order | First Order | Second Order | ||
| MS1 | 337.82 | 253.39 | 347.07 | 0.0210 | 5.12 × 10−5 | 0.9974 | 0.9983 | 9.54 | 6.35 |
| MS2 | 355.28 | 264.63 | 351.87 | 0.0236 | 6.04 × 10−5 | 0.9936 | 0.9991 | 24.32 | 3.53 |
| MS3 | 407.04 | 322.83 | 416.23 | 0.0272 | 6.31 × 10−5 | 0.9879 | 0.9988 | 62.19 | 6.28 |
| MS4 | 307.22 | 210.45 | 299.66 | 0.0176 | 4.61 × 10−5 | 0.9989 | 0.9992 | 2.71 | 2.21 |
| Adsorbent | ΔH° (kJ/mol) | ΔS° (kJ/mol Kelvin) | ΔG° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| 298 | 308 | 318 | 328 | |||
| MS1 | −42.85 | 0.1326 | −82.37 | −83.69 | −85.02 | −86.35 |
| MS2 | −43.57 | 0.1331 | −83.22 | −84.55 | −85.88 | −87.21 |
| MS3 | −45.66 | 0.1349 | −85.85 | −87.20 | −88.55 | −89.90 |
| MS4 | −43.90 | 0.1368 | −84.68 | −86.05 | −87.42 | −88.79 |
| Adsorbent | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | kL (L/mg) | RSS | R2 | Qmax (mg/g) | kFr (mg/g)(L/mg)1/n | RSS | R2 | |
| MS1 | 342.47 | 1.0069 | 2.11 × 10−6 | 0.9996 | 354.94 | 275.92 | 7.77 × 10−4 | 0.9533 |
| MS2 | 362.32 | 0.7095 | 6.53 × 10−6 | 0.9998 | 380.34 | 273.46 | 3.97 × 10−4 | 0.9775 |
| MS3 | 411.52 | 4.7178 | 6.65 × 10−7 | 0.9997 | 445.02 | 336.94 | 0.0224 | 0.5161 |
| MS4 | 310.56 | 0.7541 | 2.72 × 10−5 | 0.9996 | 311.25 | 274.58 | 3.62 × 10−4 | 0.8394 |
| Adsorbent | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | kL (L/mg) | R2 | χ2 | 1/n | kFr (mg/g) (L/mg)1/n | R2 | χ2 | |
| MS1 | 339.76 | 1.5149 | 0.9880 | 9.06 | 0.0449 | 276.61 | 0.9661 | 25.67 |
| MS2 | 356.73 | 1.2894 | 0.9642 | 49.75 | 0.0589 | 274.26 | 0.9831 | 23.51 |
| MS3 | 414.40 | 5.4610 | 0.9753 | 104.57 | 0.0461 | 341.23 | 0.6708 | 1395.98 |
| MS4 | 305.47 | 2.4586 | 0.7295 | 40.73 | 0.0229 | 274.38 | 0.8922 | 16.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, E.A.; Algethami, F.K.; AlSalem, H.S.; Binkadem, M.S.; Saad, F.A.; El-Sayyad, G.S.; Raza, N.; Rehman, K.u. Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye from Aqueous Media. Inorganics 2023, 11, 339. https://doi.org/10.3390/inorganics11080339
Abdelrahman EA, Algethami FK, AlSalem HS, Binkadem MS, Saad FA, El-Sayyad GS, Raza N, Rehman Ku. Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye from Aqueous Media. Inorganics. 2023; 11(8):339. https://doi.org/10.3390/inorganics11080339
Chicago/Turabian StyleAbdelrahman, Ehab A., Faisal K. Algethami, Huda S. AlSalem, Mona S. Binkadem, Fawaz A. Saad, Gharieb S. El-Sayyad, Nadeem Raza, and Khalil ur Rehman. 2023. "Facile Synthesis and Characterization of Novel Nanostructures for the Efficient Disposal of Crystal Violet Dye from Aqueous Media" Inorganics 11, no. 8: 339. https://doi.org/10.3390/inorganics11080339