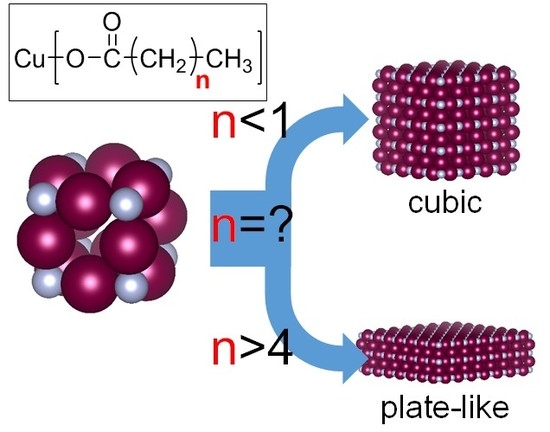
Influence of Fatty Acid Alkyl Chain Length on Anisotropy of Copper Nitride Nano-Crystallites
Abstract
:1. Introduction
2. Results
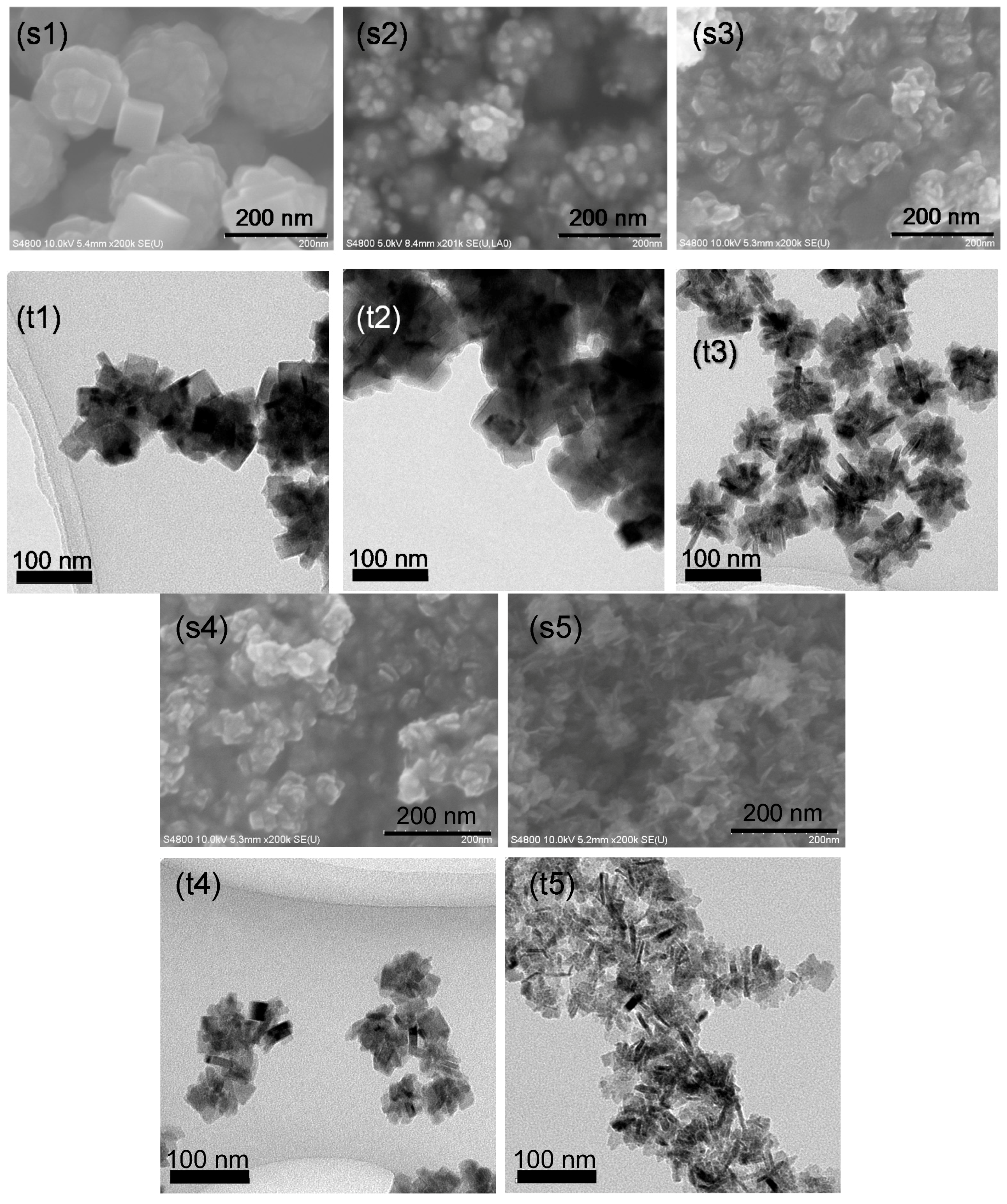
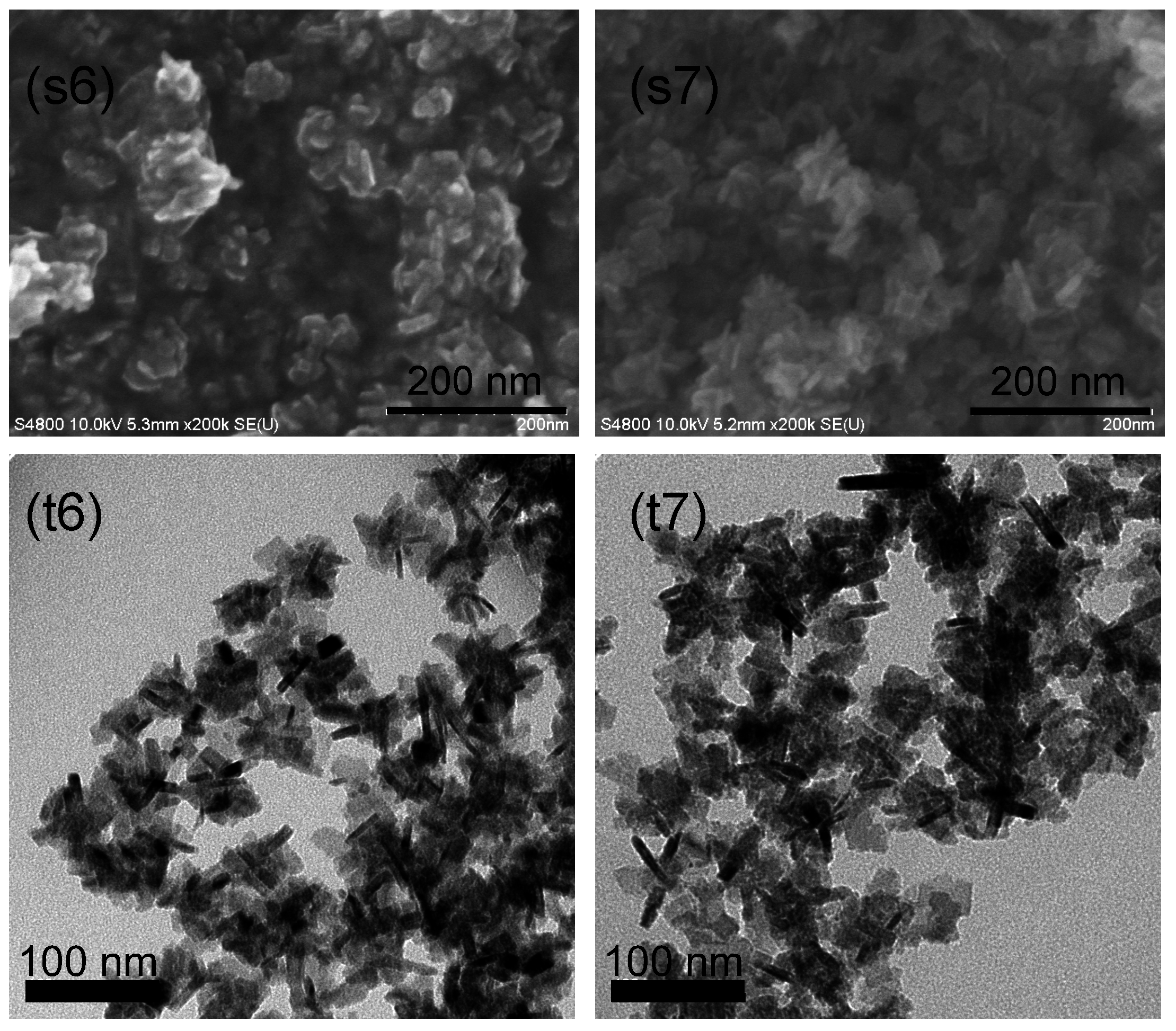
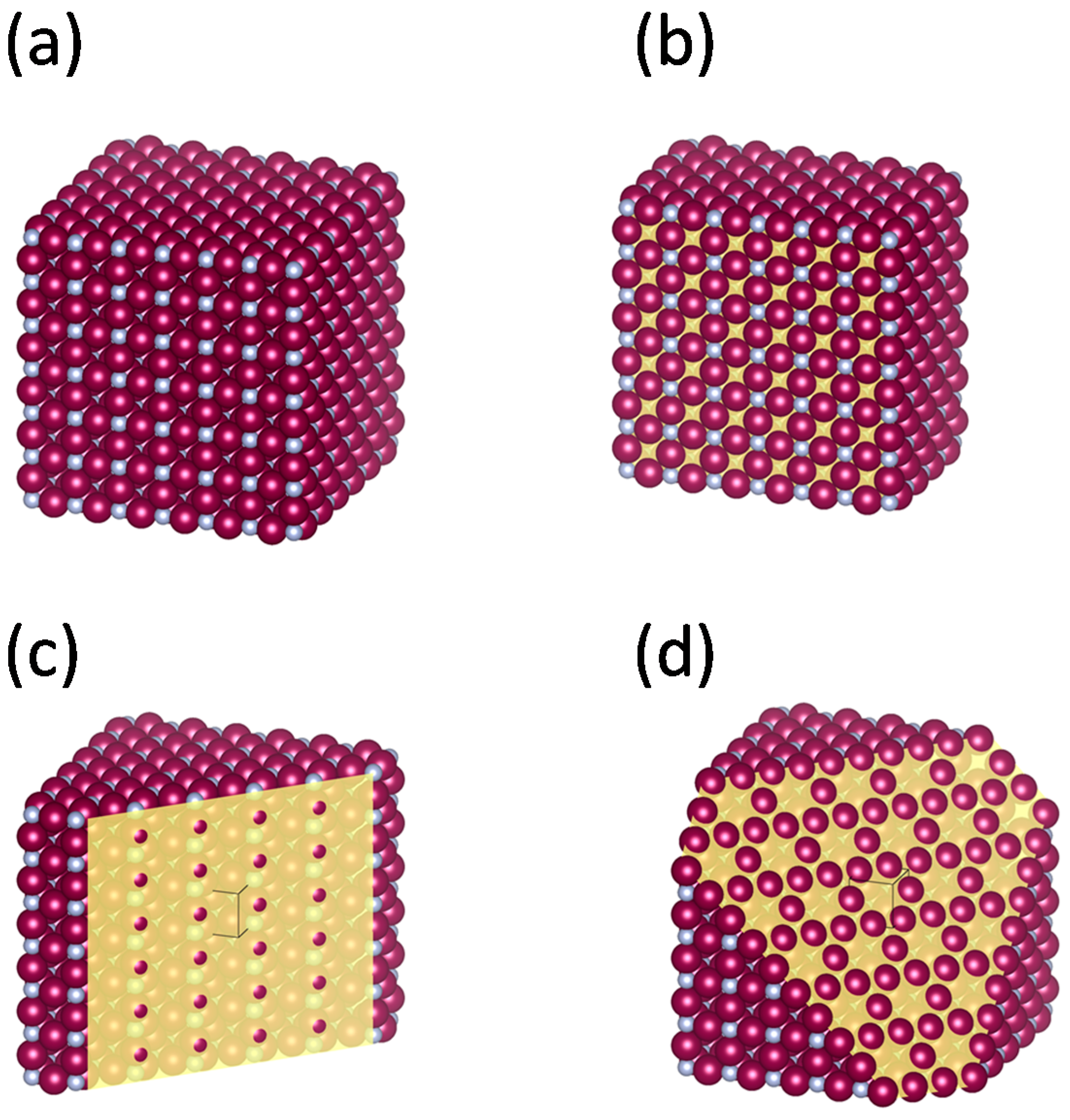
2.1. Observations of Particle and Crystallite Shape
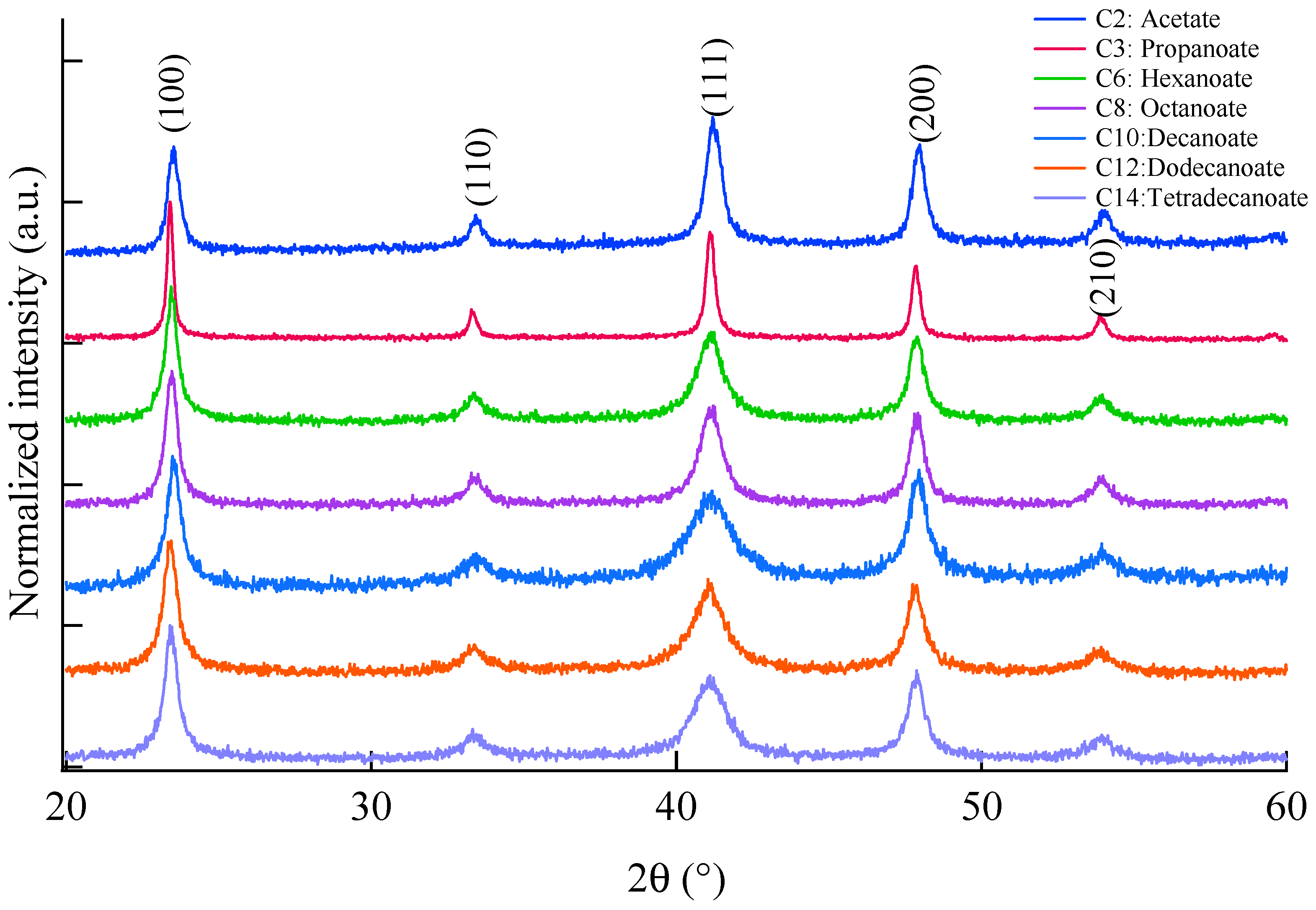
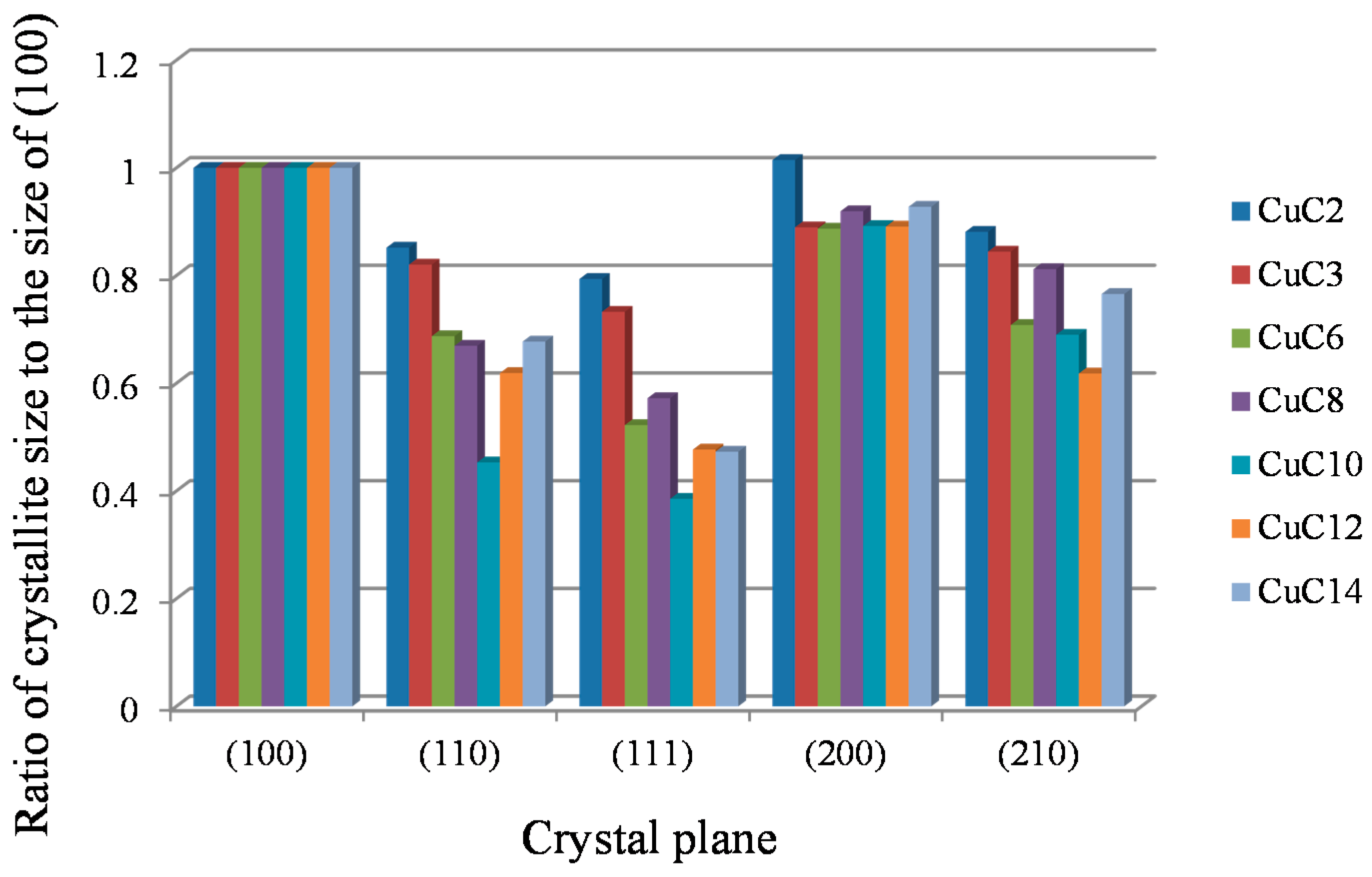
2.2. Powder XRD Measurement and Analysis of Crystallite Size
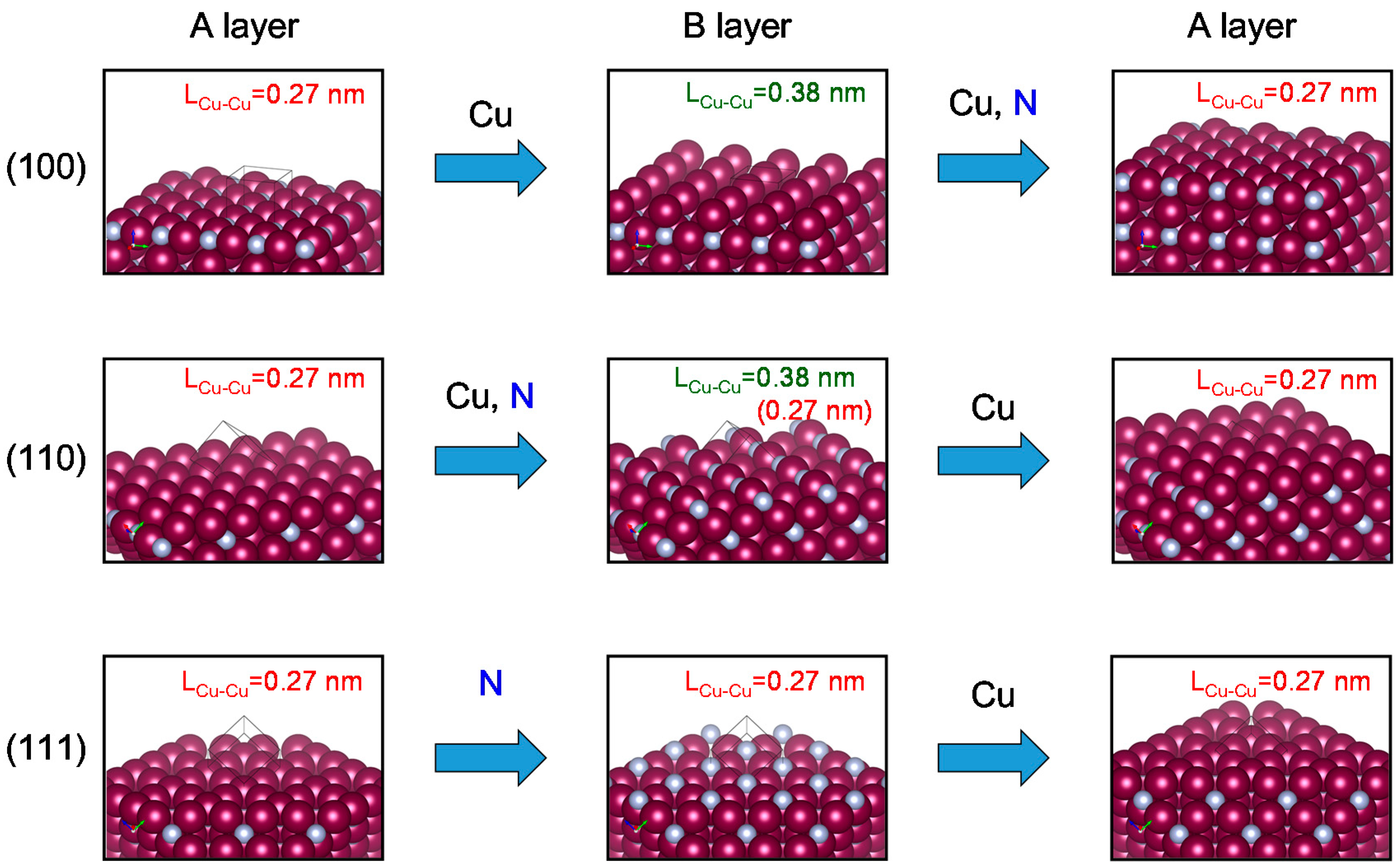
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of the Fatty Acid Copper Salt as a Starting Reagent
4.3. Preparation and Characterization of Copper Nitride Fine Particles
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | attenuated total reflection |
| FWHM | full width at half-maximum |
| MALDI-TOF MS | matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| XRD | x-ray diffractometry |
References
- Zhang, H.; Jin, M.S.; Xiong, Y.J.; Lim, B.; Xia, Y.N. Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Acc. Chem. Res. 2013, 46, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.X. Crystal plane-dependent surface reactivity and catalytic property of oxide catalysts studied with oxide nanocrystal model catalysts. Top. Catal. 2013, 56, 1363–1376. [Google Scholar] [CrossRef]
- Bensebaa, F. Clean energy. In Interface Science and Technology; Farid, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 19, pp. 279–383. [Google Scholar]
- Feng, W.L.; Huang, P.; Wang, B.C.; Wang, C.W.; Wang, W.G.; Wang, T.L.; Chen, S.F.; Lv, R.L.; Qin, Y.H.; Ma, J.Y. Solvothermal synthesis of ZnO with different morphologies in dimethylacetamide media. Ceram. Int. 2016, 42, 2250–2256. [Google Scholar] [CrossRef]
- Chen, R.R.; Han, B.N.; Yang, L.; Yang, Y.M.; Xu, Y.; Mai, Y.H. Controllable synthesis and characterization of CdS quantum dots by a microemulsion-mediated hydrothermal method. J. Lumin. 2016, 172, 197–200. [Google Scholar] [CrossRef]
- Bensebaa, F. Optoelectronics. In Interface Science and Technology; Farid, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 19, pp. 429–479. [Google Scholar]
- Turco Liveri, V. Controlled Synthesis of Nanoparticles in Microheterogeneous Systems; Springer: Berlin, Germany, 2006. [Google Scholar]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Yarulin, A.; Yuranov, I.; Cárdenas-Lizana, F.; Abdulkin, P.; Kiwi-Minsker, L. Size-effect of Pd-(poly(N-vinyl-2-pyrrolidone)) nanocatalysts on selective hydrogenation of alkynols with different alkyl chains. J. Phys. Chem. C 2013, 117, 13424–13434. [Google Scholar] [CrossRef]
- Liu, J.; Jin, J.; Deng, Z.; Huang, S.Z.; Hu, Z.Y.; Wang, L.; Wang, C.; Chen, L.H.; Li, Y.; Van Tendeloo, G.; et al. Tailoring CuO nanostructures for enhanced photocatalytic property. J. Colloid Interface Sci. 2012, 384, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, J.; Li, X.M.; Li, J.D.; Zhou, J.X.; Zhang, Z.H.; Guo, W.L. Large single-crystal hexagonal boron nitride monolayer domains with controlled morphology and straight merging boundaries. Small 2015, 11, 4497–4502. [Google Scholar] [CrossRef] [PubMed]
- Chirico, P.; Hector, A.L.; Mazumder, B. Solvothermal synthesis of group 5 and 6 nitrides via reactions using LiNH2 and ammonia nitrogen sources. Dalton Trans. 2010, 39, 6092–6097. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kumta, P.N. Synthesis, structure, and electrochemical characterization of nanocrystalline tantalum and tungsten nitrides. J. Am. Ceram. Soc. 2007, 90, 3113–3120. [Google Scholar] [CrossRef]
- Juza, R.; Hahn, H. Crystal structures of Cu3N, GaN and InN—Metallic amides and metallic nitrides. Z. Anorg. Allg. Chem. 1938, 239, 282–287. [Google Scholar] [CrossRef]
- Juza, R. Remarks on the crystal structure of Cu3N. Z. Anorg. Allg. Chem. 1941, 248, 118–120. [Google Scholar] [CrossRef]
- Li, X.J.; Hector, A.L.; Owen, J.R. Evaluation of Cu3N and CuO as negative electrode materials for sodium batteries. J. Phys. Chem. C 2014, 118, 29568–29573. [Google Scholar] [CrossRef]
- Szczęsny, R.; Szłyk, E.; Wiśniewski, M.A.; Hoang, T.K.A.; Gregory, D.H. Facile preparation of copper nitride powders and nanostructured films. J. Mater. Chem. C 2016, 4, 5031–5037. [Google Scholar] [CrossRef]
- Choi, J.L.; Gillan, E.G. Solvothermal synthesis of nanocrystalline copper nitride from an energetically unstable copper azide precursor. Inorg. Chem. 2005, 44, 7385–7393. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, W. Copper nitride nanocubes: Size-controlled synthesis and application as cathode catalyst in alkaline fuel cells. J. Am. Chem. Soc. 2011, 133, 15236–15239. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y. Controllable synthesis of Cu-based nanocrystals in ODA solvent. Chem. Commun. 2011, 47, 3604–3606. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hayashi, H.; Hanaoka, T.; Ebina, T. Preparation of copper nitride (Cu3N) nanoparticles in long-chain alcohols at 130–200 °C and nitridation mechanism. Inorg. Chem. 2014, 53, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hayashi, H.; Ebina, T. Preparation of copper nitride nanoparticles using urea as a nitrogen source in a long-chain alcohol. J. Nanopart. Res. 2014, 16, 2699. [Google Scholar] [CrossRef]
- Nakamura, T.; Hiyoshi, N.; Hayashi, H.; Ebina, T. Preparation of plate-like copper nitride nanoparticles from a fatty acid copper(II) salt and detailed observations by high resolution transmission electron microscopy and high-angle annular dark-field scanning transmission electron microscopy. Mater. Lett. 2015, 139, 271–274. [Google Scholar] [CrossRef]
- Nakamura, T.; Tsukahara, Y.; Sakata, T.; Mori, H.; Kanbe, Y.; Bessho, H.; Wada, Y. Preparation of monodispersed Cu nanoparticles by microwave-assisted alcohol reduction. Bull. Chem. Soc. Jpn. 2007, 80, 224–232. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; Wiley: New York, NY, USA, 1997. [Google Scholar]
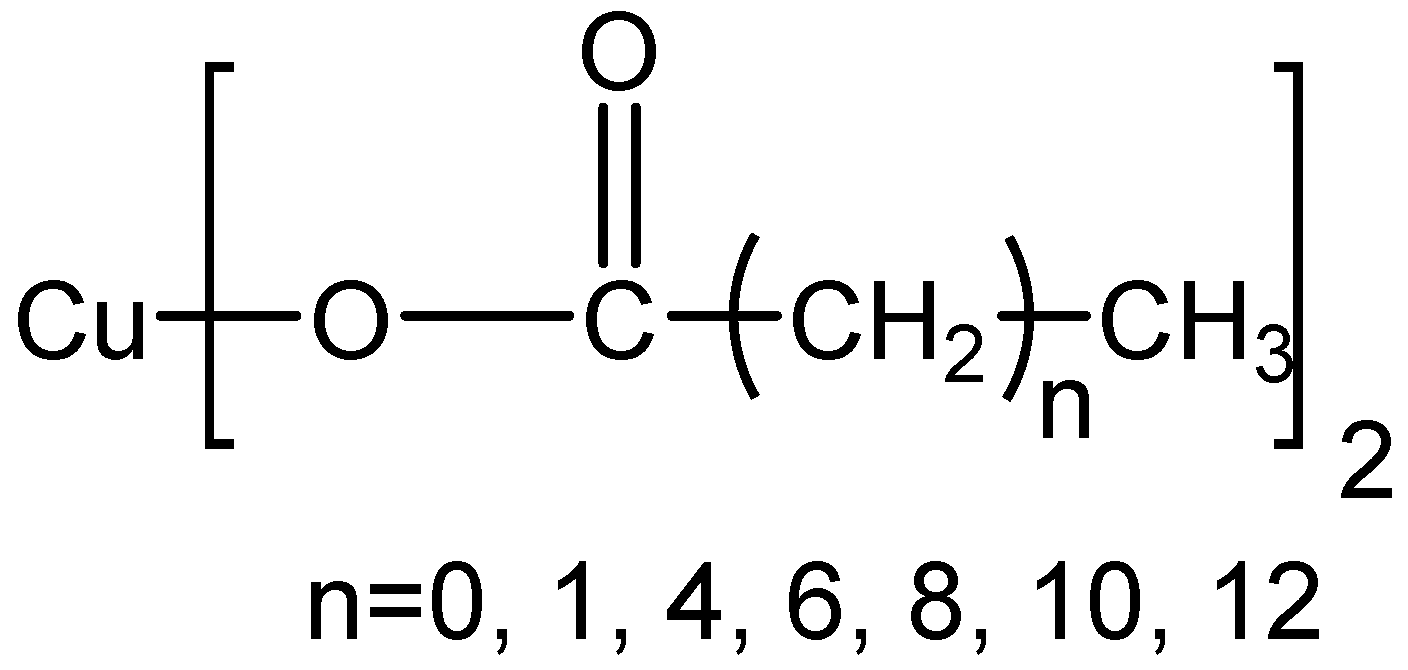
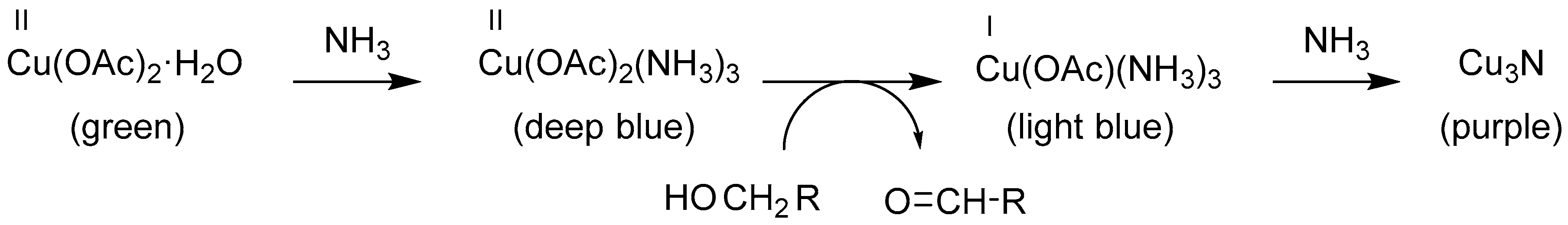
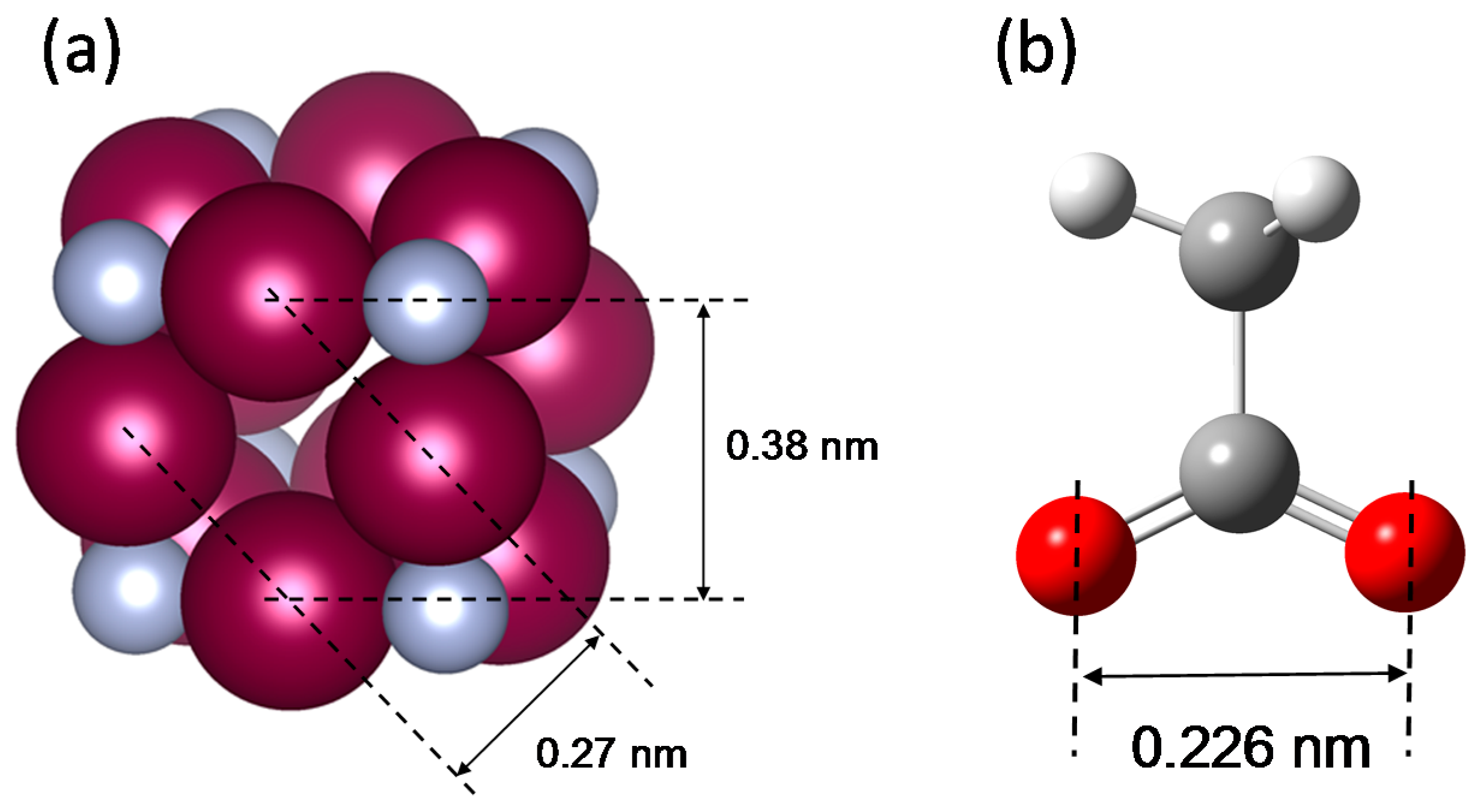
| IUPAC Name | Common Name | Length of Alkyl Chain | Abbreviation for Reagent |
|---|---|---|---|
| Copper ethanoate | Copper acetate | 1 | CuC2 |
| Copper propanoate | Copper propionate | 2 | CuC3 |
| Copper hexanoate | Copper caproate | 5 | CuC6 |
| Copper octanoate | Copper caprylate | 7 | CuC8 |
| Copper decanoate | Copper caprinate | 9 | CuC10 |
| Copper dodecanoate | Copper laurate | 11 | CuC12 |
| Copper tetradecanoate | Copper myristate | 13 | CuC14 |
| Starting Reagents | 2θ (°) | Lattice Spacing (nm) | ||||||||
| (100) | (110) | (111) | (200) | (210) | (100) | (110) | (111) | (200) | (210) | |
| CuC2 | 23.466 | 33.367 | 41.180 | 47.895 | 54.070 | 0.3788 | 0.2683 | 0.2190 | 0.1898 | 0.1695 |
| CuC3 | 23.384 | 33.277 | 41.087 | 47.810 | 53.891 | 0.3801 | 0.2690 | 0.2195 | 0.1901 | 0.1700 |
| CuC6 | 23.427 | 33.335 | 41.083 | 47.818 | 53.930 | 0.3794 | 0.2686 | 0.2195 | 0.1901 | 0.1699 |
| CuC8 | 23.437 | 33.306 | 41.088 | 47.841 | 53.977 | 0.3793 | 0.2688 | 0.2195 | 0.1900 | 0.1697 |
| CuC10 | 23.500 | 33.446 | 41.110 | 47.885 | 53.949 | 0.3783 | 0.2677 | 0.2194 | 0.1898 | 0.1698 |
| CuC12 | 23.383 | 33.288 | 41.055 | 47.795 | 53.752 | 0.3801 | 0.2689 | 0.2197 | 0.1902 | 0.1704 |
| CuC14 | 23.416 | 33.244 | 41.065 | 47.840 | 53.780 | 0.3796 | 0.2693 | 0.2196 | 0.1900 | 0.1703 |
| FWHM (°) | Crystallite Size (nm) | |||||||||
| (100) | (110) | (111) | (200) | (210) | (100) | (110) | (111) | (200) | (210) | |
| CuC2 | 0.4673 | 0.4909 | 0.5889 | 0.4873 | 0.5771 | 13.44 | 11.45 | 10.67 | 13.64 | 11.84 |
| CuC3 | 0.2050 | 0.2756 | 0.2957 | 0.2523 | 0.2295 | 27.08 | 22.22 | 19.84 | 24.09 | 22.87 |
| CuC6 | 0.4717 | 0.6630 | 1.0092 | 0.5725 | 0.6630 | 11.45 | 7.87 | 5.98 | 10.16 | 8.11 |
| CuC8 | 0.4688 | 0.6607 | 0.8865 | 0.5418 | 0.6299 | 12.35 | 8.27 | 7.07 | 11.36 | 10.03 |
| CuC10 | 0.5617 | 1.1800 | 1.7235 | 0.6904 | 1.1300 | 9.25 | 4.19 | 3.56 | 8.25 | 6.38 |
| CuC12 | 0.5387 | 0.8553 | 1.2636 | 0.6472 | 1.0443 | 9.96 | 6.17 | 4.75 | 8.88 | 6.16 |
| CuC14 | 0.5301 | 0.7867 | 1.3184 | 0.6250 | 0.9190 | 10.12 | 6.86 | 4.79 | 9.39 | 7.75 |
| Ratio of the Crystallite Size of Each Crystal Plane to the Crystallite Size of the (100) Plane | ||||||||||
| (100) | (110) | (111) | (200) | (210) | ||||||
| CuC2 | 1.00 | 0.85 | 0.79 | 1.01 | 0.88 | |||||
| CuC3 | 1.00 | 0.82 | 0.73 | 0.89 | 0.84 | |||||
| CuC6 | 1.00 | 0.69 | 0.52 | 0.89 | 0.71 | |||||
| CuC8 | 1.00 | 0.67 | 0.57 | 0.92 | 0.81 | |||||
| CuC10 | 1.00 | 0.45 | 0.39 | 0.89 | 0.69 | |||||
| CuC12 | 1.00 | 0.62 | 0.48 | 0.89 | 0.62 | |||||
| CuC14 | 1.00 | 0.68 | 0.47 | 0.93 | 0.77 | |||||
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T. Influence of Fatty Acid Alkyl Chain Length on Anisotropy of Copper Nitride Nano-Crystallites. Inorganics 2017, 5, 6. https://doi.org/10.3390/inorganics5010006
Nakamura T. Influence of Fatty Acid Alkyl Chain Length on Anisotropy of Copper Nitride Nano-Crystallites. Inorganics. 2017; 5(1):6. https://doi.org/10.3390/inorganics5010006
Chicago/Turabian StyleNakamura, Takashi. 2017. "Influence of Fatty Acid Alkyl Chain Length on Anisotropy of Copper Nitride Nano-Crystallites" Inorganics 5, no. 1: 6. https://doi.org/10.3390/inorganics5010006
APA StyleNakamura, T. (2017). Influence of Fatty Acid Alkyl Chain Length on Anisotropy of Copper Nitride Nano-Crystallites. Inorganics, 5(1), 6. https://doi.org/10.3390/inorganics5010006