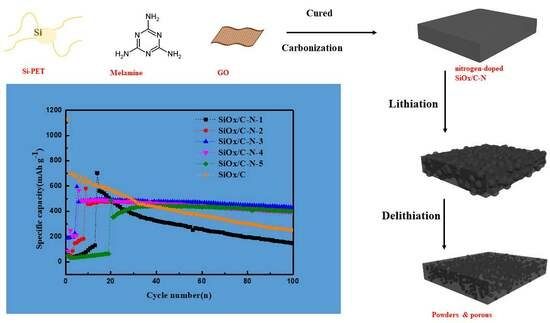
Nitrogen-Doped Carbon Matrix to Optimize Cycling Stability of Lithium Ion Battery Anode from SiOx Materials
Abstract
:1. Introduction
2. Results and Discussion
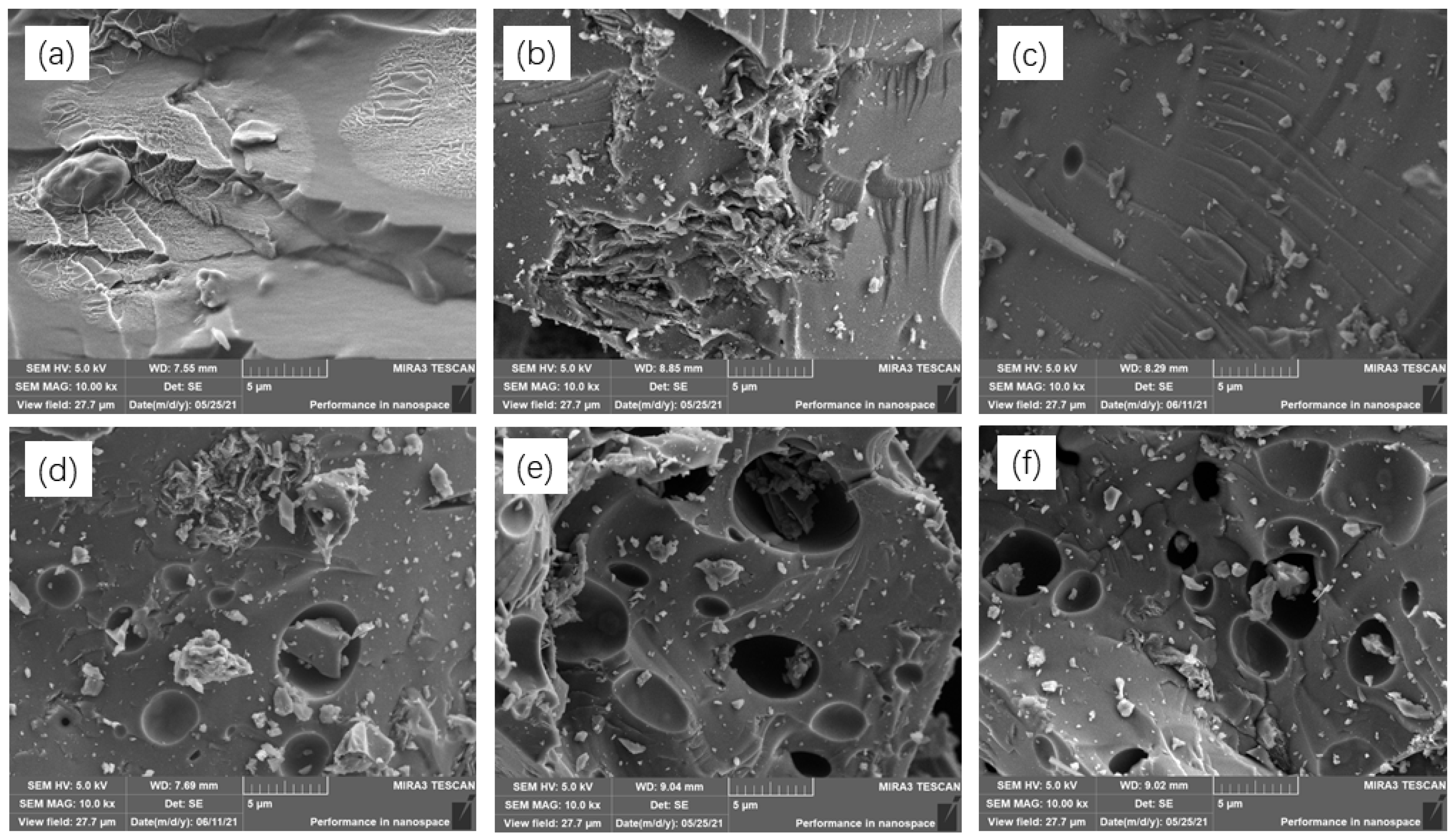
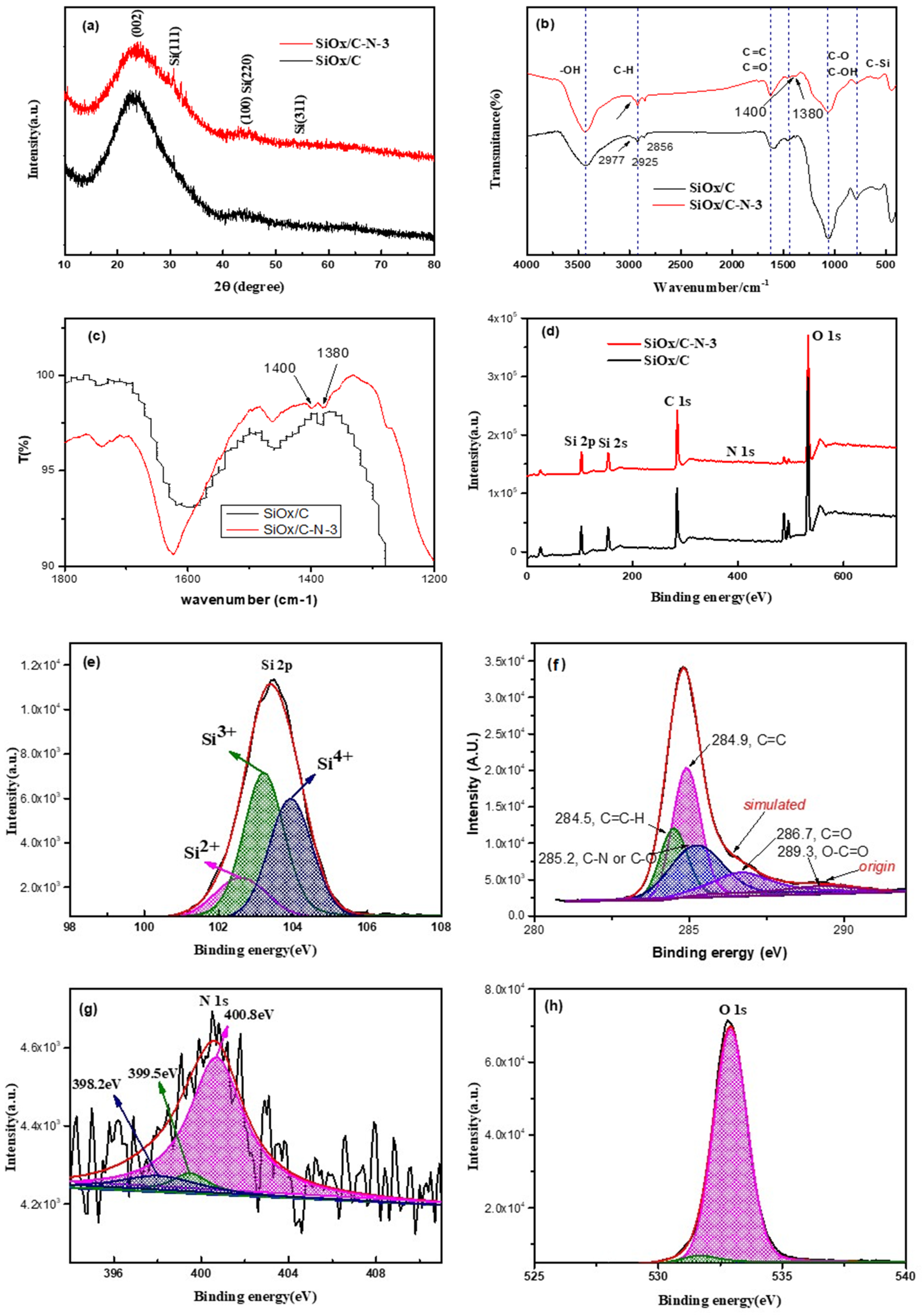
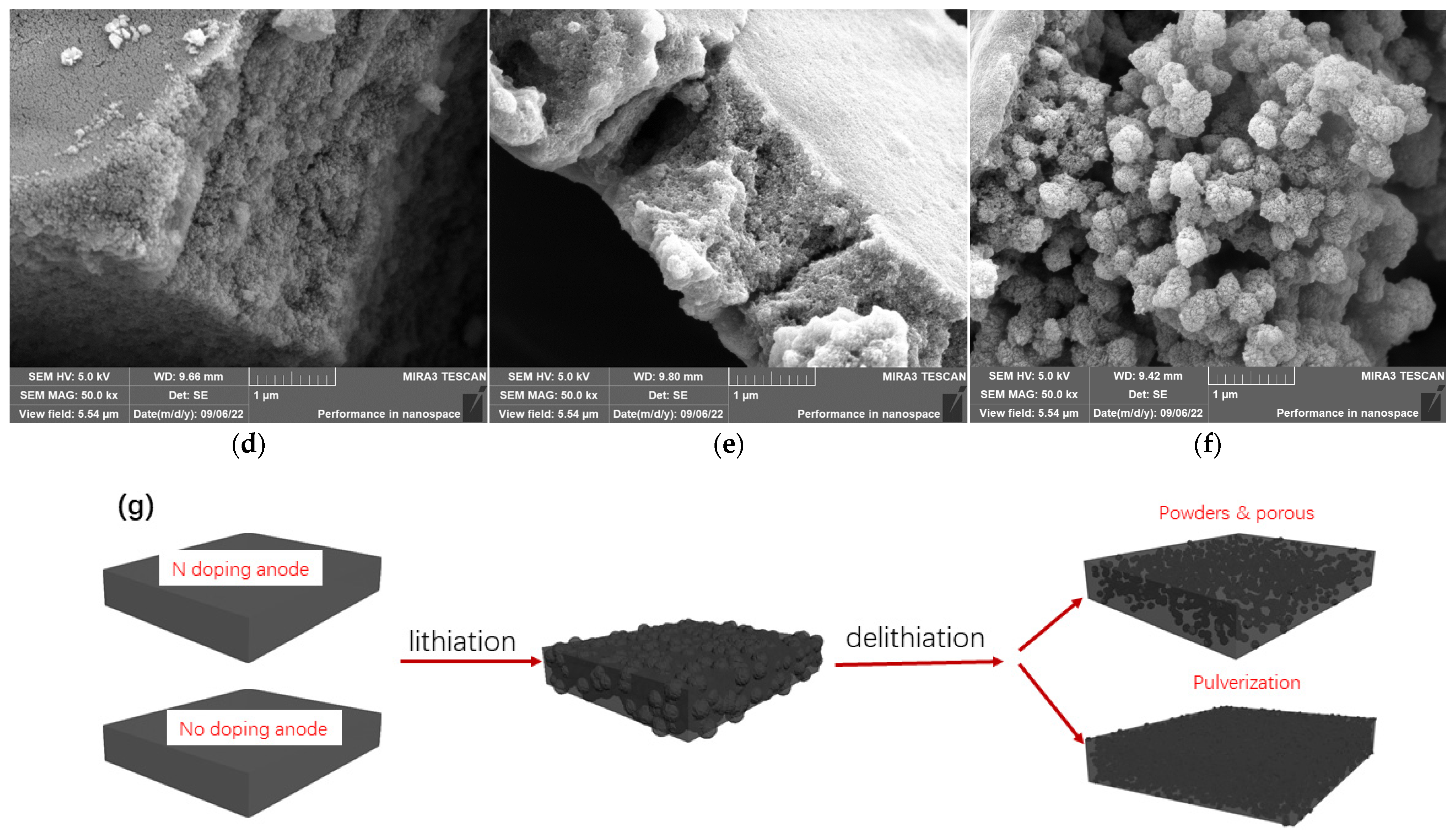
2.1. Structure Characterization
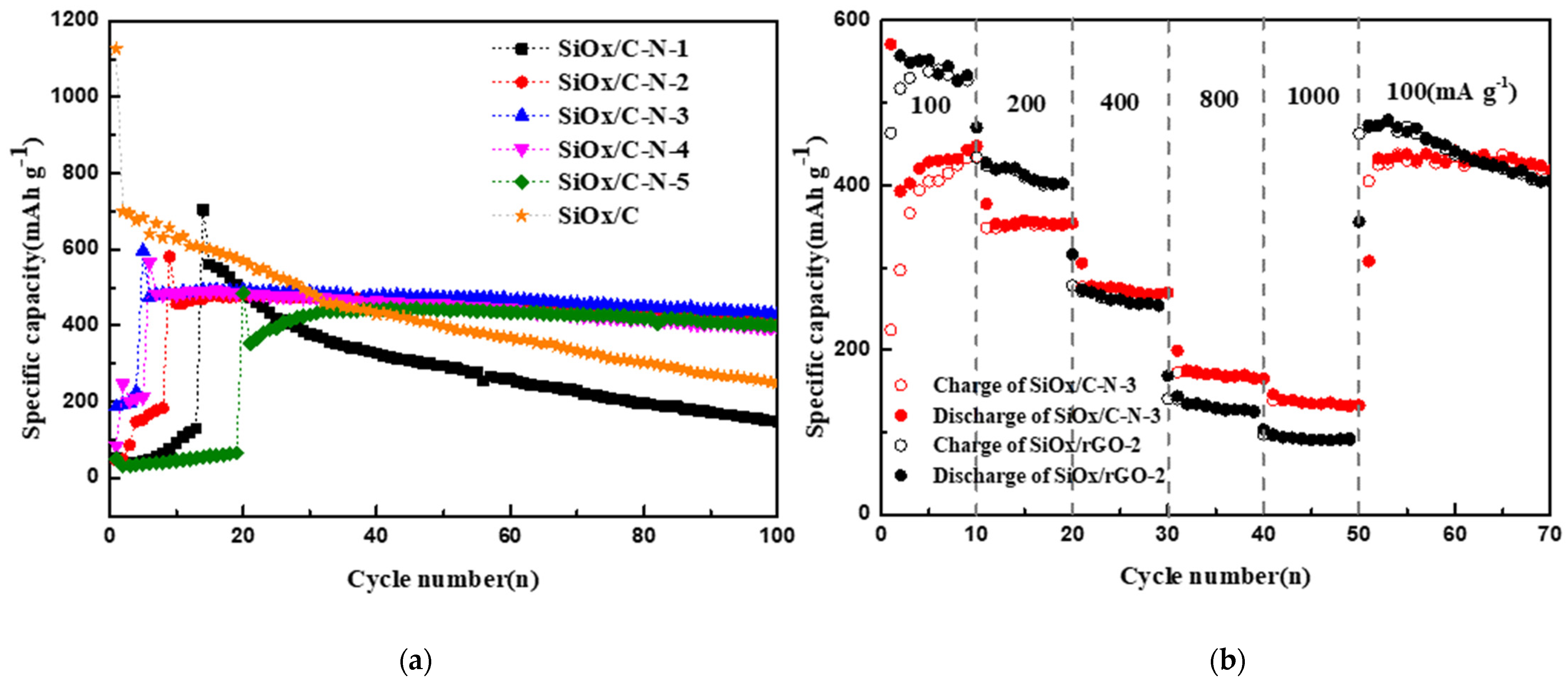
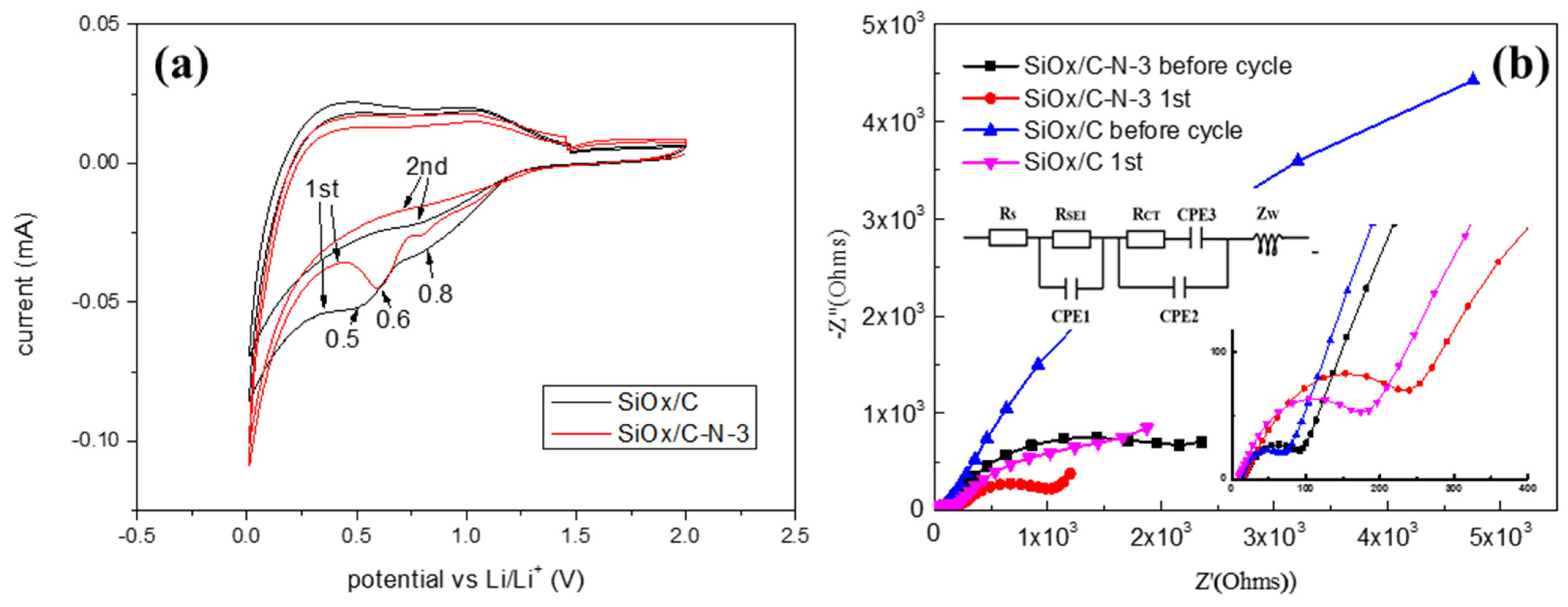
2.2. Electrochemical Performance Affected by Nitrogen Component
3. Materials
3.1. Synthesis of SiOx/C–Ns
3.2. Structural Characterization
3.3. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620. [Google Scholar] [CrossRef]
- Saint, J.; Morcrette, M.; Larcher, D.; Laffont, L.; Beattie, S.; Pérès, J.; Talaga, D.; Couzi, M.; Tarascon, J. Towards a Fundamental Understanding of the Improved Electrochemical Performance of Silicon–Carbon Composites. Adv. Funct. Mater. 2007, 17, 1765–1774. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Fang, Y.; Lv, W.; Wang, F.; Liu, Y.; Liu, H. Hydrothermal preparation of CoO/Ti3C2 composite material for lithium-ion batteries with enhanced electrochemical performance. J. Electroanal. Chem. 2018, 817, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Takeda, Y.; Imanishi, N.; Capiglia, C.; Xie, J.; Yamamoto, O. SiOx-based anodes for secondary lithium batteries. Solid State Ionics 2002, 152–153, 125–129. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2018, 48, 285–309. [Google Scholar] [CrossRef]
- Yang, J.H.; Zheng, Z.X.; Hu, L.; Tan, R.; Wang, K.; Mu, S.C.; Pan, F. FeOx and Si nano–dots as dual Li–storage centers bonded with graphene for high performance lithium ion batteries. Nanoscale 2015, 7, 14344–14350. [Google Scholar] [CrossRef]
- Zou, B.-X.; Wang, Y.; Huang, X.; Lu, Y. Hierarchical N- and O-Doped Porous Carbon Composites for High-Performance Supercapacitors. J. Nanomater. 2018, 2018, 8945042. [Google Scholar] [CrossRef]
- Jing, J.; Li, Q.; Li, C.; Yang, Z.; Yu, G.; Bai, X.; Li, T. Synchronous modification to realize micron-SiOx anode with durable and superior electrochemical performance for lithium-ion batteries. Appl. Surf. Sci. 2023, 627, 157293. [Google Scholar] [CrossRef]
- Xie, H.D.; Hou, C.P.; Qu, Y.Q.; Tian, H.; Lu, H.; Wu, J.D.; Yang, S.L.; Ma, Y. N–SiOx/graphite/rGO–CNTs@C composite with dense structure for high performance lithium–ion battery anode. J. Energy Storage 2023, 72, 108452. [Google Scholar] [CrossRef]
- Li, H.; Yu, G.; Luo, J.; Li, G.; Wang, W.; He, B.; Hou, Z.; Yin, H. Soft-template-assisted synthesis of Petals-like MoS2 nanosheets covered with N-doped carbon for long cycle-life sodium-ion battery anode. J. Electroanal. Chem. 2022, 922, 116715. [Google Scholar] [CrossRef]
- Zhang, S.S. Heteroatom-doped carbons: Synthesis, chemistry and application in lithium/sulphur batteries. Inorg. Chem. Front. 2015, 2, 1059–1069. [Google Scholar] [CrossRef]
- Ou, G.; Chen, J.; Lu, M.; Liu, J.; Zhang, X.; Lin, X.; Wu, Y.; Zeb, A.; Reddy, R.C.K.; Xu, Z. A metal–organic framework approach to engineer ZnO/Co3ZnC/N-doped carbon composite as anode material for boosting lithium storage. J. Alloys Compd. 2022, 923, 166436. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, S.J.; Choi, H.W.; Park, C.E.; Ying, J.G.; Hang, Y.; Jeong, S.Y.; Kim, J.P.; Bae, J.S.; Cho, C.R. Enhanced cycle stability of silicon nanoparticles coated with nitrogen–doped carbon layer for lithium–ion battery anode. Curr. Appl. Phys. 2017, 17, 1087–1093. [Google Scholar] [CrossRef]
- Bie, X.; Xiong, M.; Wang, B.; Dong, Y.; Chen, Z.; Huang, R. Glucose hydrothermal encapsulation of carbonized silicone polyester to prepare anode materials for lithium batteries with improved cycle stability. RSC Adv. 2022, 12, 9238–9248. [Google Scholar] [CrossRef]
- Wang, L.; Bie, X.; Dong, Y.W.; Wang, B.; Chen, Z.X.; Huang, L.; Xiong, M.; Zhang, Q.C.; Huang, R.H. Porous, Encapsulated Si−O−C lithium–ion battery anode materials from silicone–containing polyesters: Influences of graphene oxides. ACS Appl. Energy Mater. 2021, 4, 10762–10773. [Google Scholar] [CrossRef]
- Shen, T.; Xie, D.; Tang, W.; Wang, D.; Zhang, X.; Xia, X.; Wang, X.; Tu, J. Biomass-derived carbon/silicon three-dimensional hierarchical nanostructure as anode material for lithium ion batteries. Mater. Res. Bull. 2017, 96, 340–346. [Google Scholar] [CrossRef]
- Qin, Z.X.; Liu, M.L.; Li, C.F.; Cui, X.J.; Li, Y. Melamine–glyoxal–urea resin adhesive modified by polyvinyl alcohol. Pack Eng. 2022, 5, 33–38. [Google Scholar]
- Zhou, M.; Pu, F.; Wang, Z.; Cai, T.; Chen, H.; Zhang, H.; Guan, S. Facile synthesis of novel Si nanoparticles–graphene composites as high-performance anode materials for Li-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 11394–11401. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, L.; Mao, S.; Wang, X.; Wenren, H.; Xia, X.; Gu, C.; Tu, J. Core-shell structure of porous silicon with nitrogen-doped carbon layer for lithium-ion batteries. Mater. Res. Bull. 2018, 108, 170–175. [Google Scholar] [CrossRef]
- Larciprete, R.; Fabris, S.; Sun, T.; Lacovig, P.; Baraldi, A.; Lizzit, S. Dual Path Mechanism in the Thermal Reduction of Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 17315–17321. [Google Scholar] [CrossRef]
- Kato, T.; Yamada, Y.; Nishikawa, Y.; Otomo, T.; Sato, H.; Sato, S. Origins of peaks of graphitic and pyrrolic nitrogen in N1s X–ray photoelectron spectra of carbon materials: Quaternary nitrogen, tertiary amine, or secondary amine? J. Mater. Sci. 2021, 56, 15798–15811. [Google Scholar] [CrossRef]
- Yasuhiro, Y.; Haruki, T.; Shingo, K.; Satoshi, S. Unveiling bonding states and roles of edges in nitrogen–doped graphene na-noribbon by X–ray photoelectron spectroscopy. Carbon 2021, 185, 342–367. [Google Scholar]
- Yamada, Y.; Sato, H.; Gohda, S.; Taguchi, T.; Sato, S. Toward strategical bottom–up synthesis of carbon materials with excep-tionally high basal–nitrogen content: Development of screening techniques. Carbon 2023, 203, 498–522. [Google Scholar] [CrossRef]
- Pavarajarn, V.; Kimura, S. Catalytic Effects of Metals on Direct Nitridation of Silicon. J. Am. Ceram. Soc. 2004, 84, 1669–1674. [Google Scholar] [CrossRef]
- Phillipe, B.; Dedryverye, R.; Allouche, J. Nanosilicon electrodes for lithium–ion batteries: Interfacial mechanisms studied by hard and soft X–ray photoelectron spectroscopy. Chem. Mater. 2012, 24, 1107–1115. [Google Scholar] [CrossRef]
- Wang, L.; Duan, Y.; Zhang, Y.; Huang, R.; Dong, Y.; Huang, C.; Zhou, B. Surface modification of poly-(p-phenylene terephthalamide) pulp with a silane containing isocyanate group for silicone composites reinforcement. Wuhan Univ. J. Nat. Sci. 2016, 21, 505–511. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, S.; Wang, H.; Qian, J.; Yang, H.; Ai, X.; Liu, J. Surface-Bound Silicon Nanoparticles with a Planar-Oriented N-Type Polymer for Cycle-Stable Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2019, 11, 13251–13256. [Google Scholar] [CrossRef]
- Huang, S.; Cheong, L.-Z.; Wang, D.; Shen, C. Nanostructured Phosphorus Doped Silicon/Graphite Composite as Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 23672–23678. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Liu, B.; Zhao, J. Binder-Free Si Nanoparticle Electrode with 3D Porous Structure Prepared by Electrophoretic Deposition for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 7497–7504. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103. [Google Scholar] [CrossRef]
- Guo, J.; Sun, A.; Chen, X.; Wang, C.; Manivannan, A. Cyclability study of silicon–carbon composite anodes for lithium-ion batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2011, 56, 3981–3987. [Google Scholar] [CrossRef]
- Gan, C.; Zhang, C.; Wen, W.; Liu, Y.; Chen, J.; Xie, Q.; Luo, X. Enhancing Delithiation Reversibility of Li15Si4 Alloy of Silicon Nanoparticles-Carbon/Graphite Anode Materials for Stable-Cycling Lithium Ion Batteries by Restricting the Silicon Particle Size. ACS Appl. Mater. Interfaces 2019, 11, 35809–35819. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, B.; Sahore, R.; Zhang, L.; Liu, H.; Zhang, L.; Lu, W.; Zhao, B.; Zhang, Z. Surface-Functionalized Silicon Nanoparticles as Anode Material for Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 44924–44931. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zeng, Y.; Ma, T.; Wang, W.; Liu, L.; Wang, F.; Zhang, X.; Qiu, X. Quantification on Growing Mass of Solid Electrolyte Interphase and Deposited Mn(II) on the Silicon Anode of LiMn2O4 Full Lithium-Ion Cells. ACS Appl. Mater. Interfaces 2019, 11, 27839–27845. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Mu, G.; Ma, C.; Mu, D.; Wu, F. Recent progress and perspectives on silicon anode: Synthesis and prelithiation for LIBs energy storage. J. Energy Chem. 2021, 64, 615–650. [Google Scholar] [CrossRef]
- Liu, C.B.; Sun, J.C.; Zheng, P.L.; Jiang, L.; Huaiyin Liu, H.Y.; Jingchao Chai, J.C.; Liu, Q.Y.; Liu, Z.H.; Zheng, Y.; Rui, X.H. Recent advances of non–lithium metal anode materials for solid–state lithium–ion batteries. J. Mater. Chem. A 2022, 10, 16761–16778. [Google Scholar] [CrossRef]
- Zhu, S.M.; Fan, J.C.; Yang, Y.; You, L.; Wu, X.L. MoO2–Mo2C uniformly encapsulated into N, P co–doped carbon nanofibers as a freestanding anode for high and long–term lithium storage. J. Electroanal. Chem. 2022, 917, 116414. [Google Scholar] [CrossRef]
- Wu, Y.F.; Bo, S.H.; Xia, Y.Y. Solid–electrolyte interphase formation process on Li2TiSiO5 anode in LiPF6–based carbonate electrolyte. J. Power Sources 2020, 467, 228292. [Google Scholar] [CrossRef]


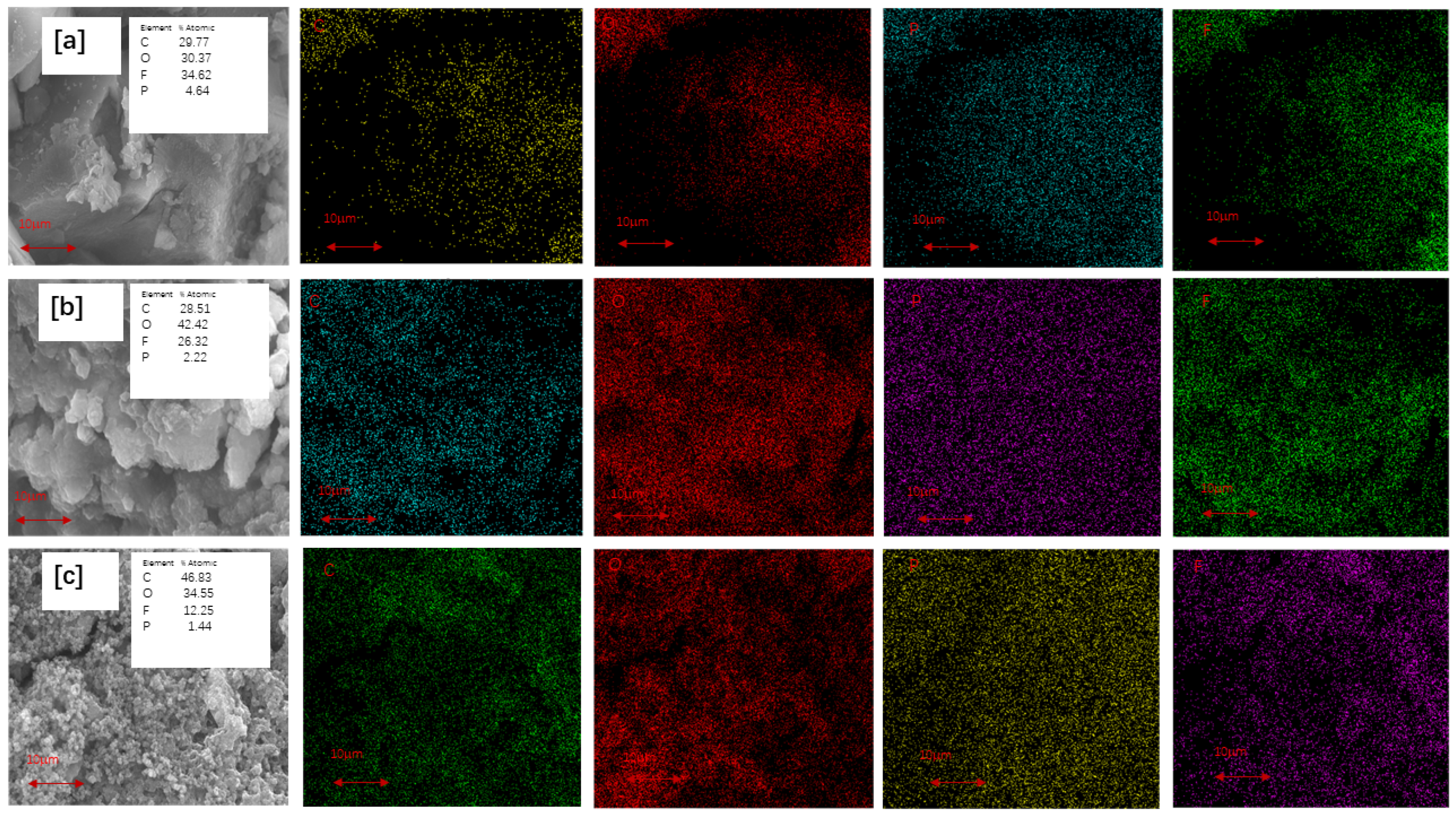
| Sample | Si (%) | O (%) | C (%) | N (%) |
| SiOx/C–N–1 | 19.84 | 32.89 | 41.94 | 5.25 |
| SiOx/C–N–3 | 12.43 | 37.88 | 42.25 | 7.43 |
| SiOx/C–N–5 | 17.39 | 26.45 | 47.78 | 8.38 |
| Sample | SiOx/C | SiOx/C–N–1 | SiOx/C–N–2 | SiOx/C–N–3 | SiOx/C–N–4 | SiOx/C–N–5 |
|---|---|---|---|---|---|---|
| Activated specific capacity (mAh/g) | 1126.7 | 560.8 | 580.7 | 493.5 | 487.2 | 484.6 |
| 100th specific capacity (mAh/g) | 249.0 | 145.9 | 429.7 | 432.8 | 394.4 | 400.8 |
| TCE (%) * | 22.1 | 26.0 | 72.3 | 87.7 | 80.1 | 82.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bie, X.; Dong, Y.; Xiong, M.; Wang, B.; Chen, Z.; Zhang, Q.; Liu, Y.; Huang, R. Nitrogen-Doped Carbon Matrix to Optimize Cycling Stability of Lithium Ion Battery Anode from SiOx Materials. Inorganics 2024, 12, 9. https://doi.org/10.3390/inorganics12010009
Bie X, Dong Y, Xiong M, Wang B, Chen Z, Zhang Q, Liu Y, Huang R. Nitrogen-Doped Carbon Matrix to Optimize Cycling Stability of Lithium Ion Battery Anode from SiOx Materials. Inorganics. 2024; 12(1):9. https://doi.org/10.3390/inorganics12010009
Chicago/Turabian StyleBie, Xuan, Yawei Dong, Man Xiong, Ben Wang, Zhongxue Chen, Qunchao Zhang, Yi Liu, and Ronghua Huang. 2024. "Nitrogen-Doped Carbon Matrix to Optimize Cycling Stability of Lithium Ion Battery Anode from SiOx Materials" Inorganics 12, no. 1: 9. https://doi.org/10.3390/inorganics12010009